Crystal and Molecular Structures of N-benzyl-C-(2-pyridyl) nitrone and its ZnBr2 Complex. A Study of Their Reactivity
Abstract
:Introduction

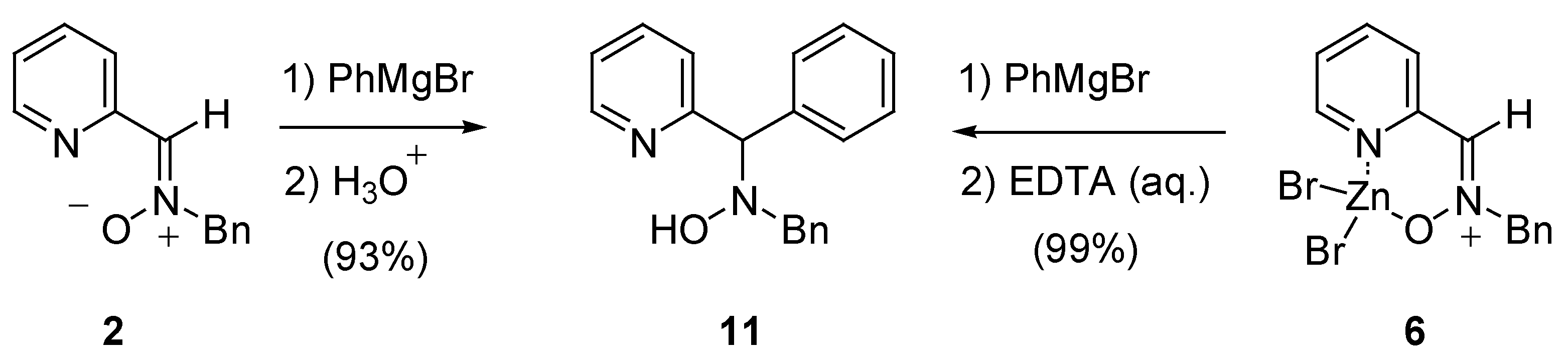
Synthesis and Structural Analysis
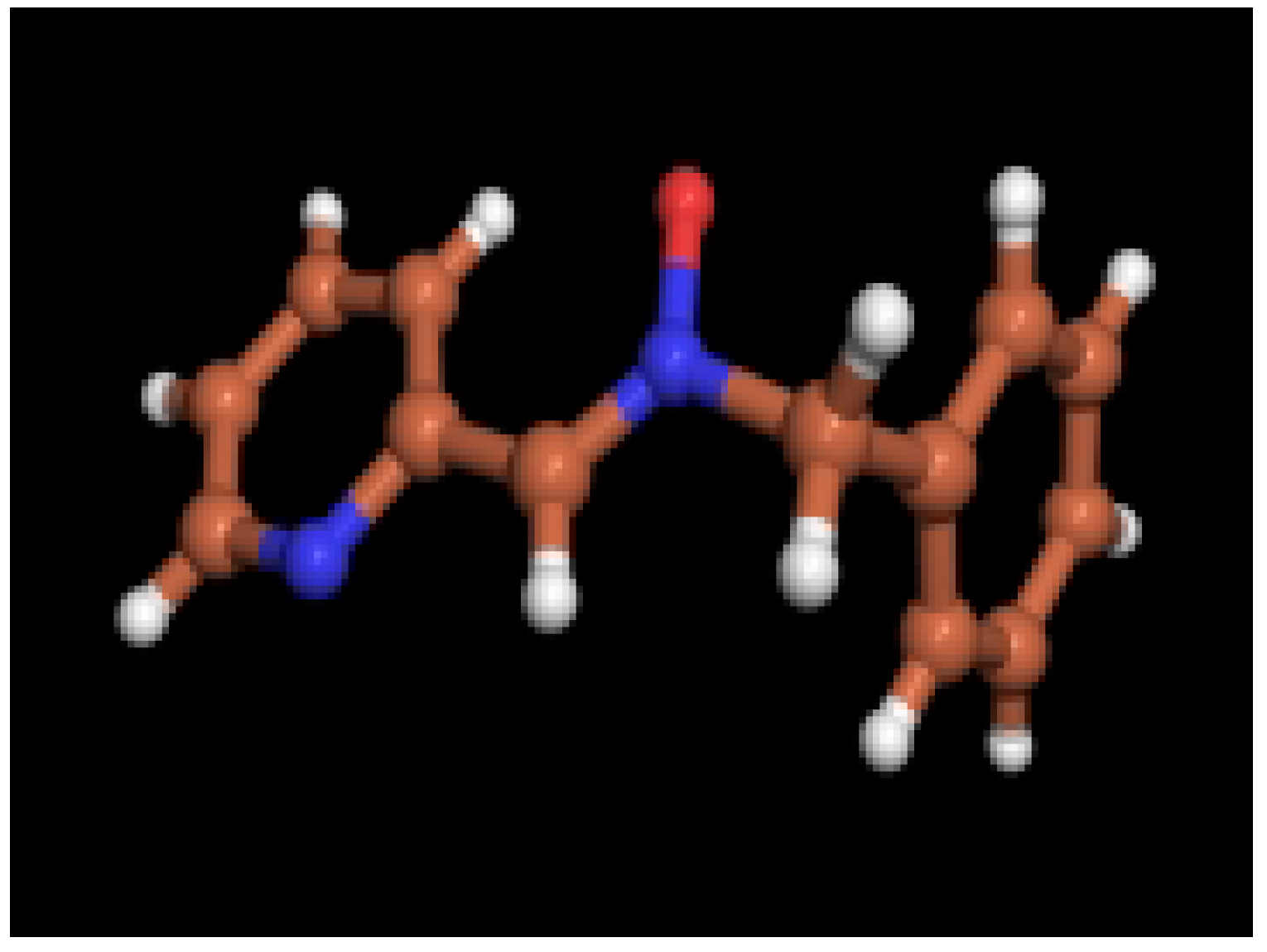
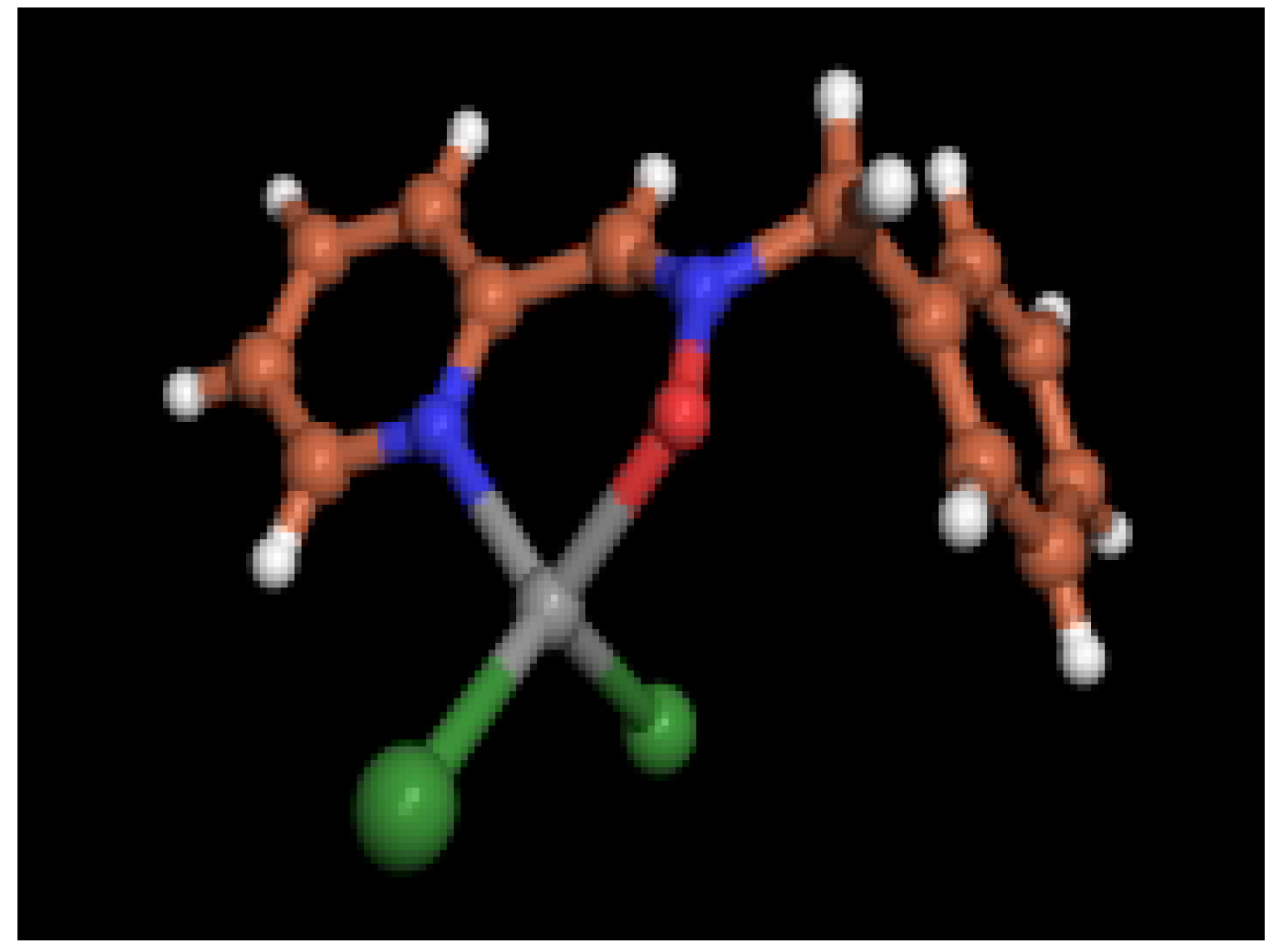
X-ray diffraction data
| 2 | 6 | |
| Formula | C13H12N2O | C13H12Br2N2OZn |
| FW | 212.25 | 437.44 |
| Crystal system | orthorhombic | triclinic |
| Space group | Pbcn | P-1 |
| a (Å) | 11.033 (5) | 7.303 (2) |
| b (Å) | 11.107 (5) | 8.135 (3) |
| c (Å) | 17.414 (5) | 12.970 (6) |
| α (deg.) | 90 | 87.01 (3) |
| β (deg.) | 90 | 80.56 (3) |
| γ (deg.) | 90 | 84.60 (3) |
| V (Å3) | 2134.0 (15) | 756.2 (5) |
| Z | 8 | 2 |
| ρcalc (g/cm3) | 1.321 | 1.921 |
| F(000) | 896 | 424 |
| μ (Mo-Kα), cm-1 | 0.086 | 6.900 |
| crystal size, mm | 0.38x0.20x0.16 | 0.24x0.10x0.08 |
| measurement T (K) | 173 (2) | 243 (2) |
| θmax (deg.) | 25.11 | 25.0 |
| crystal decay (%) | 6.86 | 15.4 |
| total reflections | 2446 | 5169 |
| total unique reflections | 1886 | 2628 |
| Rmerge | 0.072 | 0.097 |
| reflections I>2σ(I) | 1083 | 1333 |
| No. parameters | 147 | 174 |
| R | 0.0474 | 0.1007 |
| Rw | 0.0847 | 0.2432 |
| GoF (S) | 1.084 | 1.162 |
| Siemens P4 diffractometer. Mo-Ka radiation (λ=0.71609Å), normal focus sealed tube, graphite monochromator. Values given for R, Rw and GoF are based on total unique reflections. Computing data collections: Siemens XSCANS [21]. Structure solution: SIR-97 [22]. Structure refinement: SHELXL-97 [23]. Molecular Graphics: PovChem v 2.1 [24] | ||
Theoretical Calculations
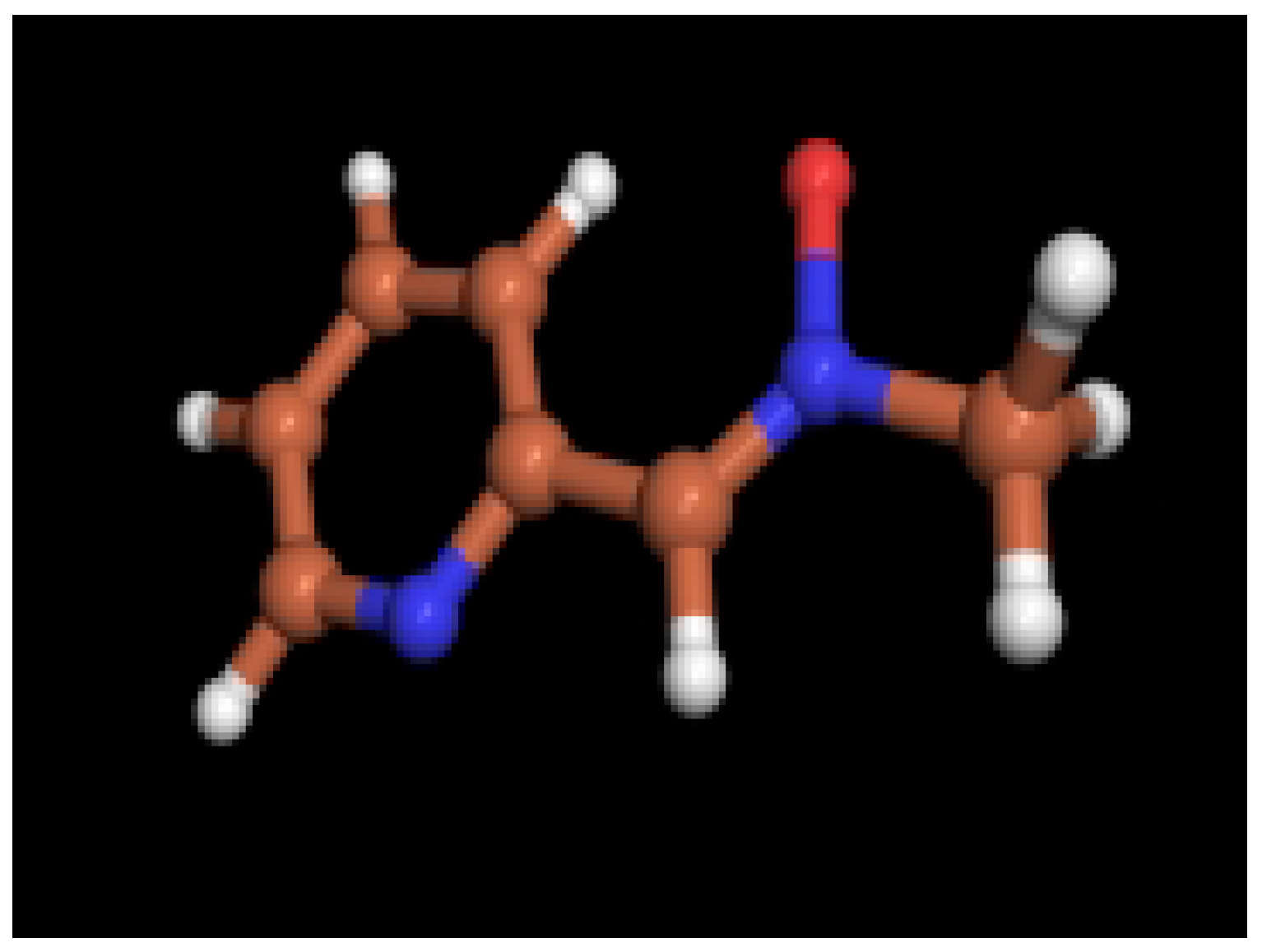

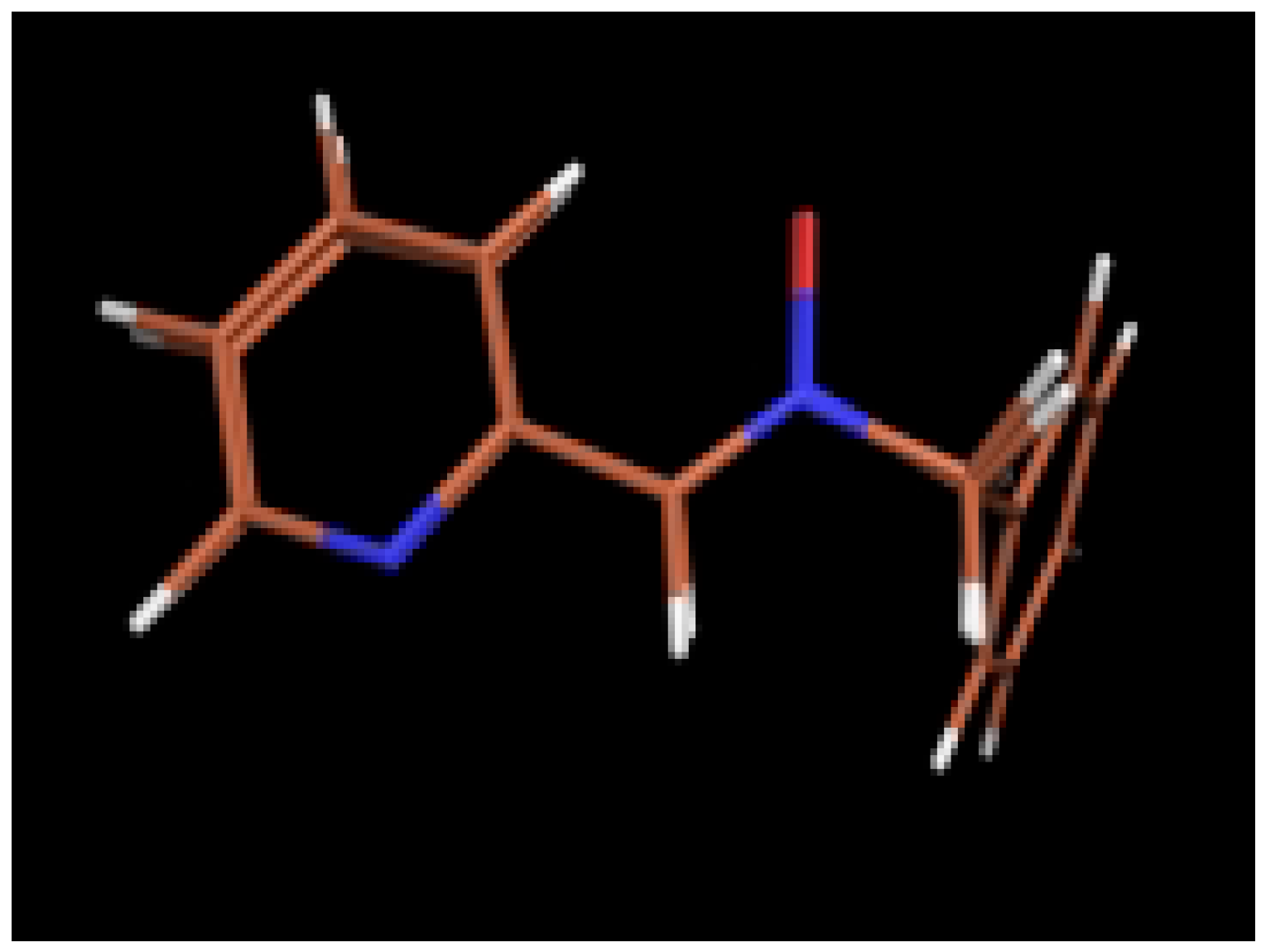
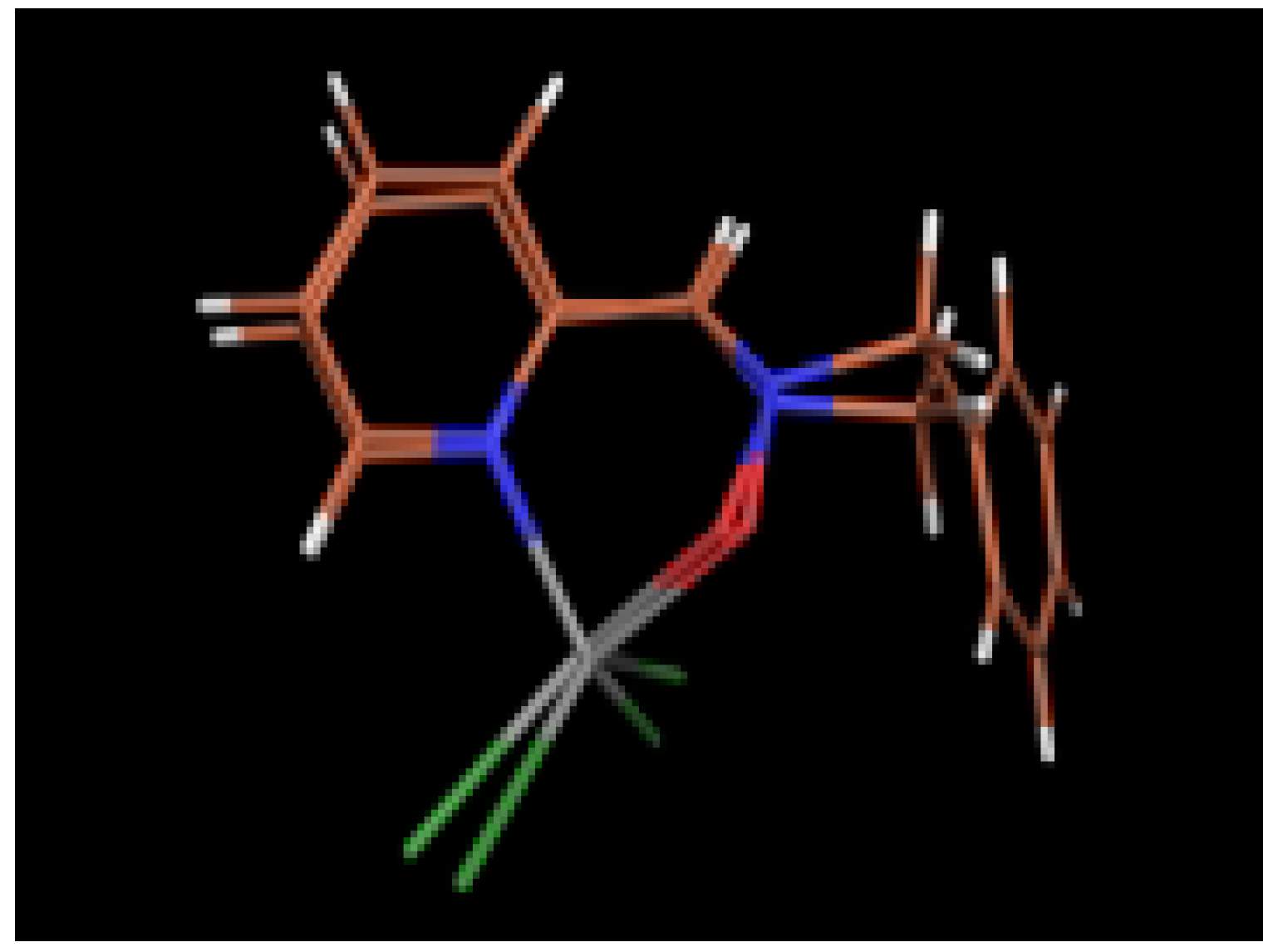
Ab initio calculations
| Nitrone 2 | Complex 6 | |||
| X-ray data | HF / 3-21G | X-ray data | HF / 3-21G | |
| bond lenghts | ||||
| O1-N2 | 1.299 | 1.383 | 1.396 | 1.326 |
| N2-C3 | 1.300 | 1.268 | 1.261 | 1.228 |
| C3-C4 | 1.451 | 1.458 | 1.469 | 1.501 |
| C4-N5 | 1.365 | 1.337 | 1.318 | 1.318 |
| N2-C10 | 1.488 | 1.472 | 1.477 | 1.500 |
| O1-Zn | -------- | -------- | 1.993 | 1.930 |
| N5-Zn | -------- | -------- | 2.055 | 2.029 |
| Zn-Br1 | -------- | -------- | 2.321 | 2.287 |
| Zn-Br2 | -------- | -------- | 2.357 | 2.333 |
| bond angles | ||||
| O1-N2-C3 | 126.1 | 125.6 | 127.3 | 123.5 |
| N2-C3-C4 | 126.3 | 126.4 | 129.8 | 125.7 |
| C3-C4-N5 | 113.2 | 113.8 | 118.6 | 119.6 |
| O1-N2-C10 | 114.4 | 111.2 | 111.1 | 111.2 |
| C3-N2-C10 | 119.5 | 123.2 | 121.0 | 125.3 |
| O1-Zn-N5 | -------- | -------- | 89.7 | 87.5 |
| N2-O1-Zn | -------- | -------- | 118.1 | 111.1 |
| N5-Zn-Br1 | -------- | -------- | 108.1 | 105.3 |
| O1-Zn-Br2 | -------- | -------- | 113.7 | 126.1 |
| dihedral angles | ||||
| O1-N2-C3-C4 | 3.1 | 0.0 | 2.6 | 2.9 |
| N2-C3-C4-N5 | 175.6 | 180.0 | 13.0 | 23.7 |
| O1-N2-C10-C11 | 112.6 | -------- | -------- | 85.0 |
| N2-O1-Zn-N5 | -------- | -------- | 122.8 | 141.5 |
| O1-Zn-N5-C4 | -------- | -------- | 37.2 | 30.7 |
Reactivity
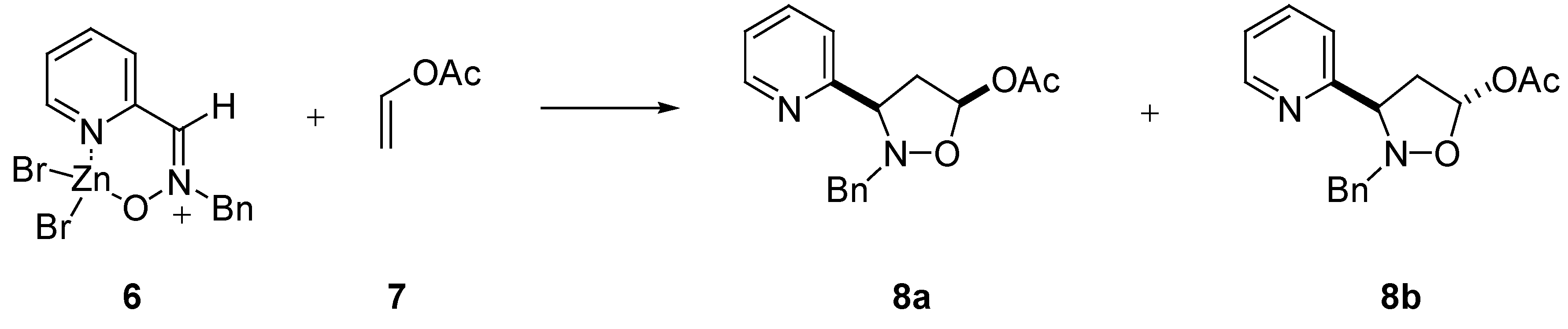
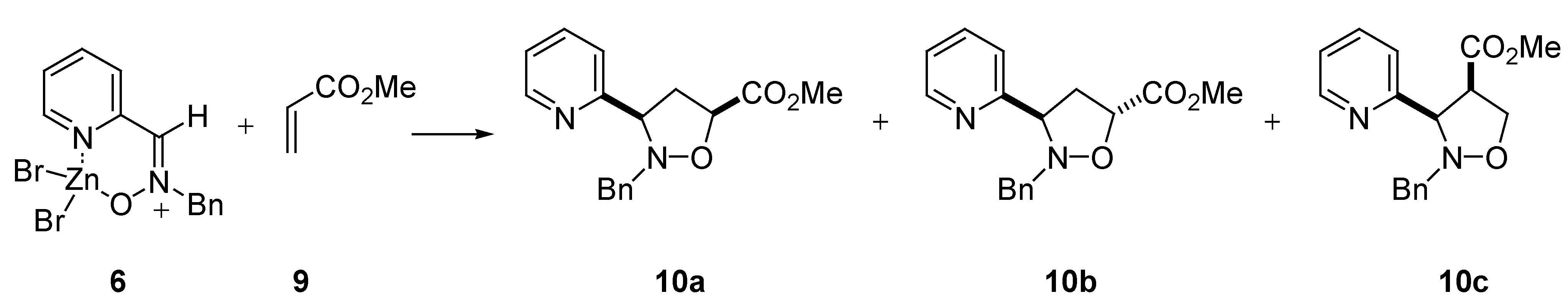
| 1,3-Dipole | dipolarophileb | time | cis : transc | yield (%) |
|---|---|---|---|---|
| 2 | 7 | 168 h | 85 : 15 | 93 |
| 2 | 9 | 72 h | 23 : 77d | 93 |
| 6 | 7 | 168 h | 89 : 11 | 92 |
| 6 | 9 | 72 h | 27 : 73e | 89 |
Conclusions
Experimental
General
Preparation of complex 6.
General Procedure for the 1,3-dipolar cycloaddition of nitrone 2 and complex 6 with dipolarophiles 7 and 9.
N-Benzyl-N-(phenyl-pyridin-2-yl-methyl)-hydroxylamine 13
Acknowledgements
References and Notes
- (a) Kobayashi, S.; Busujima, T.; Nagayama, A. Novel Classification of Lewis-Acids on the Basis of Activity and Selectivity. Chem. Eur. J. 2000, 6, 3491–3494, and references cited therein. [Google Scholar] [CrossRef] (b) Haughton, L.; Williams, J.M.J. Catalytic Applications of Transition-Metals in Organic-Synthesis. J. Chem. Soc. Perkin Trans. 1 2000, 3335–3349. [Google Scholar] [CrossRef]
- For a recent account see: Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Nucleophilic additions to chiral nitrones: New approaches to nitrogenated compounds. Synlett 2000, 442–454. [Google Scholar]
- See inter alia: (a) Merino, P.; Castillo, E.; Franco, S.; Merchan, F.L.; Tejero, T. Enantiodivergent approach to D- and L-secondary N-hydroxy-α-amino acids by using N-benzyl-2,3-O- isopropylidene-D-glyceraldehyde nitrone as an effective N-hydroxyglycine cation equivalent. J. Org. Chem. 1998, 63, 2371–2374. [Google Scholar] (b) Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Diastereoselective nucleophilic addition of acetylide to N-benzyl-2,3-O-isopropylidene-D- glyceraldehyde nitrone (BIGN). Stereodivergent synthesis of β-hydroxy-α-(hydroxyamino)- and β- hydroxy-α-aminoacids. Tetrahedron: Asymmetry 1997, 8, 3489–3496. [Google Scholar] (c) Merino, P.; Castillo, E.; Franco, S.; Merchan, F.L.; Tejero, T. Nucleophilic additions of Grignard reagents to N-benzyl-2,3- O-isopropylidene-D-glyceraldehyde nitrone (BIGN). Synthesis of (2S,3R)) and (2S,3S)-3- phenylisoserine. Tetrahedron 1998, 54, 12301–12322. [Google Scholar]
- Kanemasa, S.; Uemura, T.; Wada, E. Lewis-acid catalyzed nitrone cycloadditions to bidentate and tridentate alpha,beta-unsaturated keones. High-rate acceleration, absolutely enoselective and regioselective reactions. Tetrahedron Lett. 1992, 33, 7889–7892. [Google Scholar] Kanemasa, S.; Oderaotoshi, Y. Asymmetric cyloaddition reactions catalyzed by transition-metal complexes. New guidelines for structural design of chiral catalyst. J. Synth. Org. Chem. Jpn. 1998, 56, 368–377. [Google Scholar]
- Kanemasa, S.; Tsururoka, T. Magnesium bromide promoted E/Z isomerization of carbonyl- conjugated nitrones ad highly stereoselective and regioselective cycloadditions to allylic alcohol dipolarophiles. Chem. Lett. 1995, 49, 49–50. [Google Scholar] [CrossRef]
- (a) Gothelf, K.V.; Hazell, R.G.; Jorgensen, K.A. Control of diastereoselectivity and enantioselectivity in metal-catalyzed 1,3-dipolar cycloaddition reactions of nitrones with alkenes. Experimental and theoretical investigations. J. Org. Chem. 1996, 61, 346–355. [Google Scholar] (b) Gothelf, K.V.; Hazell, R.G.; Jorgensen, K.A. Molecular-sieve dependent absolute stereoselectivity in asymmetric catalytic 1,3-dipolar cycloaddition reactions. J. Org. Chem. 1998, 63, 5483–5488. [Google Scholar] (c) Jensen, K.B.; Gothelf, K.V.; Hazell, R.G.; Jorgensen, K.A. Improvement of taddolate-TiCl2-catalyzed 1,3- dipolar nitrone cycloaddition reactions by substitution of the oxazolidinone auxiliary of the alkene with succinimide. J. Org. Chem. 1997, 62, 2471–2477. [Google Scholar]
- Simonsen, K.B.; Bayon, P.; Hazell, R.G.; Gothelf, K.V.; Jorgensen, K.A. Catalytic enantioselective inverse-electron demand 1,3-dipolar cycloaddition reactions of nitrones with alkenes. J. Am. Chem. Soc. 1999, 121, 3845–3847. [Google Scholar] [CrossRef]
- Merino, P.; Anoro, S.; Merchan, F.L.; Tejero, T. 1,3-Dipolar cycloadditions of N-benzyl furfuryl nitrones with electron-rich alkenes. Molecules 2000, 5, 132–152. [Google Scholar] [CrossRef]
- (a) Tejero, T.; Dondoni, A.; Rojo, I.; Merchan, F.L.; Merino, P. 1,3-Dipolar cycloaddition of C-(2- thiazolyl)nitrones to chiral acrylates. Synthesis of enantiopure α-amino-2-alkylthiazoles and 5- formylpyrrolidin-2-ones. Tetrahedron 1997, 53, 3301–3318. [Google Scholar] (b) Merino, P.; Anoro, S.; Rojo, I.; Merchan, F.L.; Tejero, T. 1,3-Dipolar cycloaddition between hetaryl nitrones and methyl acrylate: Theoretical study and application to the synthesis of functionalized pyrrolidines. Heterocycles 2000, 53, 861–875. [Google Scholar]
- Merino, P.; Anoro, S.; Franco, S.; Merchan, F.L.; Tejero, T.; Tuñon, V. 1,3-Dipolar cycloaddition of furfuryl nitrones with acrylates. A convenient approach to protected 4-hydroxypyroglutamic acids. J. Org. Chem. 2000, 65, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Moinet, C.; Toupet, L. Synthesis, structure and hydrolysis of some ((eta(5)- cyclopentadienyl)(eta(6)-arene)iron(II))(PF6) complexes bearing a imine or a nitrone function in α position of the arene ligand. J. Organomet. Chem. 1997, 527, 51–64. [Google Scholar] [CrossRef]
- Pierre, F.; Stricker, A.; Moinet, C.; Sinbandhit, S.; Toupet, L. Electrochemical reduction of some ((eta(5)-cyclopentadienyl)(eta(6)-arene)iron(II))(PF6) complexes bearing an imine or a nitrone function in benzylic position of the arene ligand. J. Organomet. Chem. 1998, 553, 253–267. [Google Scholar] [CrossRef]
- Alallaf, T.A.K.; Abdulrahman, A. Diorganotin (IV) dichloride complexes of some N- arylfurfuralnitrones. Synth. React. Inorg. Metal-Org. C 1997, 27, 985–996. [Google Scholar] [CrossRef]
- (a) Villamena, F.A.; Dickman, M.-H.; Crist, D.R. Nitrones as ligands in complexes of Cu(II), Mn(II), Co(II), Ni(II), Fe(II), and Fe(III) with N-tert-butyl-α-(2-pyridyl)nitrone and 2,5,5- trimethyl-1-pyrroline-N-oxide. Inorg. Chem. 1998, 37, 1446–1453. [Google Scholar] [CrossRef] (b) Villamena, F.A.; Crist, D.R. Metal-nitrone complexes. Spin-trapping and solution characterization. J. Chem. Soc. Dalton, Trans. 1998, 4055–4064. [Google Scholar] [CrossRef]
- Dickmann, M.-H.; Ward, J.P.; Villamena, F.A.; Crist, D.R. Bis(μ-(N-((methylthio)- phenylmethylene)-methanamine-N-oxide)-O-O)bis(bis-(1,1,1,5,5,5-hexa-fluoro-pentane-2,4- dionato-O,O') nickel(II)) Acta Cryst. Sect. C Cryst. Struct. Commun. 1998, 54, 929–930. [Google Scholar] [CrossRef]
- Kliegel, W.; Metge, J.; Rettig, S.J.; Trotter, J. Structural Studies of Organoboron Compounds Lxviii - C-(2-Hydroxyaryl)-N-(2-Hydroxyphenylmethyl)Nitrones as Regioselective Bidentate Ligands in Boron Chelate Formation - Crystal and Molecular-Structures of a Diphenylboron Complex and Its Parent Ligand. Can. J. Chem. 1998, 76, 1082–1092. [Google Scholar] [CrossRef]
- Purchased from Aldrich (P6,200-3) and distilled prior to use.
- Borch, R.F.; Berstein, M.D.; Durst, M.D. The cyanoborohydridoborate anion as a selective reducing agent. J. Am. Chem. Soc. 1971, 93, 2897–2904. [Google Scholar] [CrossRef]
- Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Synthesis of N-benzyl nitrones. Synth. Commun. 1994, 24, 2537–2550. [Google Scholar] [CrossRef]
- Merino, P.; Anoro, S.; Tejero, T.; Laguna, M.; Cerrada, E.; Moreno, A. unpublished results.
- Siemens XSCANS. X-Ray Single Crystal Analysis System. Copyright (C) 1992 by SIEMENS. Siemens analytical X-ray instruments Inc. Madison, Wisconsin. USA.
- SIR-97. A Package for Crystal Structure Solution by Direct Methods and Refinement. Giacovazzo et al. 1997.
- SHELXL-97. Program for the Refinement of Crystal Structures. Sheldrick, G. M. 1997.
- PovChem v. 2.1. Copyright (C) 1999 by Paul A. Thiessen.
- Gaussian 98, Revision A.3, Frisch, J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery Jr., J. A.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al- Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian, Inc.: Pittsburgh PA, 1998.
- CS ChemOffice. Copyright (C) 1997 by Cambridge Soft Corporation. Cambridge, MA. USA.
- Samples Availability: Samples of compounds 2 and 6 are available from MDPI.
© 2001 by Molecular Diversity Preservation International (MDPI)
Share and Cite
Merino, P.; Anoro, S.; Cerrada, E.; Laguna, M.; Moreno, A.; Tejero, T. Crystal and Molecular Structures of N-benzyl-C-(2-pyridyl) nitrone and its ZnBr2 Complex. A Study of Their Reactivity. Molecules 2001, 6, 208-220. https://doi.org/10.3390/60300208
Merino P, Anoro S, Cerrada E, Laguna M, Moreno A, Tejero T. Crystal and Molecular Structures of N-benzyl-C-(2-pyridyl) nitrone and its ZnBr2 Complex. A Study of Their Reactivity. Molecules. 2001; 6(3):208-220. https://doi.org/10.3390/60300208
Chicago/Turabian StyleMerino, Pedro, Sonia Anoro, Elena Cerrada, Mariano Laguna, Ana Moreno, and Tomas Tejero. 2001. "Crystal and Molecular Structures of N-benzyl-C-(2-pyridyl) nitrone and its ZnBr2 Complex. A Study of Their Reactivity" Molecules 6, no. 3: 208-220. https://doi.org/10.3390/60300208
APA StyleMerino, P., Anoro, S., Cerrada, E., Laguna, M., Moreno, A., & Tejero, T. (2001). Crystal and Molecular Structures of N-benzyl-C-(2-pyridyl) nitrone and its ZnBr2 Complex. A Study of Their Reactivity. Molecules, 6(3), 208-220. https://doi.org/10.3390/60300208





