Nitration Of Phenols Under Mild And Heterogeneous Conditions
Abstract
:Introduction
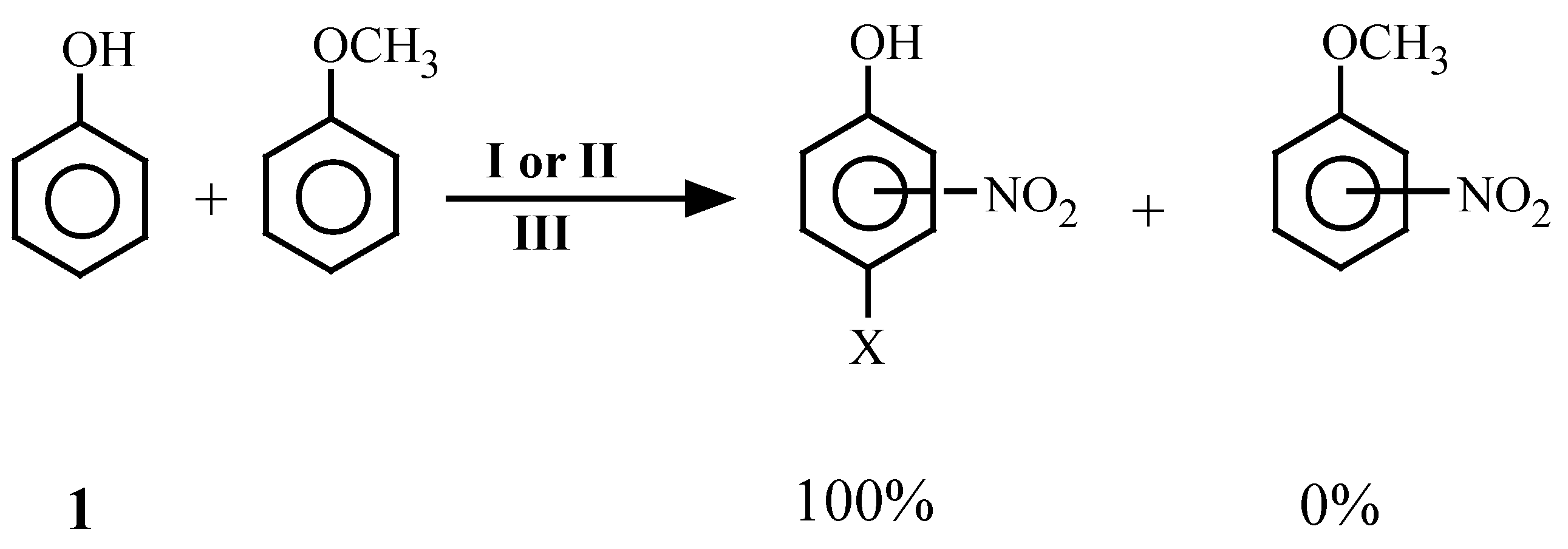
Results and Discussion
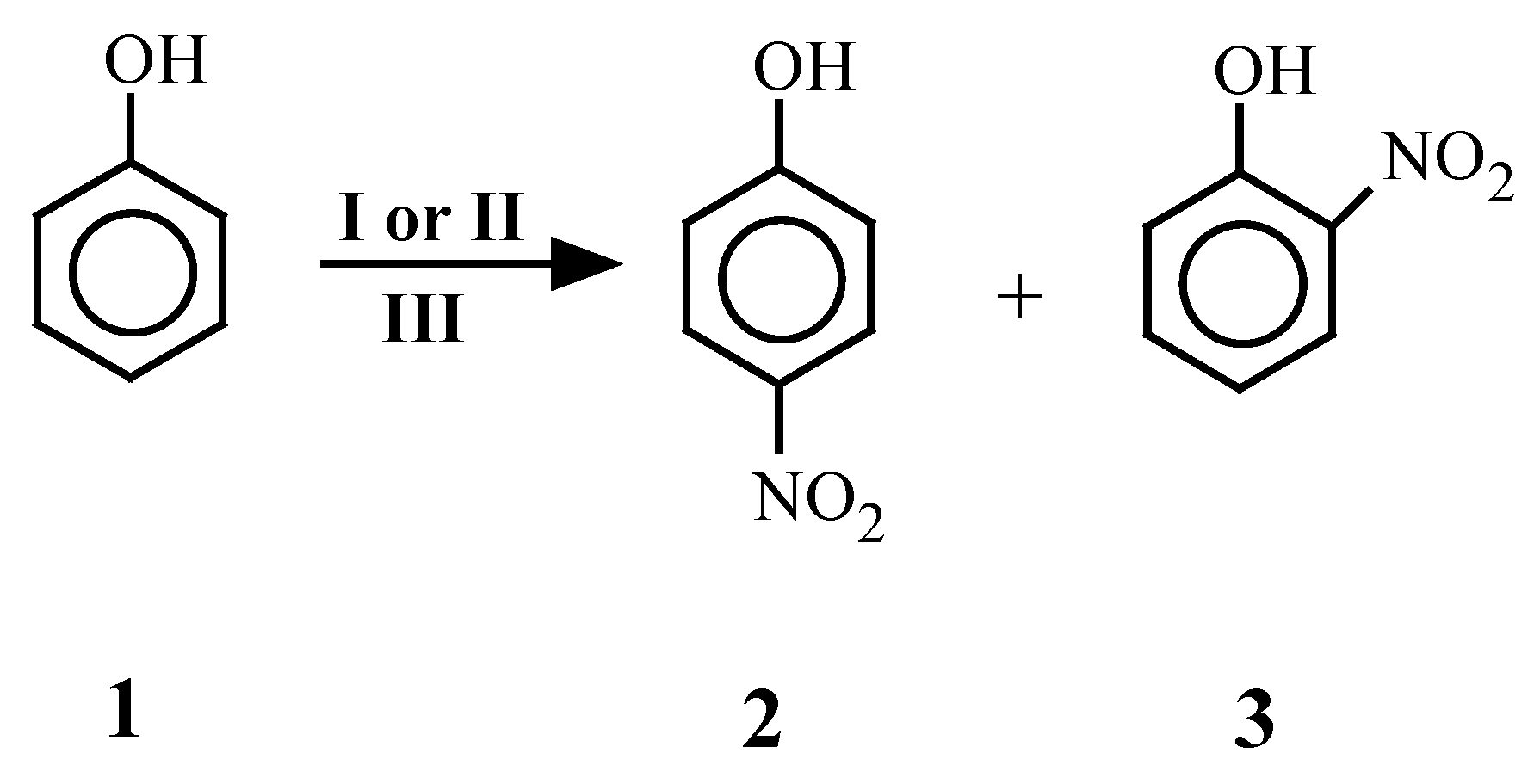
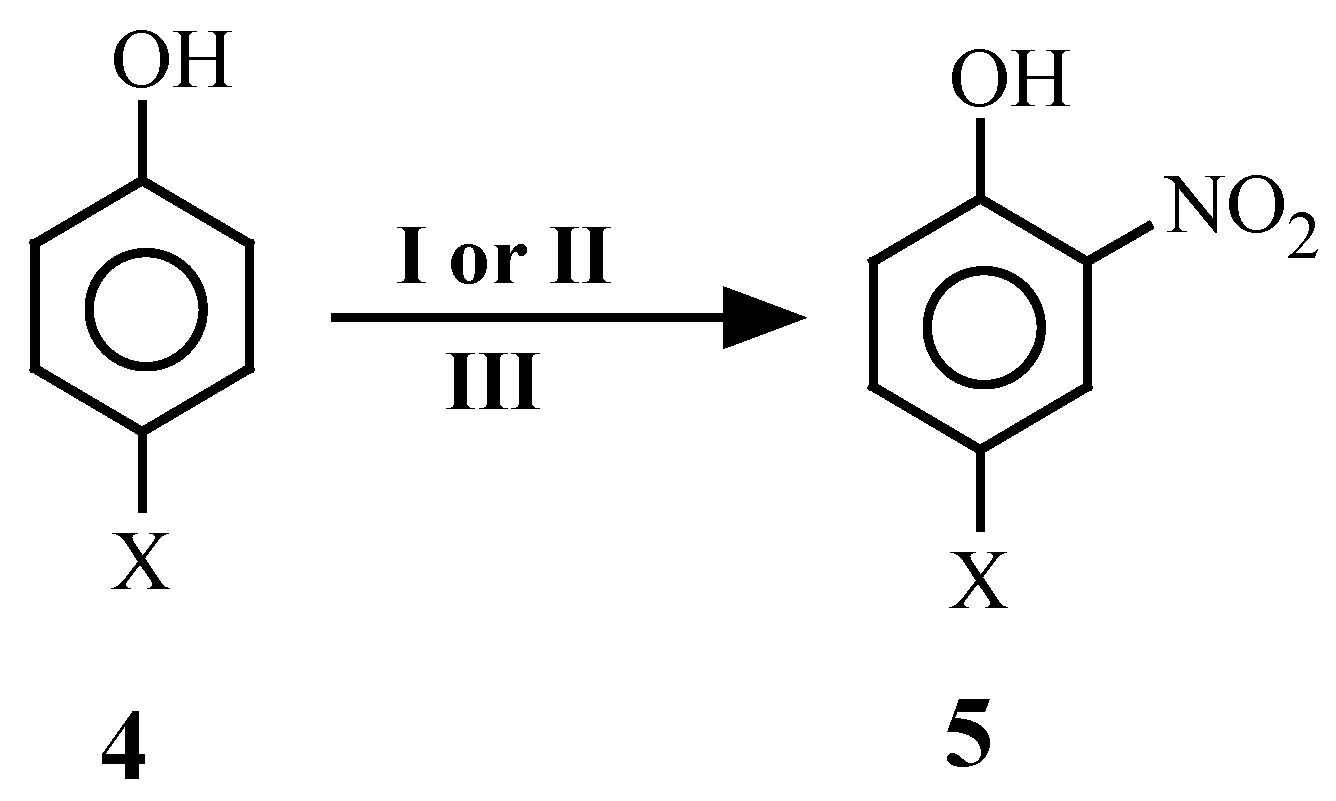
| 4 | X | 4 | X | 4 | X |
|---|---|---|---|---|---|
| a | F | e | Ph | i | COOH |
| b | Cl | f | CH3 | j | CH2Ph |
| c | Br | g | OCH3 | k | NHOAc |
| d | CN | h | COCH3 | l | 4-HOC6H4- |

| Entry | Substrate | Productsa | Reag. /Subst. (mmol)b | Time (Min) | Yieldsc % | ||
|---|---|---|---|---|---|---|---|
| I | II | III | |||||
| 1 | 1 | 2[7] | 1 | -- | 1 | 30 | 26 |
| 3[7] | 36 | ||||||
| 2 | 1 | 2[7] 3[7] | -- | 1 | 1 | 30 | 20 50 |
| 3 | 4a | 5a[4]c | 1 | -- | 1 | 90 | 54 |
| 4 | 4a | 5a[4c] | -- | 1 | 1 | 90 | 60 |
| 5 | 4b | 5b[11, 18] | 1 | -- | 1 | 90 | 70 |
| 6 | 4b | 5b[11, 18] | -- | 1 | 1 | 90 | 72 |
| 7 | 4c | 5c[11, 18] | 1 | -- | 1 | 130 | 78 |
| 8 | 4c | 5c[11, 18] | -- | 1 | 1 | 130 | 70 |
| 9 | 4d | 5d[11, 18] | 1 | -- | 1 | 180 | 88 |
| 10 | 4d | 5d[11, 18] | -- | 1 | 1 | 180 | 92 |
| 11 | 4e | 5e[11, 18] | 1 | -- | 1 | 180 | 90 |
| 12 | 4e | 5e[11, 18] | -- | 1 | 1 | 180 | 92 |
| 13 | 4f | 5f[11, 18] | 1 | -- | 1 | 180 | 72 |
| 14 | 4f | 5f[11, 18] | -- | 1 | 1 | 180 | 78 |
| 15 | 4g | 5g[18] | 1 | -- | 1 | 45 | 84 |
| 16 | 4g | 5g[18] | -- | 1 | 1 | 45 | 79 |
| 17 | 4h | 5h[11, 18] | 2 | -- | 2 | 120 | 74 |
| 18 | 4h | 5h[11, 18] | -- | 2 | 2 | 120 | 79 |
| 19 | 4i | 5i[18] | 2 | -- | 2 | 90 | 80 |
| 20 | 4i | 5i[18] | -- | 2 | 2 | 90 | 83 |
| 21 | 4j | 5j[18] | 2 | -- | 2 | 180 | 91 |
| 22 | 4j | 5j[18] | -- | 2 | 2 | 180 | 91 |
| 23 | 4k | 5k[22] | -- | 1 | 1 | 180 | 55 |
| 24 | 4k | 5k[22] | 1 | -- | 1 | 180 | 74 |
| 25 | 4l | 5l[21] | -- | 1 | 1 | 90 | 56 |
| 26 | 4l | 5l[21] | 1 | -- | 1 | 90 | 63 |
| 27 | 1 | 2, 3 | -- | -- | 1 | No Reactiond | |
Conclusions
Acknowledgments
Experimental section
General
Typical Procedure for Mononitration of Phenol (1) with Mg(HSO4)2 (I), NaNO3 (III) and wet SiO2
Typical Procedure for Mononitration of 4-Cyanophenol (4d) with Mg(HSO4)2 (I), NaNO3 (III) and wet SiO2
References and Notes
- Gu, S.; Jing, H.; Wu, J.; Liang, y. Synth. Commun. 1997, 27, 2793.
- Smith, K.; Musson, A.; DeBoos, G. A. Chem. Commun. 1996, 469.
- Waller, F. G.; Barrett, A. G. M.; Braddock, D. C.; Ramprasad, D. Chem. Commun. 1997, 613, and references cited therein.
- (a) Delaude, L.; Laszlo, P.; Smith, K. Acc. Chem. Res. 1993, 26, 607. (b) Laszlo, P. Acc. Chem. Res. 1986, 19, 121. (c) Cornelis, A.; Laszlo, P.; Pennetreau, P. Bull. Soc. Chim. Belg. 1984, 93, 961.
- Zeegers, P. J. J. Chem. Ed. 1993, 70, 1036.
- Pervas, H.; Onysiuka, S. O.; Rees, L.; Rooney, J. R.; Sukling, G. J. Tetrahedron 1988, 44, 4555.
- Furniss, B. S.; Smith, P. W. G.; Hannaford, A. J.; Tatchell, A. R. "Vogel’s Textbook of Practical Organic Chemistry", 4th Ed ed; Longman: London and New York, 1986. [Google Scholar]
- Bruice, T. C.; Gregor, M. G.; Walters, S. L. J. Am. Chem. Soc. 1986, 90, 1612.
- Crivello, J. V. J. Org. Chem. 1981, 46, 3056.
- Oueartani, M.; Girard, P.; Kagan, H. B. Tetrahedron Lett 1982, 23, 4315.
- Poirier, J. M.; Vottero, C. Tetrahedron 1989, 45, 1415.
- Thompson, M. J.; Zeegers, P. J. Tetrahedron 1989, 45, 191.
- Tapia, R.; Torres, G.; Valderrama, J. A. Synth. Commun. 1986, 16, 681.
- Gaude, D.; Goallar, R. L.; Pierre, J. L. Synth. Commun. 1986, 16, 63.
- Gigante, B.; Prazeres, A. O.; Marcelo-Curto, M. J. J. Org. Chem. 1995, 60, 3445.
- Rodrigues, J. A. R.; Filho, A. P. O.; Moran, P. J. S. Tetrahedron 1999, 55, 6733.
- Suboch, G. A.; Belyaev, E. Y. Russ. Org. Chem. 1998, 34, 288.
- Nonoyama, N.; Chiba, K.; Hisatome, K.; Suzuki, H.; Shintani, F. Tetrahedron Lett. 1999, 40, 6923.
- Lehnig, M. Tetrahedron Lett. 1999, 40, 2299.
- “Dictionary of Organic Compounds”, 3th Ed ed; Eyre & Spottiswoode: London, 1965; Volume 2, p. 620.
- Raiford, L. C.; Colbert, J. C. J. Am. Chem. Soc. 1925, 47, 1454.
- Hancock, C. K.; Clagvex, A. D. J. Am. Chem. Soc. 1964, 86, 4942.
- (a) Zolfigol, M. A.; Iranpoor, N.; Firouzabadi, H. Orient. J. Chem. 1998, 14, 369. (b) Firouzabadi, H.; Iranpoor, N.; Zolfigol, M. A. Iran. J. Chem. & Chem. Eng. 1997, 16, 48. (c) Firouzabadi, H.; Iranpoor, N.; Zolfigol, M. A. Synth. Commun. 1997, 27, 3301. (d) Iranpoor, N.; Firouzabadi, H.; Zolfigol, M. A. Synth. Commun. 1998, 28, 2773.
- (a) Firouzabadi, H.; Iranpoor, N.; Zolfigol, M. A. Synth. Commun. 1998, 28, 377. (b) Firouzabadi, H.; Iranpoor, N.; Zolfigol, M. A. Synth. Commun. 1998, 28, 1179. (c) Iranpoor, N.; Firouzabadi, H.; Zolfigol, M. A. Synth. Commun. 1998, 28, 367. (d) Firouzabadi, H.; Iranpoor, N.; Zolfigol, M. A. Bull. Chem. Soc. Jpn. 1998, 71, 2169. (e) Iranpoor, N.; Firouzabadi, H.; Zolfigol, M. A. Bull. Chem. Soc. Jpn. 1998, 71, 905.
- (a) Laszlo, P.; Cornelis, A. Aldrichimica Acta 1988, 21, 97. (b) Cornelis, A.; Laszlo, P. Synthesis 1985, 909. (c) Laszlo, P.; Cornelis, A. Synthesis 1994, 155.
- Riego, J. M.; Sedin, Z.; Zaldivar, J. M.; Marziano, N. C.; Tortato, C. Tetrahedron Lett. 1996, 37, 513.
- (a) Zolfigol, M. A.; Kiany-Borazjani, M.; Sadeghi, M. M.; Mohammadpoor-Baltork, I.; Memarian, H. R. Synth. Commun. 2000, 30, 551. (b) Zolfigol, M. A.; Kiany-Borazjani, M.; Sadeghi, M. M.; Memarian, H. R.; Mohammadpoor-Baltork, I. Synth. Commun. 2000, 30, 2954. (c) Zolfigol, M. A. Synth. Commun. 1999, 29, 905. (d) Zolfigol, M. A.; Nematollahi, D.; Mallakpour, S. E. Synth. Commun. 1999, 29, 2277. (e) Zolfigol, M. A.; Mallakpour, S. E. Synth. Commun. 1999, 29, 4061. (f) Zolfigol, M. A. Synth. Commun. 2000, 30, 1593. (g) Zolfigol, M. A.; Ghaemi, E.; Madrakian, E.; Kiany-Borazjani, M. Synth. Commun. 2000, 30, 2057. (h) Zolfigol, M. A.; Kiany-Borazjani, M.; Mallakpour, S. E.; Nassr-Isfahani, H. Synth. Commun. 2000, 30, 2573. (i) Zolfigol, M. A.; Madrakian, E.; Ghaemi, E. Indian J. Chem. 2000, 39B, 308. (j) Zolfigol, M. A.; Ghaemi, E.; Madrakian, E. Synth. Commun. 2000, 30, 1689. (k) Zolfigol, M. A.; Kiany-Borazjani, M.; Sadeghi, M. M.; Mohammadpoor-Baltork, I.; Memarian, H. R. Synth. Commun. 2000, 30, 3919. (l) Zolfigol, M. A.; Shirini, F.; Ghorbani Choghamarani, A.; Taqian-nasab, A.; Keypour, H.; Salehzadeh, S. J. Chem. Research (S). 2000, 420.
- For the application of this system to the oxidation of 1,4-dihydropyridines: Zolfigol, M. A.; Kiany-Borazjani, M.; Sadeghi, M. M.; Memarian, H. R.; Mohammadpoor-Baltork, I. J. Chem. Research (S) 2000, 167.
- Anderson, R. A.; Dalgleish, D. T.; Nonhebel, D. C.; Pauson, P. L. J. Chem. Research (S) 1977, 12.
- Sample Availability: All products reported in this paper are available from the authors.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zolfigol, M.A.; Ghaemi, E.; Madrakian, E. Nitration Of Phenols Under Mild And Heterogeneous Conditions. Molecules 2001, 6, 614-620. https://doi.org/10.3390/60700614
Zolfigol MA, Ghaemi E, Madrakian E. Nitration Of Phenols Under Mild And Heterogeneous Conditions. Molecules. 2001; 6(7):614-620. https://doi.org/10.3390/60700614
Chicago/Turabian StyleZolfigol, Mohammad Ali, Ezat Ghaemi, and Elahe Madrakian. 2001. "Nitration Of Phenols Under Mild And Heterogeneous Conditions" Molecules 6, no. 7: 614-620. https://doi.org/10.3390/60700614
APA StyleZolfigol, M. A., Ghaemi, E., & Madrakian, E. (2001). Nitration Of Phenols Under Mild And Heterogeneous Conditions. Molecules, 6(7), 614-620. https://doi.org/10.3390/60700614




