An Acylated Kaempferol Glycoside from Flowers of Foeniculum vulgare and F. Dulce
Abstract
:Introduction
Results and Discussion

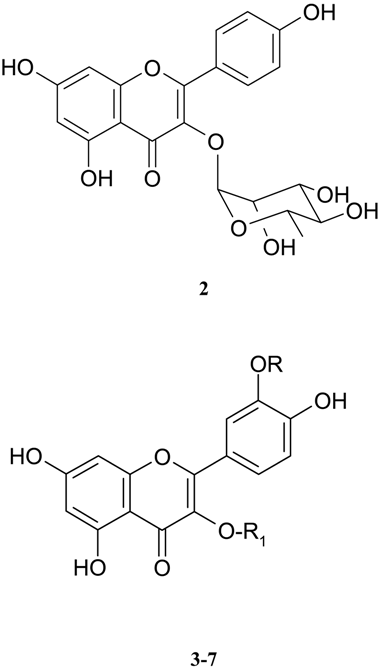
4 R=OCH3, R1=β-D-glucose
5 R=H, R1=β-D-glucose
6 R=H, R1β-D-glucose- α-L-rhamnose
7 R=H, R1= β-D-glucuronic acid
| Comp. | Rf* | F. vulgare Mill. | F. dulce DC. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| leaf | flower | fruit | stem | root | leaf | flower | fruit | stem | root | ||
| 1 | 0.96 | - | ++ | - | - | - | - | ++ | - | - | - |
| 2 | 0.89 | - | ++ | - | - | - | - | ++ | + | - | - |
| 3 | 0.86 | + | - | - | - | - | - | - | - | - | - |
| 4 | 0.81 | - | + | - | - | - | - | + | - | - | - |
| 5 | 0.79 | + | ++ | - | - | - | - | ++ | + | - | - |
| 6 | 0.58 | - | + | - | - | - | - | + | - | - | - |
| 7 | 0.42 | + | ++ | + | - | - | - | ++ | + | - | - |
Experimental
General
Plant material
Extraction and Isolation
Kaempferol-3-O-α-L-(2”,3”-E-di-p-coumaroyl)-rhamnoside (1).
Hydrolysis of the isolated compounds
Acknowledgments
References
- Marotti, M.; Piccaglia, R.; Giovanelli, E. Effects of Variety and Ontogenic Stage on the Essential Oil Composition and Biological Activity of Fennel. J. Essent. Oil Res. 1994, 6, 57–62. [Google Scholar] [CrossRef]
- Kowalchick, C.; Hylton, W.H. (Eds.) Rodales Illustrated Encyclopedia of Herbs; Rodale Press: Emmaus, Pennsylvania, 1988; Volume 188.
- Mabberley, D.J. The Plant Book, 2nd ed.; Cambridge Univ. Press: U.K, 1997; Volume 286. [Google Scholar]
- Baily, L.H.; Baily, E.Z. Hortus Third; Macmillan Publishing Co. Inc.: New York, 1976; Volume 481. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Flavonoid Pattern in the Fruits of the Umbellifera. Phytochemistry 1972, 11, 1741-50. [Google Scholar]
- Harborne, J.B.; Saleh, N.A.M. Flavonol Glycoside Variation in Fennel, Foeniculum vulgare. Phytochemistry 1971, 10, 399–400. [Google Scholar] [CrossRef]
- Crowden, R.K.; Harborne, J.B.; Heywood, V.H. Chemosystematics of the Umbelliferae - A General Survey. Phytochemistry 1969, 8, 1963-84. [Google Scholar] [CrossRef]
- Ohta, T.; Miyazaki, T. Foenicularin, a Quercetin-3-O-Arabinoside from the Leaves of F. vulgare. J. Pharm. Soc. Japan 1959, 76, 323. [Google Scholar]
- Kunzemann, J.; Herrmann, K. Z. Isolation and Identification of Flavonol-O-glycosides in Caraway (Carum carvi L.), Fennel (Foeniculum vulgare Mill.), Anise (Pimpenella anisum L.) and Coriander (Coriandrum sativum L.), and Flavonol-C-glycosides in Anise. Lebensm-Unters-Forsch 1977, 164, 194–200, [Chem. Abstr. 1977, 87, 166146y]. [Google Scholar]
- Nakaoki, T.; Morita, Y.; Nagata, Y.; Oguri, H. Flavonoids of the Leaves of Nelumbo nucifera, Cosmos bipinnatus and Foeniculum vulgare. Yakugaku Zasshi 1961, 81, 1158-59, [Chem. Abstr., 1962, 56, 1527d]. [Google Scholar]
- Ghodsi, M.B. Flavonoids of Foeniculum vulgare Mill. Maj-Daneshgah-e-Tehran Danesh kade-ye Darusazi 1976, 10, 14, [Chem. Abstr., 1980, 92, 72698f]. [Google Scholar]
- Harborne, J.B.; Mabry, T.J.; Mabry, H. (Eds.) The Flavonoids; I, Academic Press: New York, 1975.
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systemic Identification of Flavonoids; Springer-Verlag: New York, Heidelberg, Berlin, 1970. [Google Scholar]
- Aritomi, M.; Kawasaki, T. Three Highly Oxygenated Flavone Glucuronides in Leaves of Spinacia oleracea. Phytochemistry 1984, 23, 2043-47. [Google Scholar]
- Agrawal, P.K. (Ed.) Carbon-13 NMR of Flavonoids; Elsevier: Amsterdam, Oxford, New York, and Tokyo, 1989.
- Tomas-Barberan, F.A.; Gil, M.I.; Ferreres, F.; Tomas-Lorente, F. Flavonoid p-Coumaroyl and 8-Hydroxyflavone Allosylglucosides in Some Labiatae. Phytochemistry 1992, 31, 3097–3102. [Google Scholar]
- Fiorini, C.; David, B.; Fouraste, I.; Vercauteren, J. Acylated Kaempferol Glycosides from Laurus nobilis leaves. Phytochemistry 1998, 47, 821-25. [Google Scholar]
- Kaouadji, M. Acylated and Non-acylated Kaempferol Monoglycosides from Planatus acerifolia Buds. Phytochemistry 1990, 7, 2295-97. [Google Scholar]
- Sample availability: Samples of compounds 1 (2 mg), 3 (5mg), 5 (5mg) and 7 (5 mg) are available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Soliman, F.M.; Shehata, A.H.; Khaleel, A.E.; Ezzat, S.M. An Acylated Kaempferol Glycoside from Flowers of Foeniculum vulgare and F. Dulce. Molecules 2002, 7, 245-251. https://doi.org/10.3390/70200245
Soliman FM, Shehata AH, Khaleel AE, Ezzat SM. An Acylated Kaempferol Glycoside from Flowers of Foeniculum vulgare and F. Dulce. Molecules. 2002; 7(2):245-251. https://doi.org/10.3390/70200245
Chicago/Turabian StyleSoliman, Fathy M., Afaf H. Shehata, Amal E. Khaleel, and Shahera M. Ezzat. 2002. "An Acylated Kaempferol Glycoside from Flowers of Foeniculum vulgare and F. Dulce" Molecules 7, no. 2: 245-251. https://doi.org/10.3390/70200245
APA StyleSoliman, F. M., Shehata, A. H., Khaleel, A. E., & Ezzat, S. M. (2002). An Acylated Kaempferol Glycoside from Flowers of Foeniculum vulgare and F. Dulce. Molecules, 7(2), 245-251. https://doi.org/10.3390/70200245




