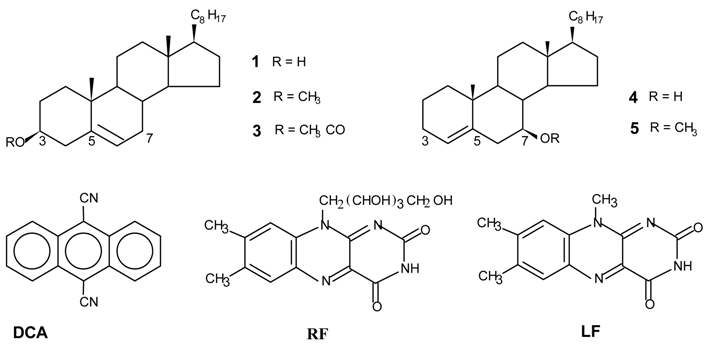
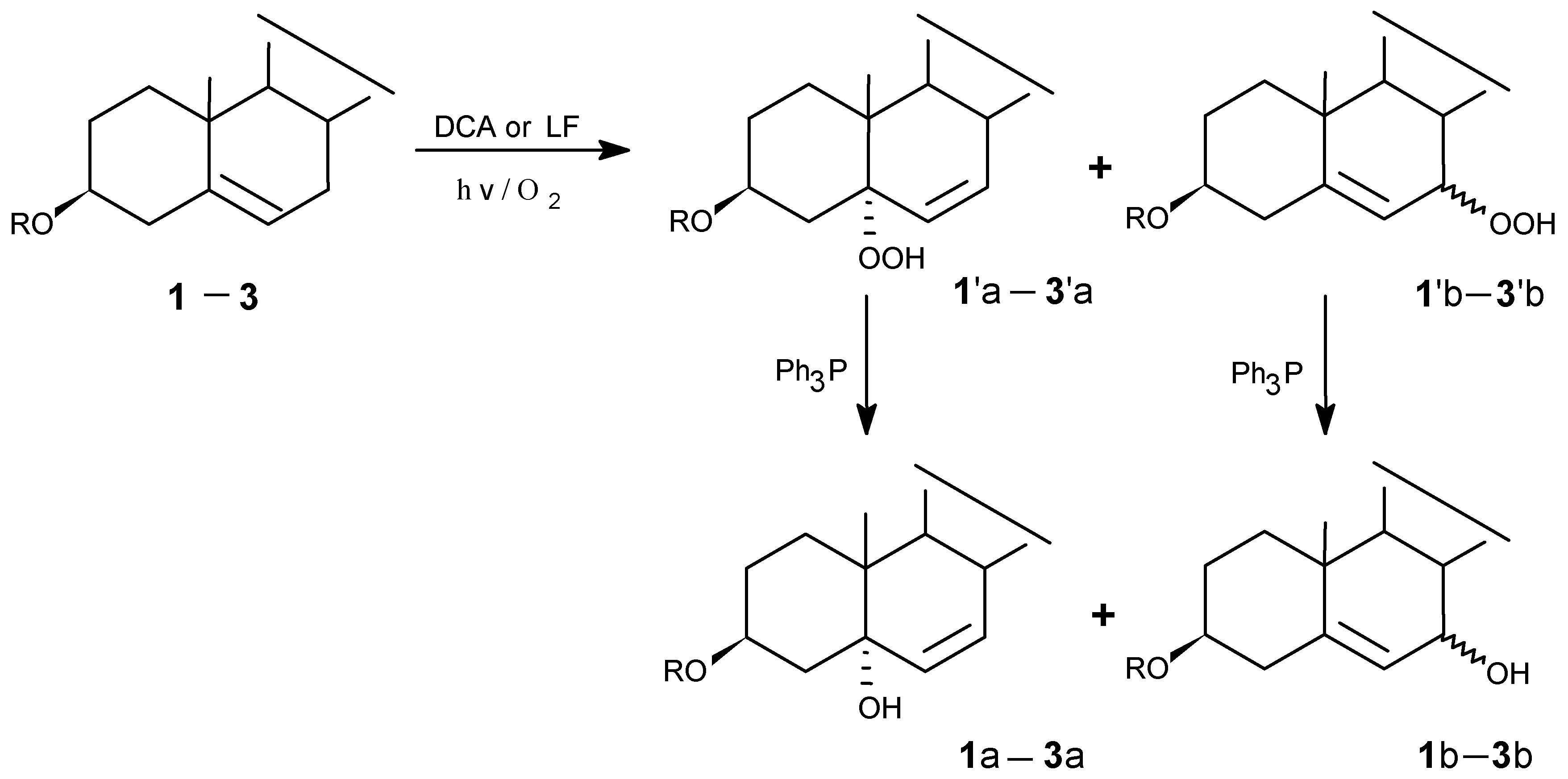
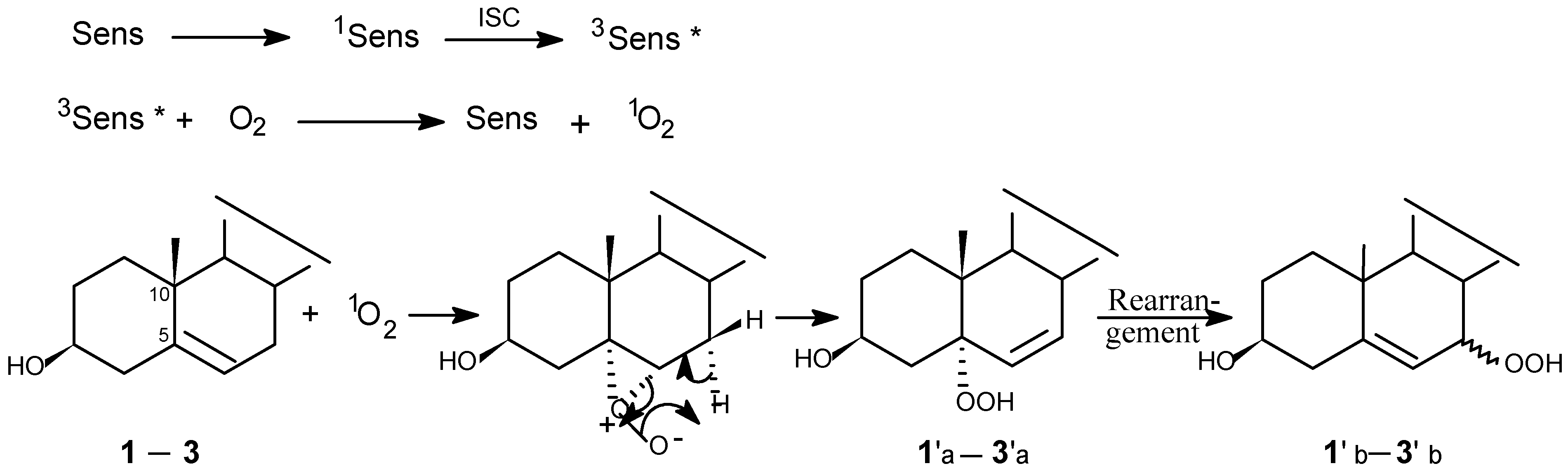
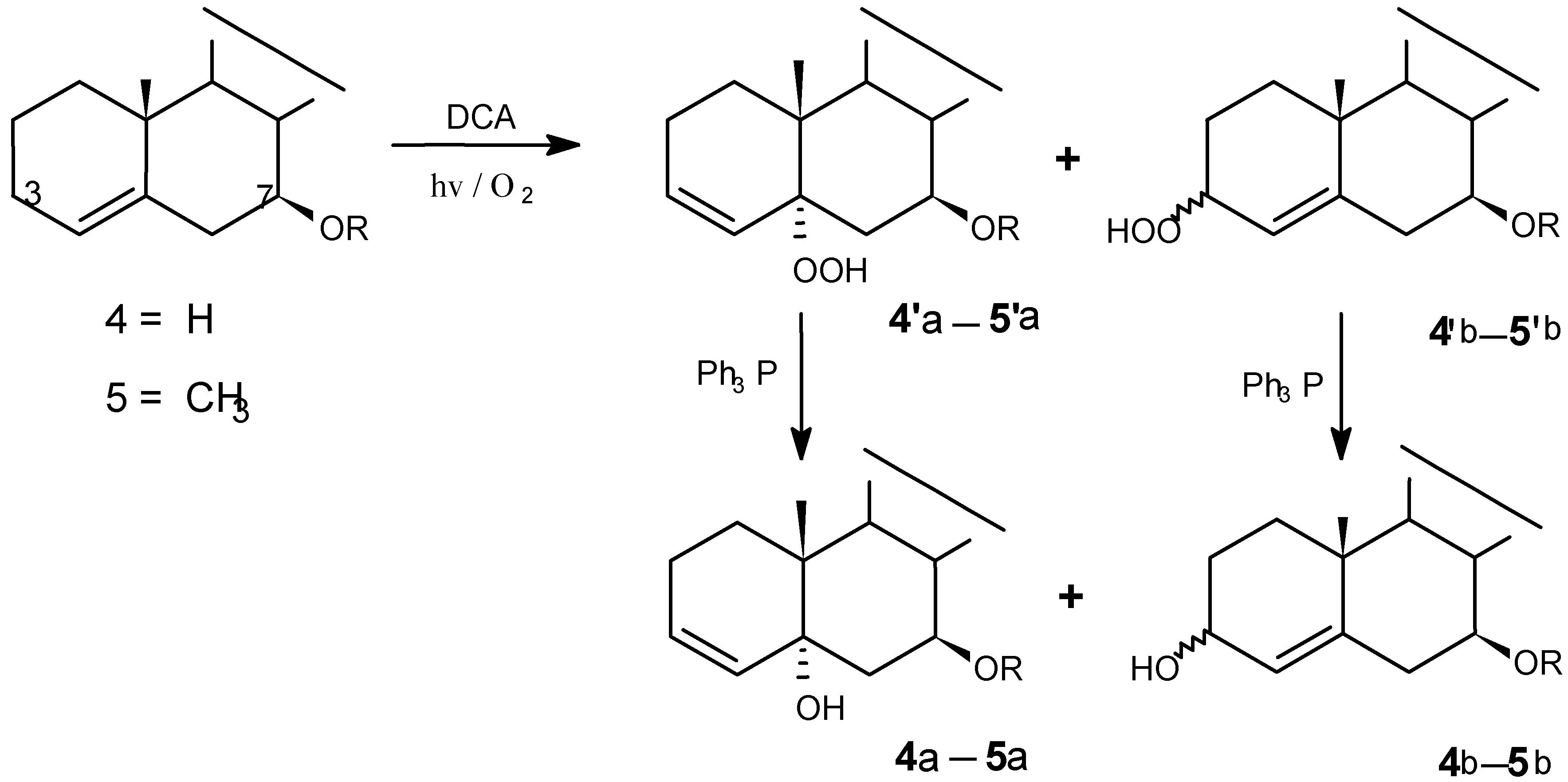
Photooxygenations and product isolation
Photolysis was carried out in a photochemical glass reactor equipped with a water-cooled jacket attached to an automatic controller. The light source was a 500 w medium-pressure mercury lamp. The mixture of steroid, sensitizer and solvent in a glass tube was irradiated with continuous oxygen bubbling. The progress of reactions was monitored by TLC.
(1) LF-sensitized photooxygenation of 1 in MeCN
77.2 mg of
1 in MeCN solution (80 mL) containing LF (5×10
-4 mol dm
-3) was irradiated for 4h with oxygen bubbling, then Ph
3P (100mg) was added and the mixture stirred for 0.5 h. The solvent was evaporated and the mixture was chromatographed on silica gel eluting with toluene/ethyl acetate. The following three purified components were obtained: 15.2 mg of recovered cholesterol (
1); 12 mg of cholest-6-en-3β, 5α-diol (
1a), recrystallized from methanol, m.p. 147-148 C (ref: 147-148 C [
15]). ν
max: 3600, 3423 (b, OH), 1636 (w, C=C) cm
-1; m/e: 384 (M
+-H
2O, 30 %), 366 (M
+-2H
2O, 100%), 351, 143, 135 (100 %); δ
H (CDCl
3): 0.71 (s, 3H, 18-CH
3), 0.93 (s, 3H, 19-CH
3), 4.13 (m, 1H, 3-CH), 5.57 (1H, dd, J=9.8, 2.5 Hz, 6-H), 5.63 (1H, dd, J=9.8, 1.7 Hz, 7-H); 47 mg of cholest-5-en-3β,7 (α,β)- diol (
1b), which was further separated by silica gel chromatograph to give cholest-5-en-3β,7β-diol
1b- (α)
,m.p. 176-178 C. ν
max: 3368 (b, OH), 2933, 2867 (s, C-H), 1662 (w, C=C), 1467, 1382 cm
-1; m/e: 402 (M
+), 384 (M
+-H
2O), 369 (M
+-H
2O-CH
3, 90 %), 351 (M
+-2H
2O-CH
3), 247, 135 (100 %); δ
H (CDCl
3): 0.69 (s, 3H, 18-CH
3), 1.02 (s, 3H, 19-CH
3), 3.60 (m, 1H, 3-CH), 3.87 (m, 1H, 7-H), 5.61 (1H, dd, J=4.8, 1.4 Hz, 6-H) and cholest-5-en-3β,7β-diol
1b-(β), m.p. 184-186 C. ν
max: 3376, 3363 (b, OH), 2943, 2867 (s, C-H), 1662 (w, C=C), 1466 cm
-1; m/e: 402 (M
+), 384 (M
+-H
2O, 100 %), 369 (M
+-H
2O-CH
3, 40 %), 351 (M
+-2H
2O-CH
3), 247, 135; δ
H (CDCl
3): 0.69 (s, 3H, 18-CH
3), 1.06 (s, 3H, 19-CH
3), 3.58 (m, 1H, 3-CH), 3.87 (m, 1H, 7-H), 5.30 (t, 1H, 6-H).
(2) LF-sensitized photooxygenation of 1 in pyridine
The same amount of 1, LF and solvent as in experiment (1) was used. Irradiation of the mixture for 6 h with oxygen bubbling was carried out, then a similar work-up and isolation as in (1) were employed. The following three purified compounds were obtained: 7.2 mg of recovered 1, 59.2 mg of 1a and 8.4 mg of 1b. Their spectra data were the same as those given in (1).
(3) DCA -sensitized photooxygenation of 1 in MeCN
Compound 1 (77.2 mg ; 0.03 mmol) in DCA-MeCN solution (80 mL, 4×10-4 mol dm-3) was irradiated for 6 h with oxygen bubbling, then a similar work-up and isolation procedure as in (1) were employed. The following three purified compounds were obtained: 8 mg of recovered 1, 30.1 mg of 1a and 39.8 mg of 1b were obtained. Their spectra data were the same as those described in (1).
(4) LF-sensitized photooxygenation of 2 in MeCN
Compound 2 (80 mg) in an MeCN solution containing LF (150 mL; 5×10-4 mol dm-3) was irradiated for 10 h with oxygen bubbling, then a similar work-up and isolation procedure as in (1) were used. The following three purified compounds were obtained: Recovered 3β−methoxy-cholest-5-ene (2) (15.8 mg), m.p. 81-83 C; 3β-methoxy-cholest-6-en-5α-ol (2a) (26.3 mg); νmax: 3430 (b, OH), 2945, 2868, 1665 (w, C=C), 1454, 1384, 1103, 1018 cm-1; m/e: 401 (M+-Me), 398 (M+-H2O, 100 %), 384 (M+-80%), 353, 301, 210, 71, 43; δH (CDCl3): 0.70 (s, 3H, 18-CH3), 1.19 (s, 19-CH3), 3.36 (s, 3H, OCH3), 3.70 (m, 1H, 3-CH), 5.65 (m, 2H, 6- and 7-H) and 3β-methoxy-cholest-5-en-7 (α,β)-ol (2b) (33.5 mg); νmax: 3430 (b, OH), 2938, 2868, 1665 (w, C=C), 1468, 1384, 1101 cm-1; m/e: 416 (M+), 401 (M+-Me), 398 (M+-H2O), 384 (M+-MeOH ,100 %), 353, 275, 210, 43; δH (CDCl3): 0.69 (s, 3H, 18-CH3), 1.25 ((s, 3H, 19-CH3), 3.18 (m, 1H, 3-CH), 3.35 (s, 3H, 3-OCH3), 3.88 (m, 1H, 7-H), 5.35 (t, 1H, 6-H with 7β-ol), 5.70 (d, 1H, 6-H with 7α-ol).
(5) DCA-sensitized photooxygenation of 2
Compound 2 (80 mg) in MeCN solution containing DCA (150 mL ; 4×10-4 mol dm-3) was irradiated for 8 h with oxygen bubbling, then a similar work-up and isolation procedure as in (1) were employed. The following three purified compounds were obtained: 2 (12 mg, recovered), 2a (28.2 mg) and 2b (39.2 mg).
(6) DCA-sensitized photooxygenation of 3
Compound 3 (88.8 mg) in MeCN solution containing DCA (100 mL; 4×10-4 mol dm-3) was irradiated for 7 h with oxygen bubbling, then a similar work-up and isolation procedure as in(1) were employed. The following three purified compounds were obtained: 3 (13.6 mg, recovered); 3β- acetyloxycholest-6-en-5α-ol (3a) (46.8 mg); νmax: 3507, 3451 (b, OH), 2945, 2868, 1728 (s, C=O), 1672 (w, C=C), 1458, 1377, 1243 cm-1; m/e: 443 (M+-1), 426 ((M+-H2O), 411 (M+-H2O-CH3), 382, 367 (100 %), 351; δH (CDCl3): 0.68 (s, 3H, 18-CH3), 1.26 (s, 3H, 19-CH3), 2.04 (s, 3H, 3-CH3COO), 2.37 (m, 1H), 4.98 (m, 1H, 3-CH), 5.62 (s, 2H, 6- and 7-H); 3β-acetyloxy-cholest-5-en-7(α,β)-ol (3b) (22.3 mg); νmax: 3479 (b, OH), 2945, 2868, 1735 (s, C=O), 1672 (w, C=C), 1451, 1370, 1032 cm-1; m/e: 443 (M+-1), 426 (M+-H2O), 411, 399, 383, 367, 351, 174, 57, 43; δH (CDCl3): 0.68 (s, 3H, 18-CH3), 1.26 (s, 3H, 19-CH3), 2.04 (s, 3H, 3-CH3COO), 2.54 (m, 1H), 3.89 (m, 1H, 7-CH), 4.76 (s, 1H, 3-CH), 5.35 (s, 1H, 6-H with 7β-ol), 5.72 (s, 1H, 6-H with 7α-ol).
(7) LF-sensitized photooxygenation of 3 in MeCN
Compound 3 (86.7 mg) in an MeCN solution containing LF (100 mL; 5×10-4 mol dm-3) was irradiated for 12 h with oxygen bubbling, then a work-up and isolation procedure similar to those described in (1) were employed. The following three purified compounds were obtained: recovered 3 (22.5 mg), 3a (42.2 mg) and 3b (18 mg).
(8) DCA-sensitized photooxygenation of 4
Compound 4 (80 mg) in MeCN solution containing DCA (100 mL; 4×10-4 mol dm-3) was irradiated for 5 h with oxygen bubbling, then a similar work-up and isolation procedure as in (1) were employed. The following three purified compounds were obtained: recovered compound 4 (42.3 mg); pseudocholest-3-en-5α-7β-diol (4a) (21 mg); νmax: 3382 (b, OH), 2931, 2840 (s, C-H), 1637 (w, C=C), 1454 cm-1; m/e: 384 ((M+-H2O, 24 %), 369 (M+-H2O-CH3, 100 %), 351 (M+-2H2O-CH3), 233, 135 (100 %); δH (CDCl3): 0.75 (s, 3H, 18-CH3), 1.22 (s, 3H, 19-CH3), 4.09 (m, 1H, 7-CH), 5.74 (m, 2H, 3-,4-H); Pseudocholest-4-en-3 (α,β)-7β-diol (4b) (5.2 mg); νmax: 3353 (b, OH), 2938, 2850 (s, C-H), 1645 (w, C=C), 1454 cm-1; m/e: 402 (M+), 384 (M+-H2O,100 %), 369 (M+-H2O-CH3), 351 (M+-2H2O-CH3), 247, 135; δH (CDCl3): 0.72 (s, 3H, 18-CH3), 1.22 (s, 3H, 19-CH3), 3.54 (m, 1H, 7-CH), 3.80 (m, 1H, 3-CH), 5.35 (s, 1H, 4-H with 3β-ol), 5.78 (d, 1H, 4-H with 3α-ol).
(9) DCA-sensitized photooxygenation of 5
Compound 5 (80 mg) in MeCN solution containing DCA (100 mL; 4×10-4 mol dm-3) was irradiated for 7 h with oxygen bubbling, then a similar work-up and isolation gave three compounds: recovered 5 (40.5 mg); 7β-methoxy-pseudocholest-3-en-5α-diol (5a) (21.7 mg); νmax: 3410 (b, OH), 2942, 2870, 1662 (w, C=C), 1382 cm-1; m/e: 401 (M+-Me), 398 (M+-H2O, 100 %), 384 (M+-MeOH), 366, 351; δH (CDCl3): 0.72 (s, 3H, 18-CH3), 1.21 (s, 3H, 19-CH3), 3.36 (s, 3H, 7-OCH3), 3.74 (m, 1H, 7-CH), 5.64 (m, 2H, 3-, 4-H); 7β-methoxy-pseudocholest-4-en-3 (α,β)-ol (5b) (6.8 mg); νmax: 3410 (b, OH), 2935, 2868 (s, C-H), 1670 (w, C=C), 1380 cm-1; m/e: 416 (M+), 401 (M+-Me), 398( (M+-H2O), 384 (M+- MeOH, 100 %), 366, 351; δH (CDCl3): 0.73 (s, 3H, 18-CH3), 1.21 (s, 3H, 19-CH3), 3.20 (m, 1H, 7- CH), 3.37 (s, 3H, 7-OCH3), 3.84 (m, 1H, 7-CH), 5.30 (s, 1H, 4-H with 3β-ol), 5.65 (d, 1H, 4-H with 3α-ol).