One-Pot Quinazolin-4-ylidenethiourea Synthesis via N-(2-Cyanophenyl)benzimidoyl isothiocyanate
Abstract
:Introduction
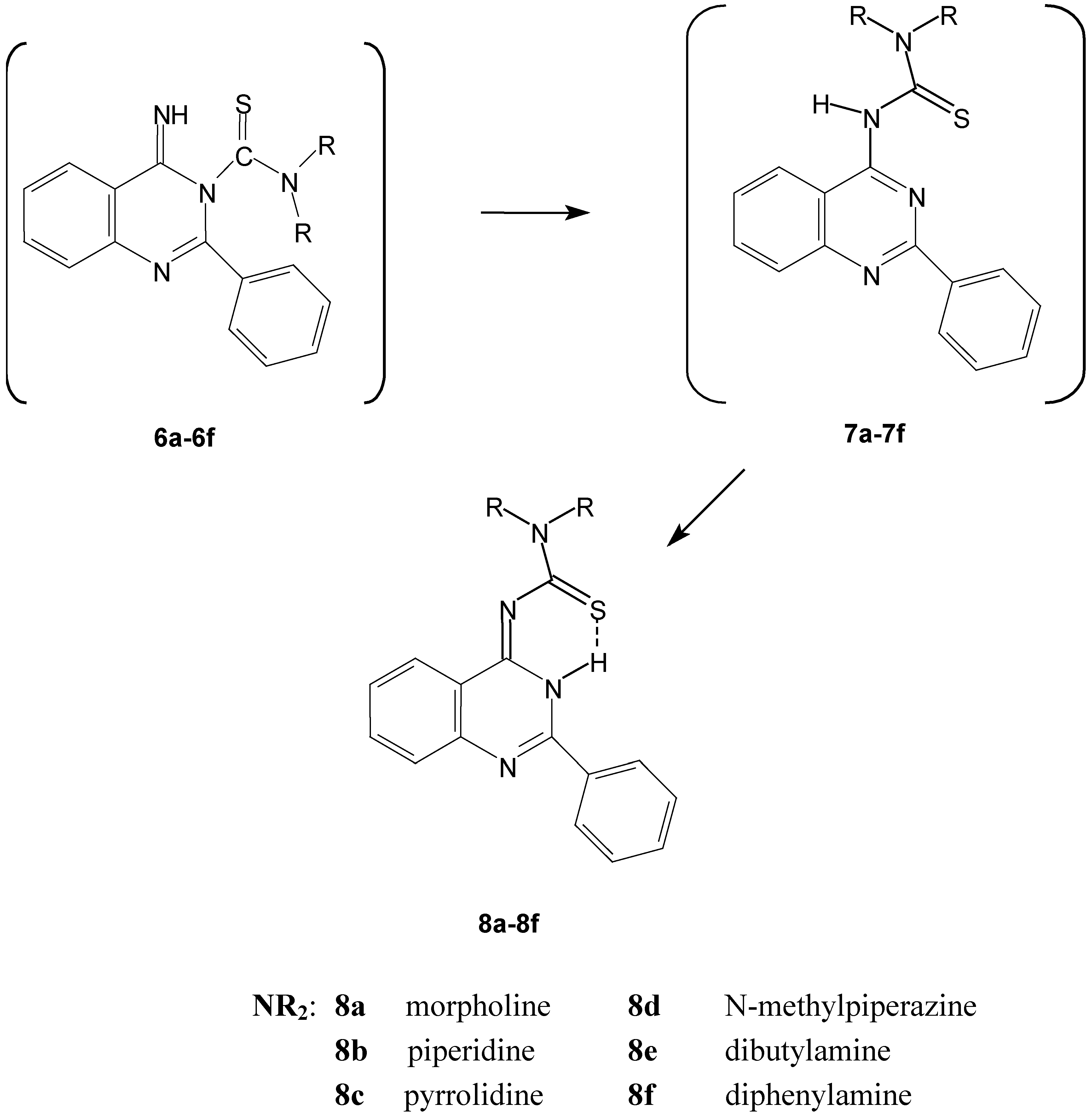
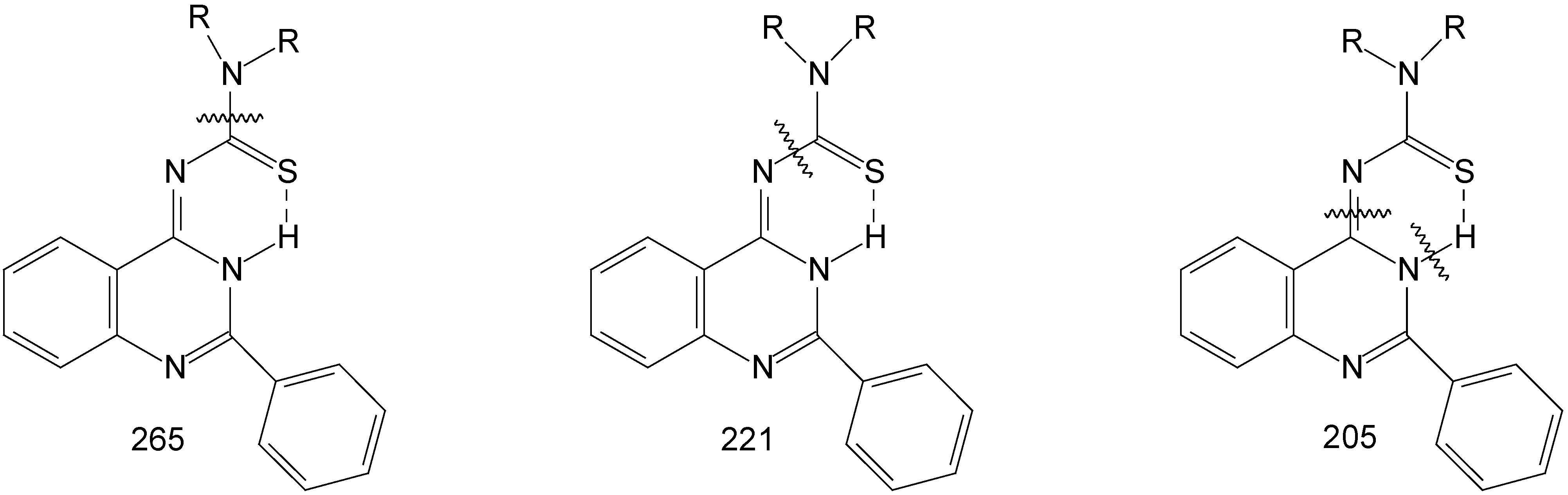
Results and Discussion
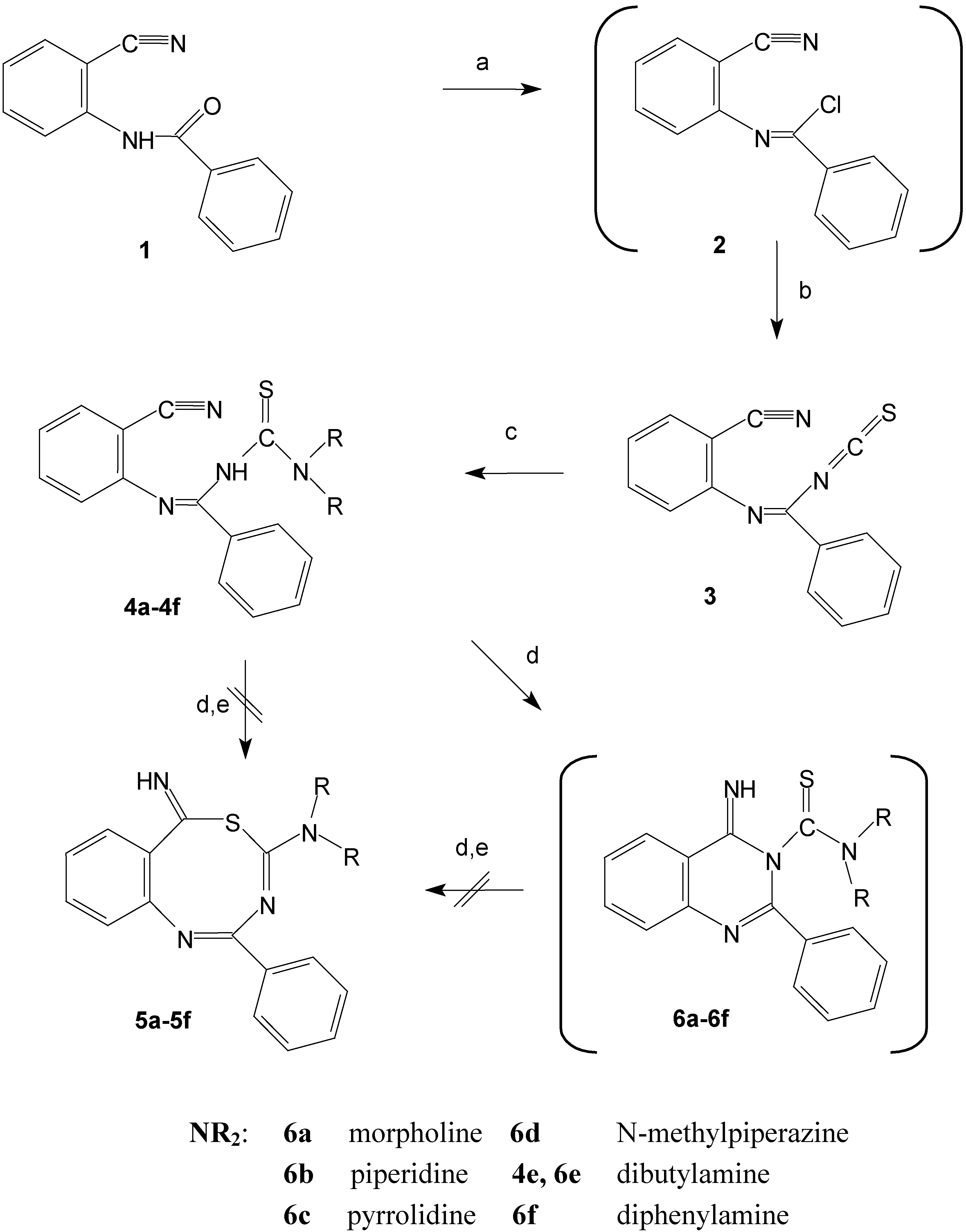
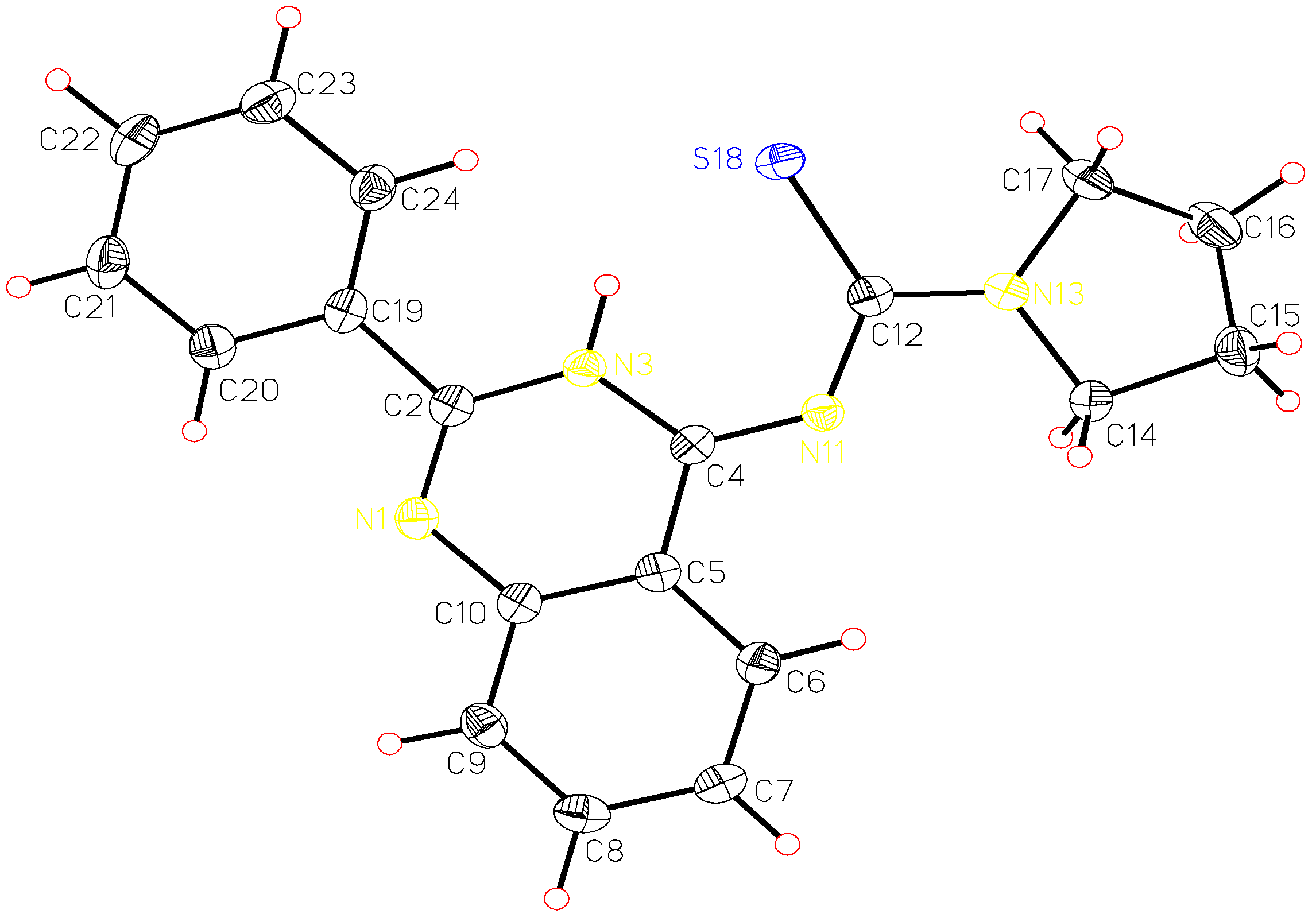
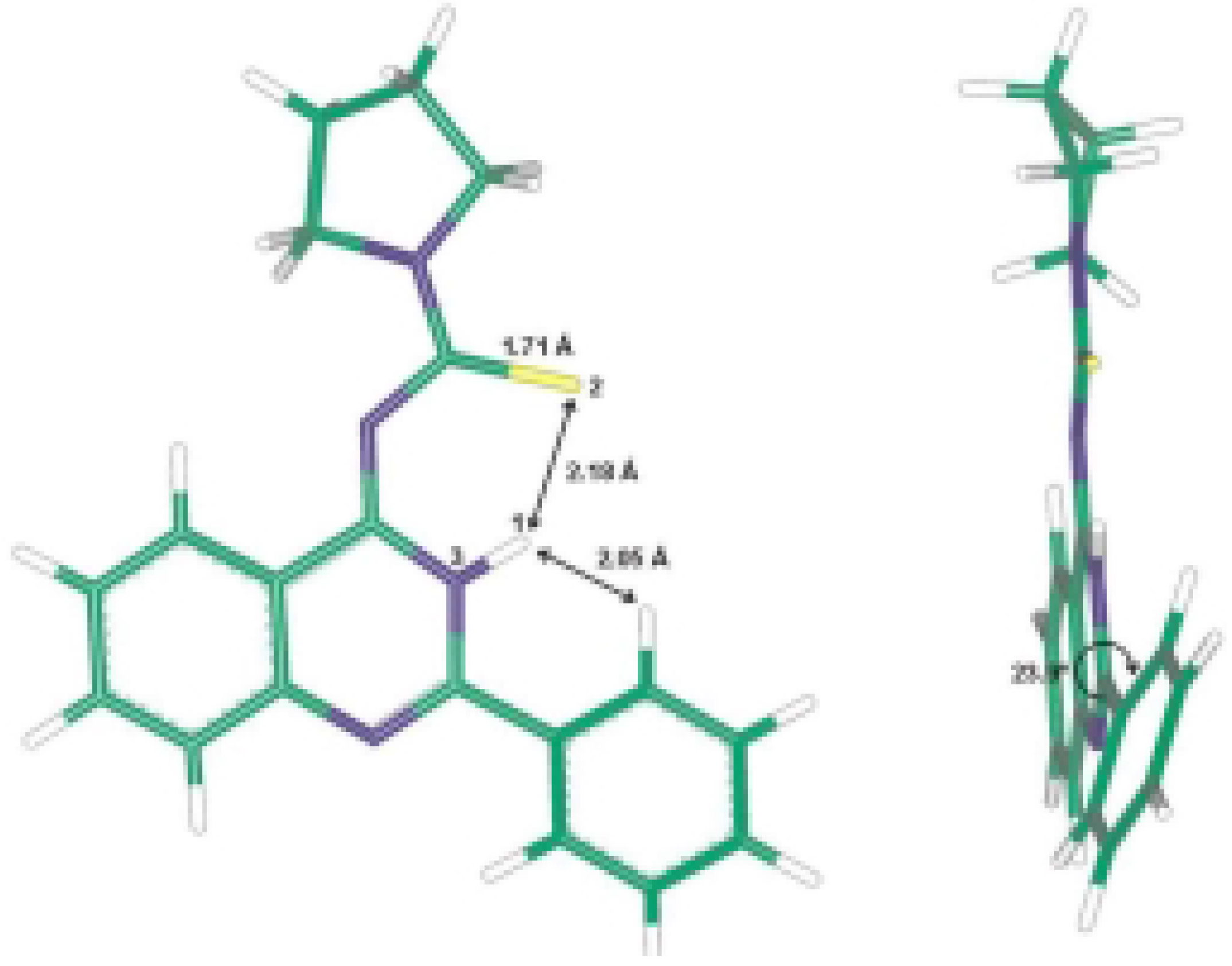
| Bond | Bond Length [Å] | Bond | Bond Length [Å] | ||
|---|---|---|---|---|---|
| X-ray | HF/6-31G** | X-ray | HF/6-31G** | ||
| N1-C2 | 1.30 | 1.27 | C12-N13 | 1.34 | 1.33 |
| N1-C10 | 1.39 | 1.38 | C12-S18 | 1.72 | 1.71 |
| C2-N3 | 1.38 | 1.37 | N13-C14 | 1.48 | 1.47 |
| C2-C19 | 1.49 | 1.49 | N13-C17 | 1.47 | 1.47 |
| N3-C4 | 1.36 | 1.35 | C14-C15 | 1.52 | 1.52 |
| C4-N11 | 1.32 | 1.30 | C15-C16 | 1.52 | 1.52 |
| N11-C12 | 1.37 | 1.36 | C16-C17 | 1.52 | 1.52 |
| Angle [°] | X-ray | HF/6-31G** | Angle [°] | X-ray | HF/6-31G** |
|---|---|---|---|---|---|
| N1-C2-C19 | 119.80 | 120.14 | C14-N13C17 | 111.84 | 112.15 |
| C2-N1-C10 | 117.34 | 117.99 | N13-C17-C16 | 103.17 | 103.47 |
| N1-C2-N3 | 123.17 | 122.99 | N1-C10-C5 | 122.81 | 122.56 |
| C2-N3-C4 | 123.63 | 124.19 | C10-C5-C6 | 120.25 | 120.07 |
| N3-C4-N11 | 125.66 | 126.65 | C5-C6-C7 | 119.86 | 120.10 |
| N11-C12-N13 | 111.85 | 112.38 | C6-C7-C8 | 119.88 | 119.80 |
| N13-C12-S18 | 119.05 | 119.49 | C7-C8-C9 | 120.82 | 120.79 |
| C12-N13-C17 | 125.13 | 124.17 | C2-C19-C24 | 122.91 | 122.15 |
| N13-C14-C15 | 103.49 | 103.46 | C2-N3-H3 | 121.32 | 120.53 |
| Torsion angle [°] | X-ray | HF/6-31G** | Torsion angle [°] | X-ray | HF/6-31G** |
|---|---|---|---|---|---|
| C14-N13-C17-C16 | 15.73 | 12.06 | N11-C4-N3-C2 | -178.35 | -178.96 |
| C17-N13-C14-C15 | 6.92 | 11.68 | C4-N3-C2-N1 | -0.56 | -1.43 |
| C16-C17-N13-C12 | -163.11 | -168.09 | N3-C2-N1-C10 | 0.93 | 023 |
| C17-N13-C12-S18 | -1.31 | -2.84 | C2-N1-C10-C5 | -0.63 | 0.92 |
| C17-N13-C12-C11 | 178.84 | 176.89 | C12-N11-C4-N3 | 2.05 | -0.44 |
| S18-C12-N11-C4 | 7.02 | -3.05 | N1-C2-N3-H3 | 179.60 | 175.29 |
Conclusions
Acknowledgments
Experimental
General
| Empirical formula | C19H18N4S |
| Molecular weight | 334.43 |
| Temperature | 120(2) K |
| Wavelength | 0.71073 Å |
| Crystal system, space group | orthorhombic, Pbca |
| Unit cell dimensions | |
| a, α | 9.51557(6) Å, α= 90 ° |
| b, β | 15.8703(10) Å, β= 90 ° |
| c, γ | 21.6351(12) Å, γ= 90 ° |
| Volume | 3267.3(3) Å3 |
| Z; density calculated | 8 mg.m-3 |
| Absorption coefficient | 0.206 mm-1 |
| F(000) | 1408 |
| Crystal size | 0.65 X 0.25 X 0.10 mm |
| θ , Range for data collection | 3.47- 25.00° |
| Range of h, k, l | -8< = h< =12, -20< = k< = 20, -28< = l< = 27, |
| Reflections collected | 15684 |
| Independent reflections | 2865 |
| Refinement method | full-matrix least-squares on F2 |
| Data; restraints; parameters | 2818 / 0 / 289 |
| Goodness-of-fit on F2 | 1.020 |
| Final R indices [I > 2σ(I)] | R1= 0.0354, wR2 = 0.0827 |
| R indices (all data) | R1= 0.0426, wR2 = 0.0856 |
| Largest diff. Peak and hole | 0.240 and –0.262 e. Å-3 |
N-(2-Cyanophenyl)benzimidoyl isothicyanate - acetone solution (3).
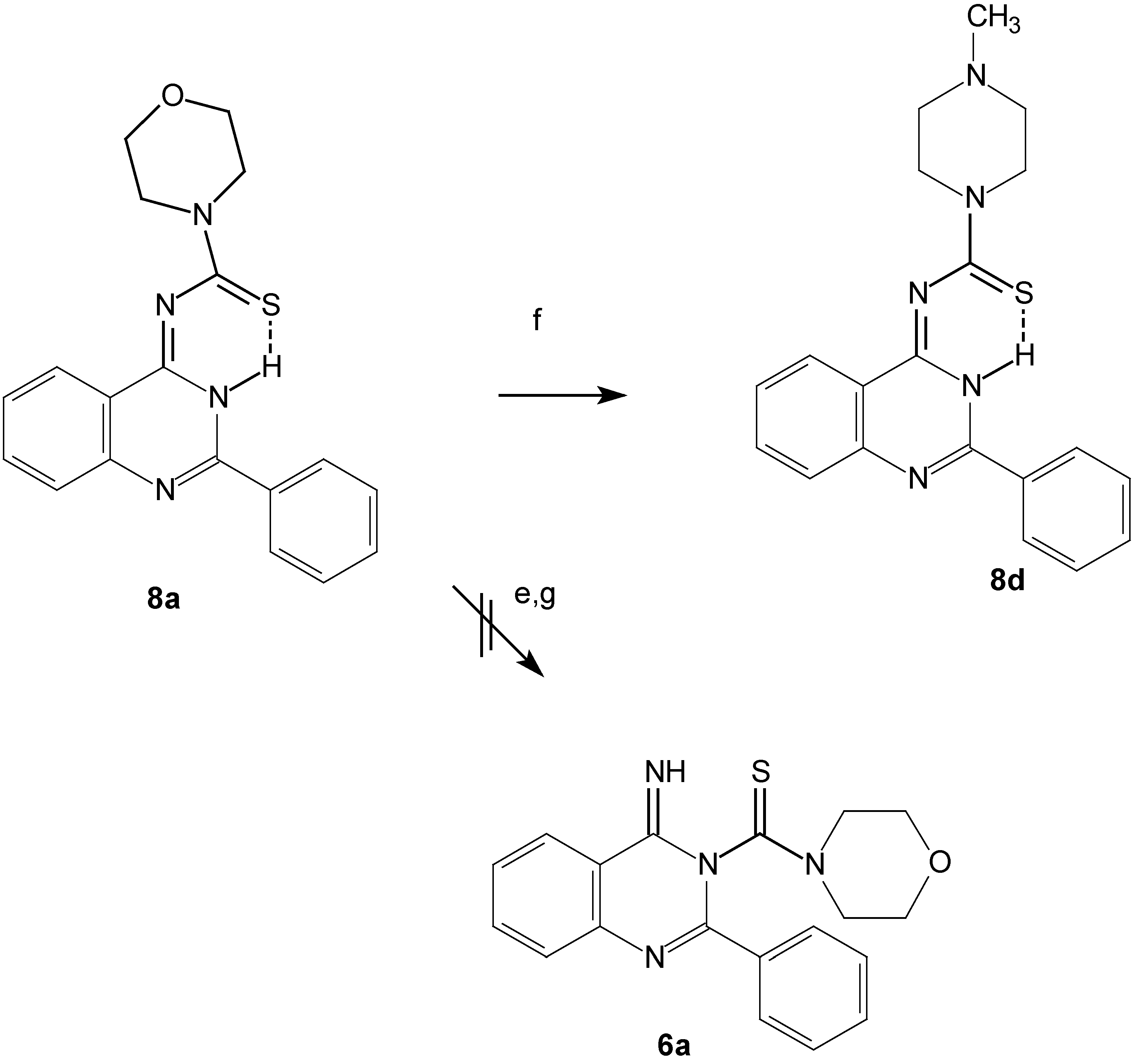
1,1-Disubstituted-3-(2-phenyl-3H-quinazolin-4-ylidene)thioureas (8).
Procedure A
Procedure B
Procedure C
Morpholine-1-carbothioic acid (2-phenyl-3H-quinazolin-4-ylidene) amide (8a).
Piperidine-1-carbothioic acid (2-phenyl-3H-quinazolin-4-ylidene) amide (8b).
Pyrrolidine-1-carbothioic acid (2-phenyl-3H-quinazolin-4-ylidene) amide (8c).
4-Methylpiperazine-1-carbothioic acid (2-phenyl-3H-quinazolin-4-ylidene) amide (8d).
1,1--Dibutyl-3-(2-phenyl-3H-quinazolin-4-yliden) thiourea (8e).
1,1-Diphenyl-3-(2-phenyl-3H-quinazolin-4-ylidene) thiourea (8f)
1,1-Dibutyl-3-[(2-cyanophenylimino)phenylmethyl] thiourea (4e).
References
- Grasso, S.; Zappalá, M.; Chimirri, A. Heterocycles 1987, 26(9), 2477.
- Brown, D. J. Quinazolines, Supplement I (The Chemistry of Heterocyclic Compounds vol. 55); John Wiley & Sons: Chichester (U.K.), 1996. [Google Scholar]
- Abdel-Megeed, M.; Teniou, A. Rev. Roum. Chim. 1988, 33, 981.
- Ravina, A. J. Organic Preparations and Procedures Int. 1973, 5, 174.
- Stevens, G. Diuretics; Academic Press Inc.: New York, 1963; p. 12. [Google Scholar]
- Hayo, S.; Harera, H. J.; Strycher, W. G.; Honge, E. J. Med. Chem. 1969, 12, 936.
- Maillard, J.; Benard, M.; Vinent, M.; Von-Van-Tri, J. R.; Morin, R.; Benharkate, C.; Mennilet, C. Chim. Ther. 1967, 2, 231.
- Malhotra, S.; Kouls, K.; Sharma, R. L.; Anand, K. K.; Gupta, O. P.; Dhar, K. L. Ind. J. Chem. 1988, 27b, 937.
- Hess, H. J.; Cronin, T. H.; Sciabine, A. J. Med. Chem. 1968, 11, 130. [PubMed]
- Waisser, K.; Dostál, H.; Kubicová, L.; Kolář, K. Čes. a Slov. Farm. 2000, 49, 113.
- Murav’eva, K. M.; Akhangle, S. N. V.; Schukina, M. N.; Zykova, T. N.; Perchin, T. N. Khim. Farm. Zh. 1967, 1, 29.
- Leszkuvszky, C.; Erdely, I.; Tardos, L. Acta. Physiol. Acad. Sci. Hung. 1965, 27, 81.
- Gupta, C. M.; Hussain, S. T.; Bhaduri, A. P.; Khana, N. M.; Mukherjee, S. K. Nature 1969, 223, 524.
- Bodajla, M.; Stankovský, Š.; Špirková, K. Collect. Czech. Chem. Commun. 1995, 60, 1415. [CrossRef]
- Bodajla, M.; Stankovský, Š.; Špirková, K.; Jantová, S. Collect. Czech. Chem. Commun. 1996, 61, 1681. [CrossRef]
- Bodajla, M.; Stankovský, Š.; Jantová, S.; Hudecová, D.; Špirková, K. Chem. Papers 1996, 50(1), 28.
- Pazdera, P.; Nováček, E.; Kalviňš, I.; Trapencieris, P.; Pugovics, O. Chem. Papers 1991, 45, 527.
- Pazdera, P.; Ondráček, D.; Nováček, E. Chem. Papers 1989, 43(6), 771.
- The complete computational analysis of all the predicted structures and the similar structures reported by Stankovsky [14,15,16] are available from the authors.
- Sheldrick, G. M. Acta Crystallogr. Sect. A. 1990, 46, 467.
- Sheldrick, G. M. SHELXL93: Program for Structure Refinement; University of Göttingen: Göttingen, 1993. [Google Scholar]
- Osamu, K.; Shigeo, Y.; Toshiro, K. Agric. Biol. Chem. 1980, 44, 2143.
- Houghton, P. G.; Pipe, D. F.; Rees, C. W. J. Chem. Soc. Perkin Trans. I 1985, 7, 1471. [CrossRef]
- Sample Availability: Available from the authors.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Fathalla, W.M.; Čajan, M.; Marek, J.; Pazdera, P. One-Pot Quinazolin-4-ylidenethiourea Synthesis via N-(2-Cyanophenyl)benzimidoyl isothiocyanate. Molecules 2001, 6, 574-587. https://doi.org/10.3390/60700574
Fathalla WM, Čajan M, Marek J, Pazdera P. One-Pot Quinazolin-4-ylidenethiourea Synthesis via N-(2-Cyanophenyl)benzimidoyl isothiocyanate. Molecules. 2001; 6(7):574-587. https://doi.org/10.3390/60700574
Chicago/Turabian StyleFathalla, Walid M., Michal Čajan, Jaromír Marek, and Pavel Pazdera. 2001. "One-Pot Quinazolin-4-ylidenethiourea Synthesis via N-(2-Cyanophenyl)benzimidoyl isothiocyanate" Molecules 6, no. 7: 574-587. https://doi.org/10.3390/60700574




