Synthetic Studies on Optically Active Schiff-base Ligands Derived from Condensation of 2-Hydroxyacetophenone and Chiral Diamines
Abstract
:Introduction
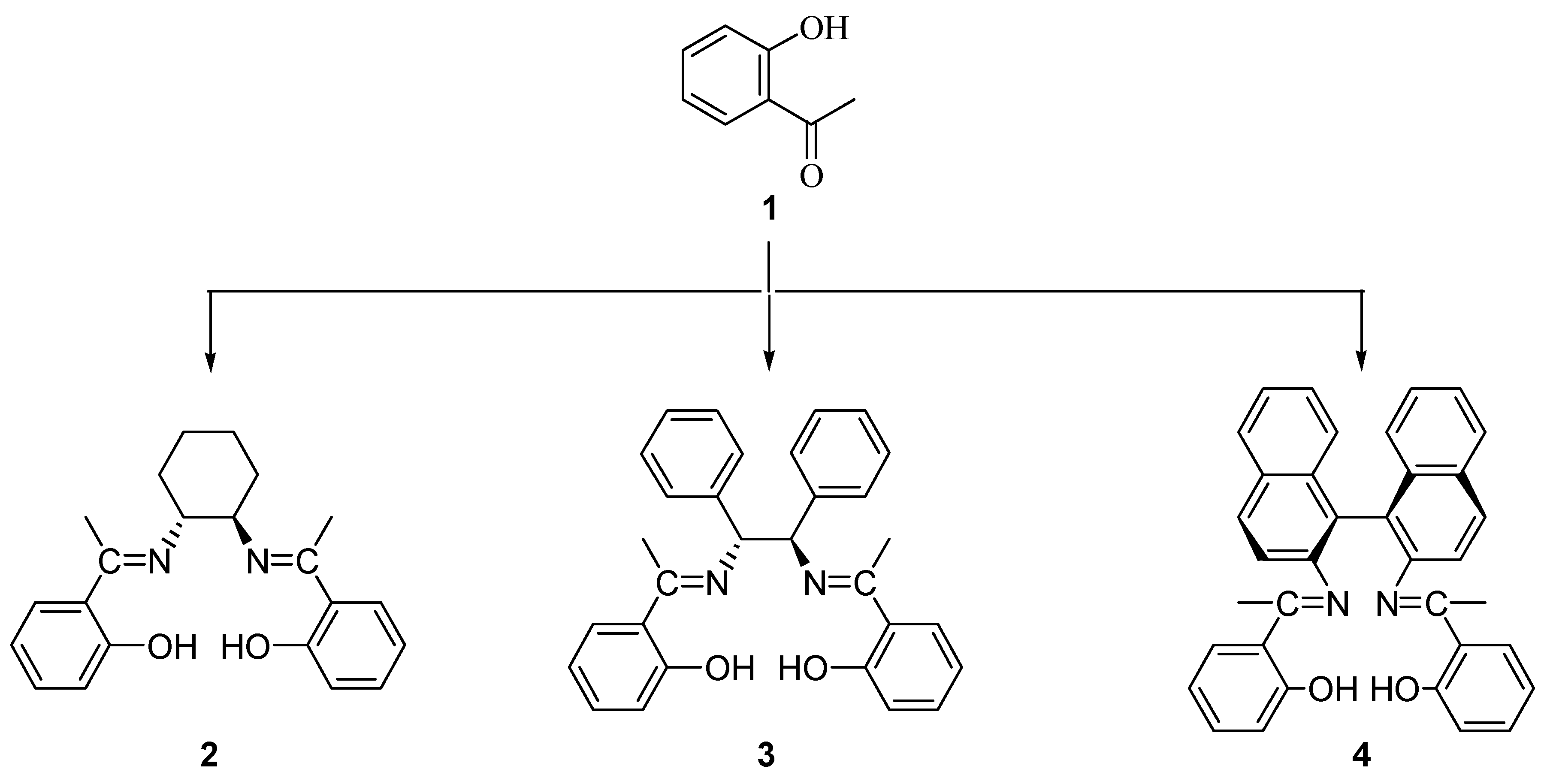
Results and discussion
Experimental
General
Synthesis of N,N’-(1R,2R)-(-)-1,2-cyclohexylenebis(2-hydroxyacetophenonylideneimine) (2).
Synthesis of (1S,2S)-(-)- 1,2-diphenylethylenebis(2-hydroxyacetophenonylideneimine) (3).
Synthesis of R-(+)-1,1’-binaphthalene-2,2’-diaminobis(2-hydroxyacetophenonylideneimine) (4).
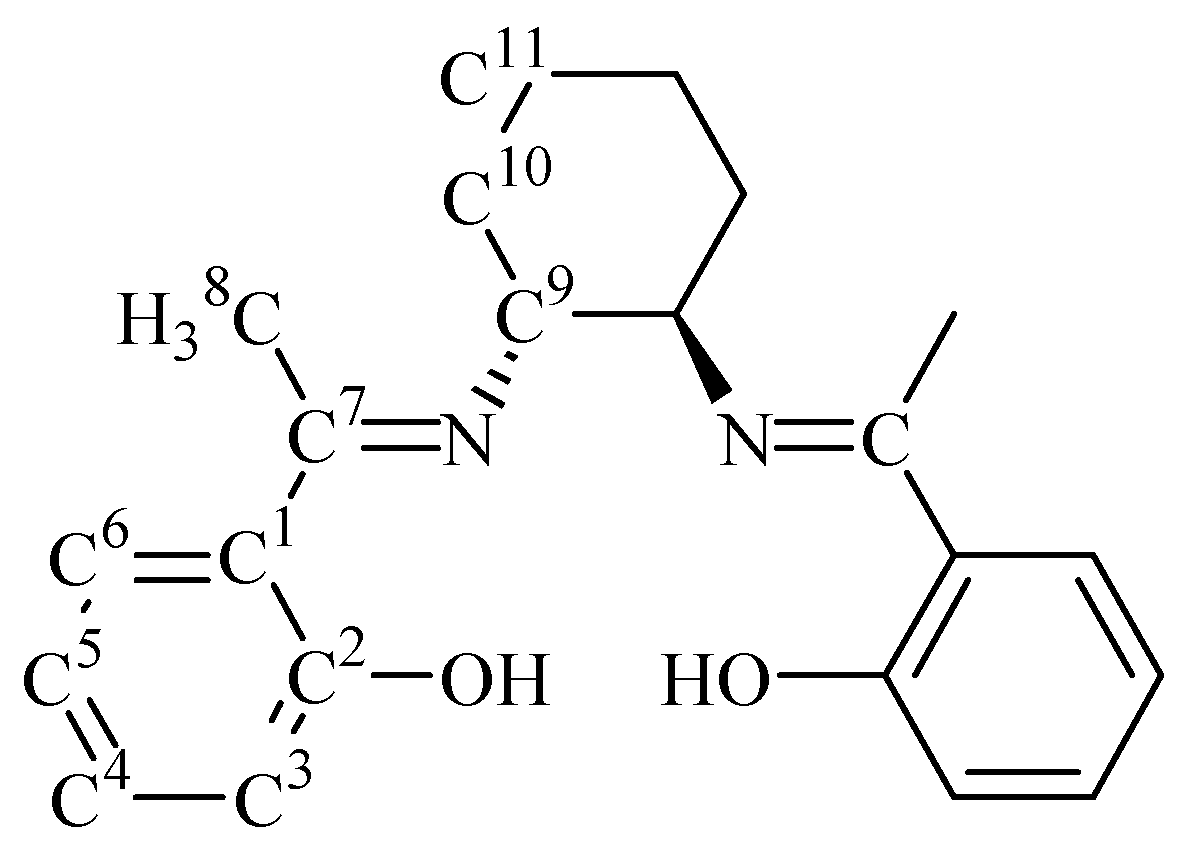
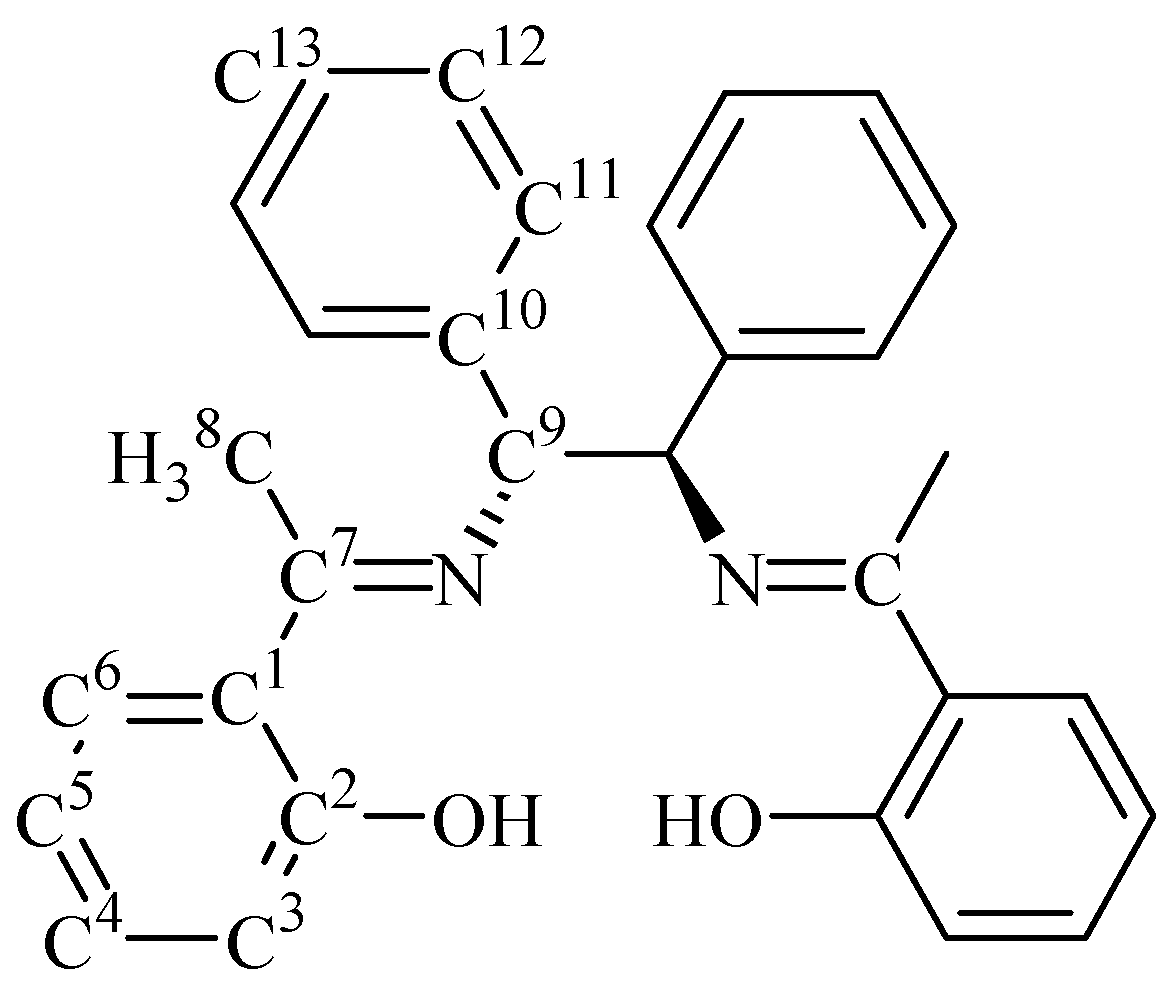

Acknowledgements
References and Notes
- Zhang, J.X.; Zhou, Y.; Cai, G. Preparation of Schiff base-Cu complex and their application in the asymmetric synthesis of chrysanthemate. J. Mol. Catal (China) 1997, 11, 41–44. [Google Scholar] Holland, D; Laidler, D.A.; Milner, D.J. Catalytic asymmetric synthesis of cyclopropane carboxylates: Ligand-reagent interaction in diazoacetate reactions catalysed by copper(II)species bearing sugar-Schiff base ligands. J. Mol. Catal. 1981, 11, 119–127. [Google Scholar] Chen, G.M.; Chen, F.; Zhou, C. Synthesis of chiral copper(II) compounds and their application in asymmetric cyclopropanation of olefins. Chem. J. Chin. Univ. 1995, 16, 216–219. [Google Scholar] Qiu, M.; Liu, G.; Yiao, X. Chiral copper (II)-Schiff base complexes as catalysts for asymmetric cyclopropanation of styrene. Chin. J. Catal. 2001, 22, 77–80. [Google Scholar] Li, C.; Zhang, W.; Yao, X. Synthesis of a new C2-symmetric chiral Schiff base and its catalytic performance in asymmetric cyclopropanation. Chin. J. Catal. 2000, 21, 77–80. [Google Scholar] Li, Z.N.; Liu, G.; Zheng, Z. Asymmetric cyclopropanation of styrene catalyzed by Cu-(chiral Schiff-base) complexes. Tetrahedron 2000, 56, 7187–7191. [Google Scholar]
- Kenneth, J.O.; Shiow, J.W.; Cynthia, J.B. Alkene aziridination and epoxidation catalyzed by chiral metal salen complexes. Tetrahedron Lett. 1992, 33, 1001–1004. [Google Scholar]
- Jacobsen, E.J.; Zhang, W.; Guler, M.L. Electronic tuning of asymmetric catalysts. J. Am. Chem. Soc. 1991, 113, 6703–6704. [Google Scholar] [CrossRef] Jacobsen, E.J.; Zhang, W.; Muci, A.R. Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane. J. Am. Chem. Soc. 1991, 113, 7063–7064. [Google Scholar] Kureshy, R.I.; Khan, N.H.; Abdi, S.T. Synthesis, Physico-chemical studies and solvent-dependent enantioselective epoxidation of 1,2-dihydronaphthalene catalyzed by chiral ruthenium(II) Schiff base complexes. I. J. Mol. Catal. 1999, 150, 163–173. [Google Scholar] Kureshy, R.I.; Khan, N.H.; Abdi, S.T. Synthesis, physico-chemical studies and solvent-dependent enantio-selective epoxidation of 1,2-dihydronaphthalene catalyzed by chiral ruthenium(II) Schiff base complexes. II. J. Mol. Catal. 1999, 150, 175–183. [Google Scholar] Rukhsana, I.K.; Noor-ul, H.K.; Sayed, H.R. Asymmetric epoxidation of styrene by novel chiral ruthenium(II) Schiff base complexes-Synthesis and characterization. Tetrahedron: Asymmetry 1993, 4, 1693–1701. [Google Scholar] Hosoya, N.; Hatayama, A.; Irie, R. Rational design of Mn-Salen epoxidation catalysts: Preliminary results. Tetrahedron 1994, 50, 4311–4322. [Google Scholar] Fernandez, I.; Pedro, J.R.; Salud, R. Aerobic catalytic epoxidation of unfunctionalized olifens using a new (Salen)manganese(III) complex bearing sesquiterpene salicyladehyde derivative. Tetrahedron 1996, 52, 12031–12038. [Google Scholar] Kureshy, R.I.; Khan, N.H.; Abdi, S.T. Enantioselective catalytic epoxidation of nonfunctionalized prochiral olefins by dissymmetric chiral Schiff base complexes of Mn(III) and Ru(III) metal ions. J. Mol. Catal. 1997, 120, 101–108. [Google Scholar] Kureshy, R.I.; Khan, N.H.; Abdi, S.T. Synthesis, physicochemical studies and aerobic Enantioselective epoxidation of nonfunctionalized olefins catalyzed by new Co (II) chiral salen complexes. J. Mol. Catal. 1997, 121, 25–31. [Google Scholar] Kureshy, R.I.; Khan, N.H.; Abdi, S.T. Eenantioselective catalytic epoxidation of styrenes by iodosylbenzene using chiral ruthenium (II) Schiff base complexes. J. Mol. Catal. 1995, 96, 117–122. [Google Scholar] Kureshy, R.I.; Khan, N.H.; Abdi, S.H.R.; Patel, S.T.; Lyer, P. Asymmetric catalytic epoxidation of styrene by dissymmetric Mn(III) and Ru(III) chiral Schiff base complexes. Synthesis and physicochemical studies. J. Mol. Catal. 1996, 110, 33–40. [Google Scholar]
- Kim, G.J.; Shin, J.H. Application of new unsymmetrical chiral Mn(III), Co(II,III) and Ti(IV) salen complexes in enantioselective catalytic reactions. Cat. Lett. 1999, 63, 83–89. [Google Scholar] [CrossRef]
- Sasaki, C.; Nakajima, K.; Kojima, M. Preparation and characterization of optically active quadridentate Schiff base-Titanium (IV) complexes and the catalytic properties of these complexes on asymmetric oxidation of methyl phenyl sulfide with organic hydro peroxides. Bull. Chem. Soc. Jpn. 1991, 64, 1318–1324. [Google Scholar] [CrossRef]
- Waldemar, A.; Rainer, T.; Veit, R.S. Synthesis of optically active carbonyl compounds by the catalytic, Enantiselective oxidation of silyl enol ethers and ketene acetals with (salen) manganese (III) complexes. J. Am. Chem. Soc. 1998, 120, 708–714. [Google Scholar]
- Hayashi, M.; Inoue, T.; Miyamoto, Y. Asymmetric carbon-carbon bond formation reactions catalyzed by chiral Schiff base-Titanium alkoxide complexes. Tetrahedron 1994, 50, 4385–4398. [Google Scholar] [CrossRef]
- Aratani, T.; Yoneyoshi, Y.; Nagase, T. Tetrahedron Lett. 1975, 1707. 1977, 2599. 1982, 23, 685. Aratani, T. Pure Appl. Chem. 1985, 57, 1839.
- Sample Availability: Samples of the ligands 2 and 3 are available from the MDPI.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Gao, W.T.; Zheng, Z. Synthetic Studies on Optically Active Schiff-base Ligands Derived from Condensation of 2-Hydroxyacetophenone and Chiral Diamines. Molecules 2002, 7, 511-516. https://doi.org/10.3390/70700511
Gao WT, Zheng Z. Synthetic Studies on Optically Active Schiff-base Ligands Derived from Condensation of 2-Hydroxyacetophenone and Chiral Diamines. Molecules. 2002; 7(7):511-516. https://doi.org/10.3390/70700511
Chicago/Turabian StyleGao, Wen Tao, and Zhuo Zheng. 2002. "Synthetic Studies on Optically Active Schiff-base Ligands Derived from Condensation of 2-Hydroxyacetophenone and Chiral Diamines" Molecules 7, no. 7: 511-516. https://doi.org/10.3390/70700511



