Novel Behavior of Thiiranium Radical Cation Intermediates. Reactions of Dimethyl Disulfide with Alkenes in the Presence of Pd(OAc)2
Abstract
:Introduction
Results and Discussion
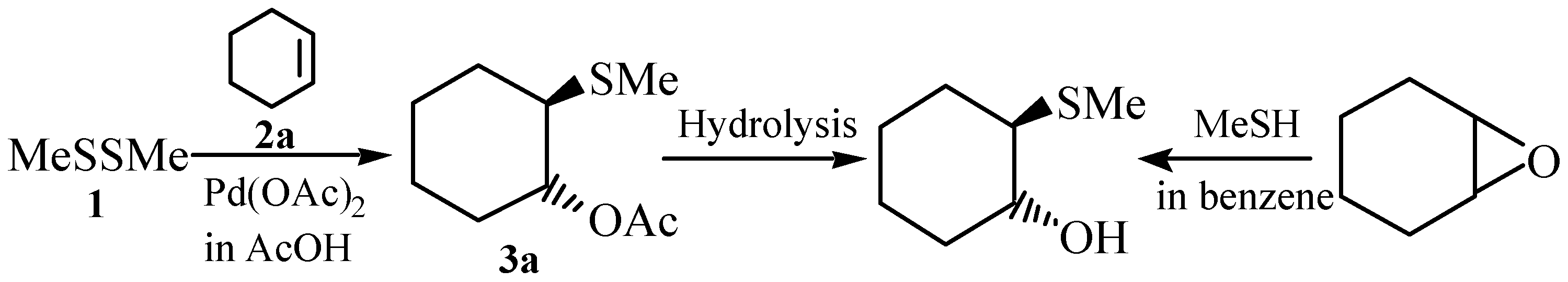
Reactions with Cyclohexene (2a)
Reactions with Hex-1-ene (2b)
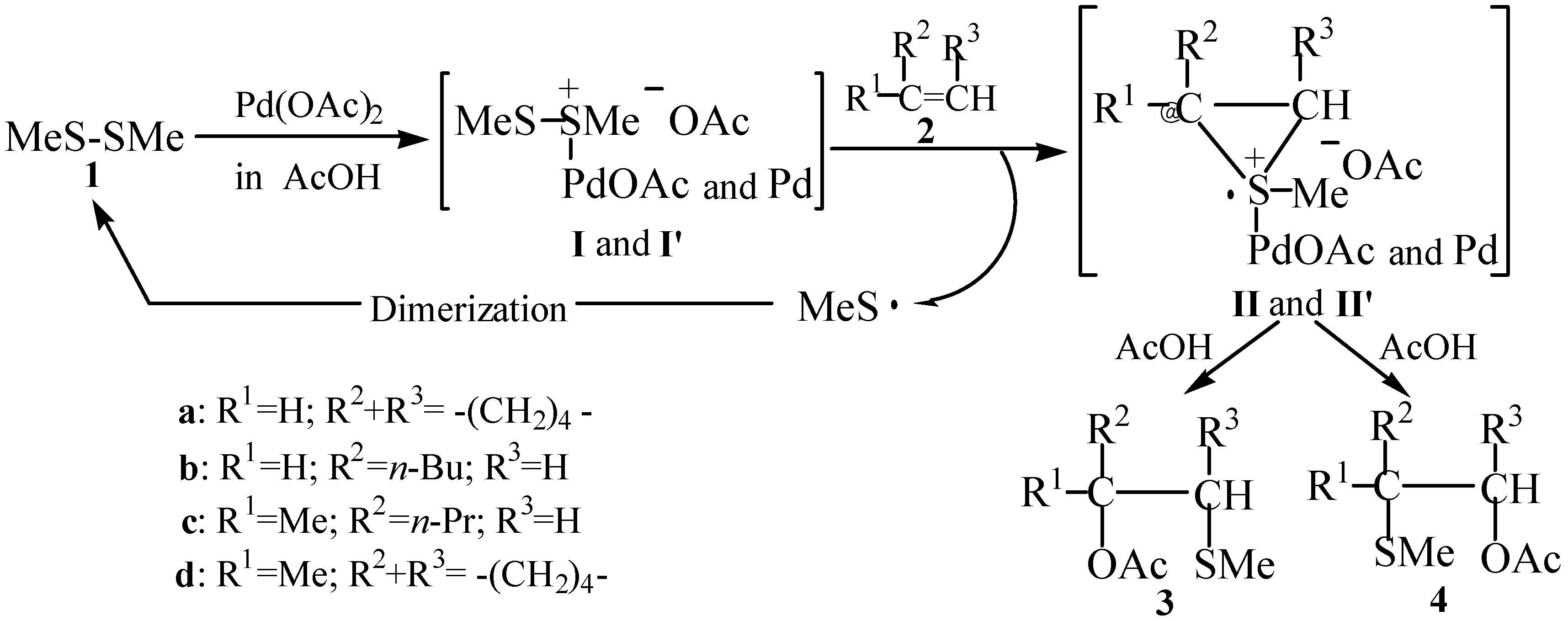
Formation of Thiiranium Radical Cations II and II’ from Sulfonium Salts I and I’
Reactions with 2-Methylpent-1-ene (2c)
Reactions with 1-Methylcyclohex-1-ene (2d)
Effects of Solvent and Concentration of AcOH
Effects of Oxidants
Experimental
General
Experimental procedures
Reactions of dimethyl disulfide (1) with cyclohexene (2a), hex-1-ene (2b), 2-methylpent-1-ene (2c) and 1-methylcyclohex-1-ene (2d) in AcOH containing Ac2O in the presence of Pd(OAc)2
Trans-1-acetoxy-2-methylthiocyclohexane (3a)
2-Acetoxy-2-methyl-1-methylthiopentane (3c)
Effects of solvents and concentration of AcOH on the reaction of dimethyl disulfide (1) with hex-1-ene (2b) or 2-methylpent-1-ene (2c)
Effects of oxidants in the reaction of dimethyl disulfide (1) with hex-1-ene (2b)
References and Notes
- Smit, W. A.; Zefirov, N. S.; Bodrikov, I. V.; Krimer, M. Z. Acc. Chem. Res. 1979, 12, 282.
- Trost, B. M.; Ochiai, M.; McDougal, P. G. J. Am. Chem. Soc. 1978, 100, 7103.
- Bewick, A.; Mellor, J. M.; Owton, W. M. J. Chem. Soc., Perkin Trans. 1 1985, 1039.
- E. Samii, Z. K. M. A.; Ashmawy, M. I. A.; Mellor, J. M. Tetrahedron Lett. 1986, 27, 52859.
- Bewick, A.; Mellor, J. M.; Milano, D.; Owton, W. M. J. Chem. Soc., Perkin Trans. 1 1985, 1045.
- Bewick, A.; Coe, D. E.; Mellor, J. M.; Owton, W. M. J. Chem. Soc., Perkin Trans. 1 1985, 1033.
- It has been reported that the disulfide radical cation is highly reactive in solution, regardless of whether it is generated by chemical, photochemical or electrochemical oxidation, and thus the spectral confirmation is difficult in solution; Marti, V.; Fernandez, L.; Garcia, H.; Roth, H. D. J. Chem. Soc., Perkin Trans. 2 1999, 145. We have recently observed that the disulfide radical cation (from MeSSMe) with λmax 375 nm is not capable of dissociation into a sulfenium ion, and undergoes an aromatic methylthiolation; the details will be published in the near future.
- A sulfenium ion seems to be formed in the first instance, but this formation would be impossible because a very unstable sulfenium ion can be generated when it interacts directly with the unshared electron-pair of a compound formed by dissociation from the precursor; In fact, arylsufenium ions are generated by interaction with both the unshared electron-pair of amine and the counterion: Takeuchi, H.; Oya, H.; Yanase, T.; Itou, K.; Adachi, T.; Sugiura, H.; Hayashi, N. J. Chem. Soc., Perkin Trans. 2 1994, 827.
- Unpublished data; in the near future we will publish the details in which the reaction of 1 with alkene using AgOAc proceeds via the thiiranium ion formed from [MeS-S+(Me)Ag]–OAc because of the formation of MeSAg.
- Mueller, H. Angew. Chem. Int. Ed. Engl. 1969, 8, 482.
- Lucchini, V.; Modena, G.; Pasquato, L. J. Am. Chem. Soc. 1988, 110, 6900.
- Lucchini, V.; Modena, G.; Pasquato, L. J. Am. Chem. Soc. 1991, 113, 6600.
- Huang, X.; Batchelor, R. J.; Einstein, F. W. B.; Bennet, A. J. J. Org. Chem. 1994, 59, 7108.
- Luccchini, V.; Modena, G.; Pasi, M.; Pasquato, L. J. Org. Chem. 1997, 62, 7018.
- Ogawa, A.; Tanaka, H.; Yokoyama, H.; Obayashi, R.; Yokoyama, K.; Sonoda, N. J. Org. Chem. 1992, 57, 111.
- Fristad, W. E.; Peterson, J. R. J. Org. Chem. 1985, 50, 10.
- Sample Availability: Available from the authors.

| Alkene | T/oC | t/h | Yielda(%) | Ratio | |
|---|---|---|---|---|---|
| 3 | 4 | 3/4 | |||
| 2a | 25 | 24 | 69b | - | - |
| 2a | 116 | 24 | 68b | - | - |
| 2b | 0 | 2.0 | 5.6 | 15 | 0.37 |
| 2b | 0 | 4.0 | 8.8 | 24 | 0.37 |
| 2b | 25 | 2.0 | 14 | 32 | 0.44 |
| 2b | 25 | 24 | 16 | 37 | 0.43 |
| 2b | 25 | 96 | 16 | 36 | 0.44 |
| 2b | 60 | 1.0 | 13 | 29 | 0.45 |
| 2b | 60 | 2.0 | 20 | 40 | 0.50 |
| 2b | 116 | 1.0 | 17 | 23 | 0.74 |
| 2b | 116 | 2.0 | 36 | 34 | 1.1 |
| 2b | 116 | 24 | 76 | 17 | 4.5 |
| Alkene | T/oC | t/h | Yielda(%) | Ratio | ||
|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 3/4 | |||
| 2c | 0 | 2.0 | 15 | 8.5 | 0 | 1.8 |
| 2c | 0 | 4.0 | 20 | 11 | 0 | 1.8 |
| 2c | 25 | 2.0 | 18 | 10 | 0 | 1.8 |
| 2c | 25 | 24 | 34 | 17 | 0 | 2.0 |
| 2c | 60 | 2.0 | 24 | 13 | 0 | 1.8 |
| 2c | 60 | 4.0 | 47 | 26 | 0 | 1.8 |
| 2d | 0 | 2.0 | 15 | 4.1 | 3.2 | 3.7 |
| 2d | 0 | 4.0 | 26 | 7.4 | 2.8 | 3.5 |
| 2d | 25 | 2.0 | 30 | 10 | 5.3 | 3.0 |
| 2d | 25 | 24 | 39 | 14 | 3.5 | 2.8 |
| 2d | 60 | 2.0 | 38 | 14 | 3.5 | 2.7 |
| 2d | 60 | 4.0 | 44 | 20 | 4.1 | 2.2 |
| Alkene | Solvent (cm3) | Yielda(%) | Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| AcOH | CH2Cl2 | n-C6H14 | MeCN | MeNO2 | 3 | 4 | 3/4 | |
| 2b | 10 | 0 | 0 | 0 | 0 | 16 | 37 | 0.43 |
| 2b | 9.0 | 1.0 | 0 | 0 | 0 | 12 | 29 | 0.41 |
| 2b | 5.0 | 5.0 | 0 | 0 | 0 | 10 | 26 | 0.38 |
| 2b | 1.0 | 9.0 | 0 | 0 | 0 | 6.5 | 19 | 0.34 |
| 2b | 5.0 | 0 | 5.0 | 0 | 0 | 5.3 | 14 | 0.38 |
| 2b | 0 | 0 | 0 | 10 | 0 | 4.1 | 17 | 0.24 |
| 2b | 0 | 0 | 0 | 0 | 10 | 3.2 | 17 | 0.19 |
| 2b | 5.0 | 0 | 0 | 0 | 5.0 | 13 | 42 | 0.31 |
| 2bb | 10 | 0 | 0 | 0 | 0 | 5.2 | 19 | 0.27 |
| 2bb | 5.0 | 0 | 0 | 0 | 5.0 | 5.4 | 16 | 0.34 |
| 2c | 10 | 0 | 0 | 0 | 0 | 34 | 17 | 2.0 |
| 2c | 9.0 | 1.0 | 0 | 0 | 0 | 31 | 18 | 1.7 |
| 2c | 5.0 | 5.0 | 0 | 0 | 0 | 24 | 18 | 1.3 |
| 2c | 1.0 | 9.0 | 0 | 0 | 0 | 16 | 19 | 0.84 |
| 2c | 9.0 | 0 | 1.0 | 0 | 0 | 32 | 20 | 1.6 |
| 2c | 5.0 | 0 | 5.0 | 0 | 0 | 15 | 12 | 1.3 |
| 2c | 1.0 | 0 | 9.0 | 0 | 0 | 17 | 13 | 1.3 |
| 2c | 5.0 | 0 | 0 | 0 | 5.0 | 47 | 27 | 1.7 |
| Oxidant | Oxidant/mmol | Yielda(%) | Ratio | |
|---|---|---|---|---|
| 3b | 4b | 3b/4b | ||
| Pd(OAc)2+Cu(OAc)2 | b | 7.7 | 17 | 0.45 |
| Cu(OAc)2 | 0.68 | 0 | 0 | - |
| H2SO4 | 0.68 | trace | trace | - |
| PdSO4 | 0.68 | trace | trace | - |
| AgOAc | 1.36 | 5.2 | 19 | 0.27 |
| Mn(OAc)3 | 2.4 | 0 | 0 | - |
| Pb(OAc)4 | 2.4 | 0 | 0 | - |
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Takeuchi, H.; Takatori, J.; Iizuka, S. Novel Behavior of Thiiranium Radical Cation Intermediates. Reactions of Dimethyl Disulfide with Alkenes in the Presence of Pd(OAc)2. Molecules 2000, 5, 916-926. https://doi.org/10.3390/50700916
Takeuchi H, Takatori J, Iizuka S. Novel Behavior of Thiiranium Radical Cation Intermediates. Reactions of Dimethyl Disulfide with Alkenes in the Presence of Pd(OAc)2. Molecules. 2000; 5(7):916-926. https://doi.org/10.3390/50700916
Chicago/Turabian StyleTakeuchi, Hiroshi, Junnichi Takatori, and Shingo Iizuka. 2000. "Novel Behavior of Thiiranium Radical Cation Intermediates. Reactions of Dimethyl Disulfide with Alkenes in the Presence of Pd(OAc)2" Molecules 5, no. 7: 916-926. https://doi.org/10.3390/50700916




