Introduction
The utility of microwaves in heterocyclic synthesis is now receiving considerable attention [
1,
2,
3,
4] and, although enaminones has been recently extensively utilized as precursors for the synthesis of heteroaromatics, the solventless reaction of enaminones with nucleophilic reagents under microwave irradiation has not, to our knowledge, been previously investigated. As a part of a recent project, aiming to explore potential utility of microwaves as an energy source for heterocyclic synthesis, I report here on synthesis of 3-heteroarylsubstituted coumarins and benzocoumarins of potential interest as pharmaceuticals and/or photochromic dyes [
5,
6,
7] and investigate the possibility of conducting these reactions under microwave irradiation in addition to the standard thermal conditions.
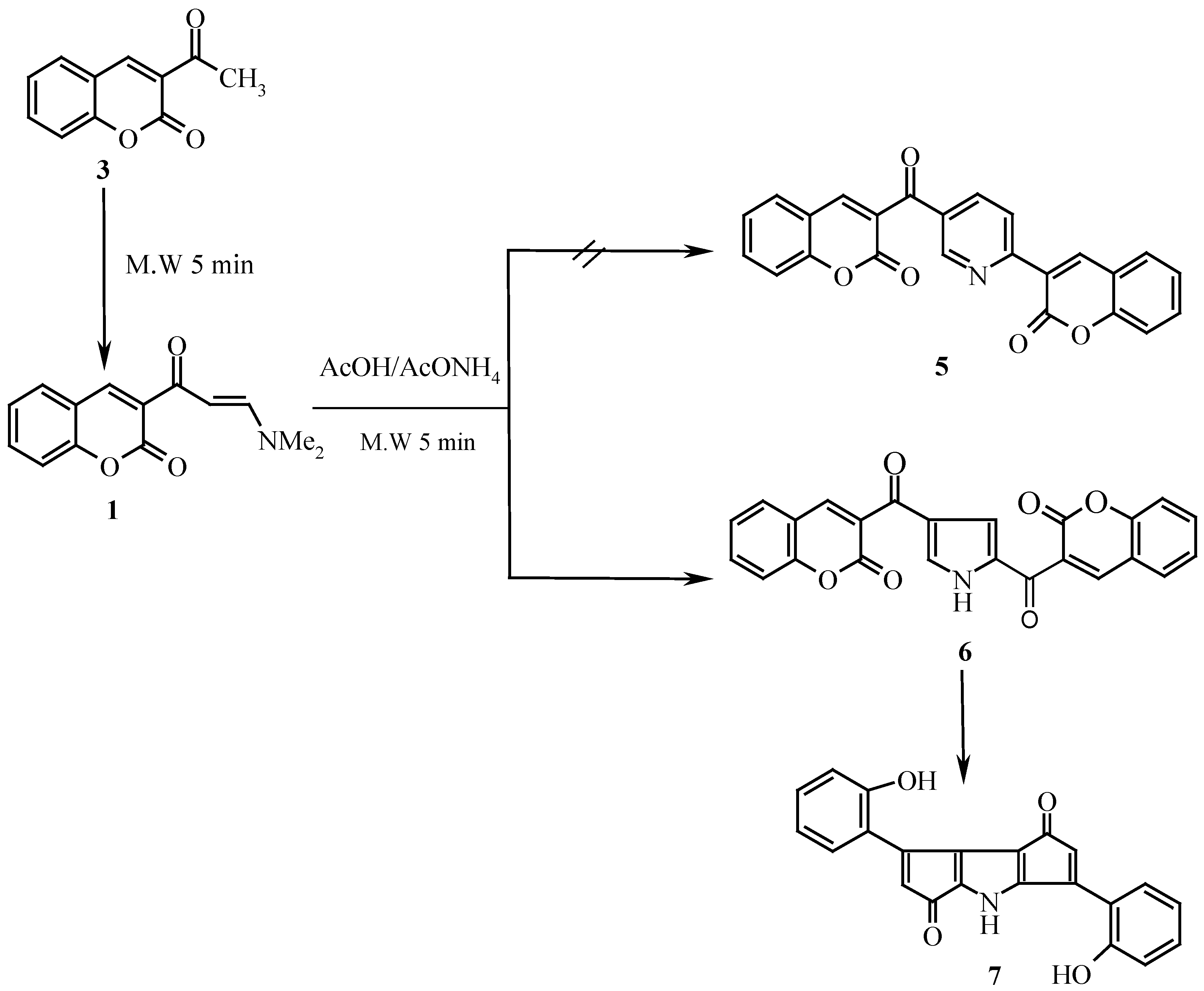
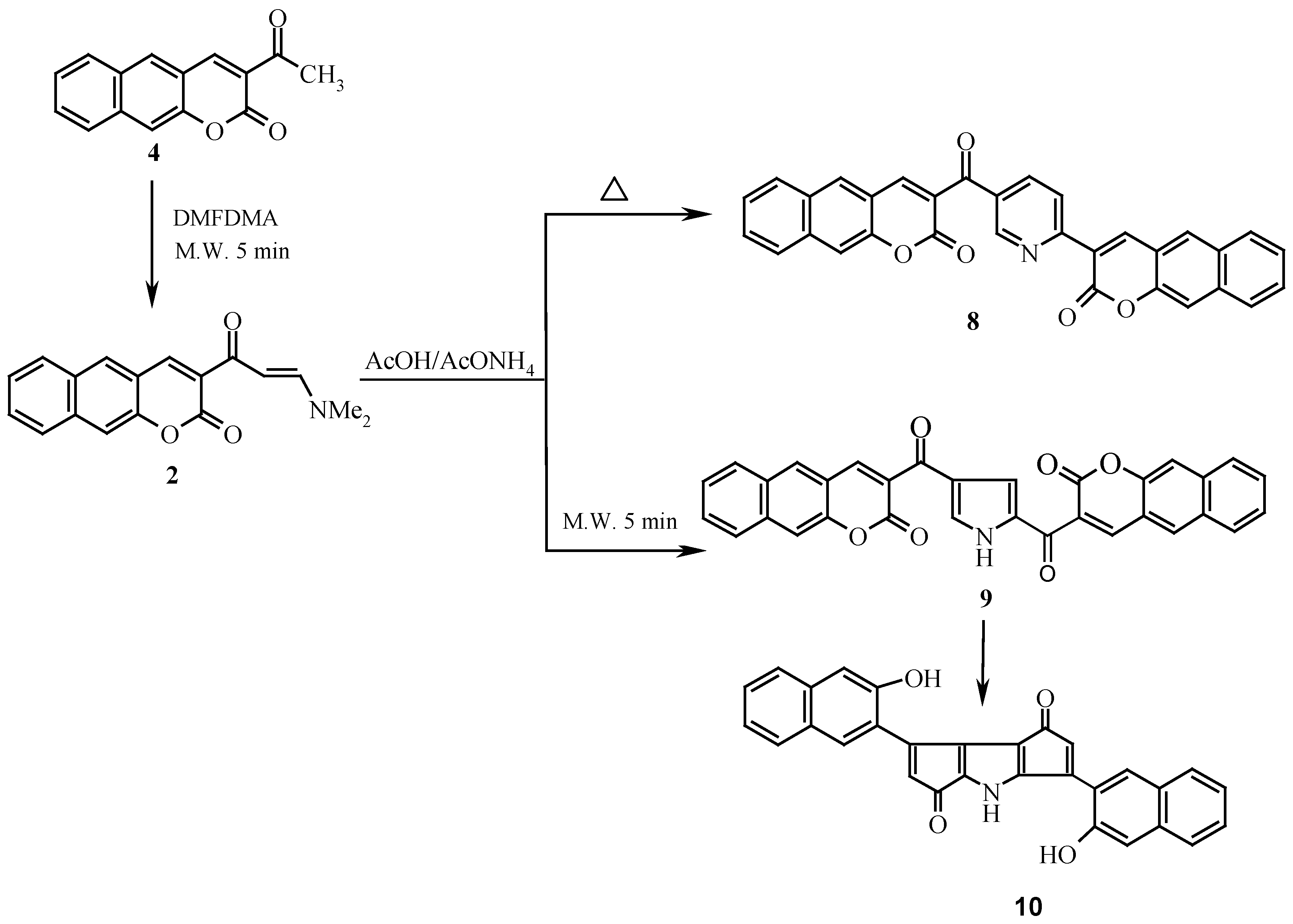
Enaminones
1 and
2 were smoothly obtained by reacting compounds
3 and
4 with dimethylformamide dimethylacetal (DMFDMA) in a domestic microwave for a very short time, the yield being much higher than that obtained by conventional heating with a solvent. Compound
1 has been recently synthesized by Elnagdi
et al., [
8] by refluxing 3-acetylcoumarin with DMFDMA in xylene solution. However, under such conditions the yield of the enaminone was much lower than that obtained by the solventless procedure. Although the enaminones obtained may exist also in
cis-form only the
trans-forms
1 and
2 were obtained, as revealed by
1H-NMR, which showed olefinic protons at δ = 6.53 ppm and δ = 8.17 ppm with
J = 14Hz.
Although enaminone
1 has been reported earlier in literature [
8] to yield pyridine derivative
5 on reflux in acetic acid in presence of ammonium acetate, treatment of
1 with acetic acid and ammonium acetate in a domestic microwave oven at full power has afforded a product of molecular formula C
22H
13NO
4, for which structure
7 is suggested. It is assumed that an initially formed 2,4-dicoumarinoyl pyrrole
6 undergoes a Nenitzescu like cyclization [
9] and decarbonylation thus yielding
7 (
Scheme 1).
Similar to the behavior of
1, compound
2 yielded pyridine derivative
8 on reflux in acetic acid in presence of ammonium acetate, while treatment of
2 with acetic acid and ammonium acetate in a domestic microwave oven at full power has afforded compound
10 (
Scheme 2).
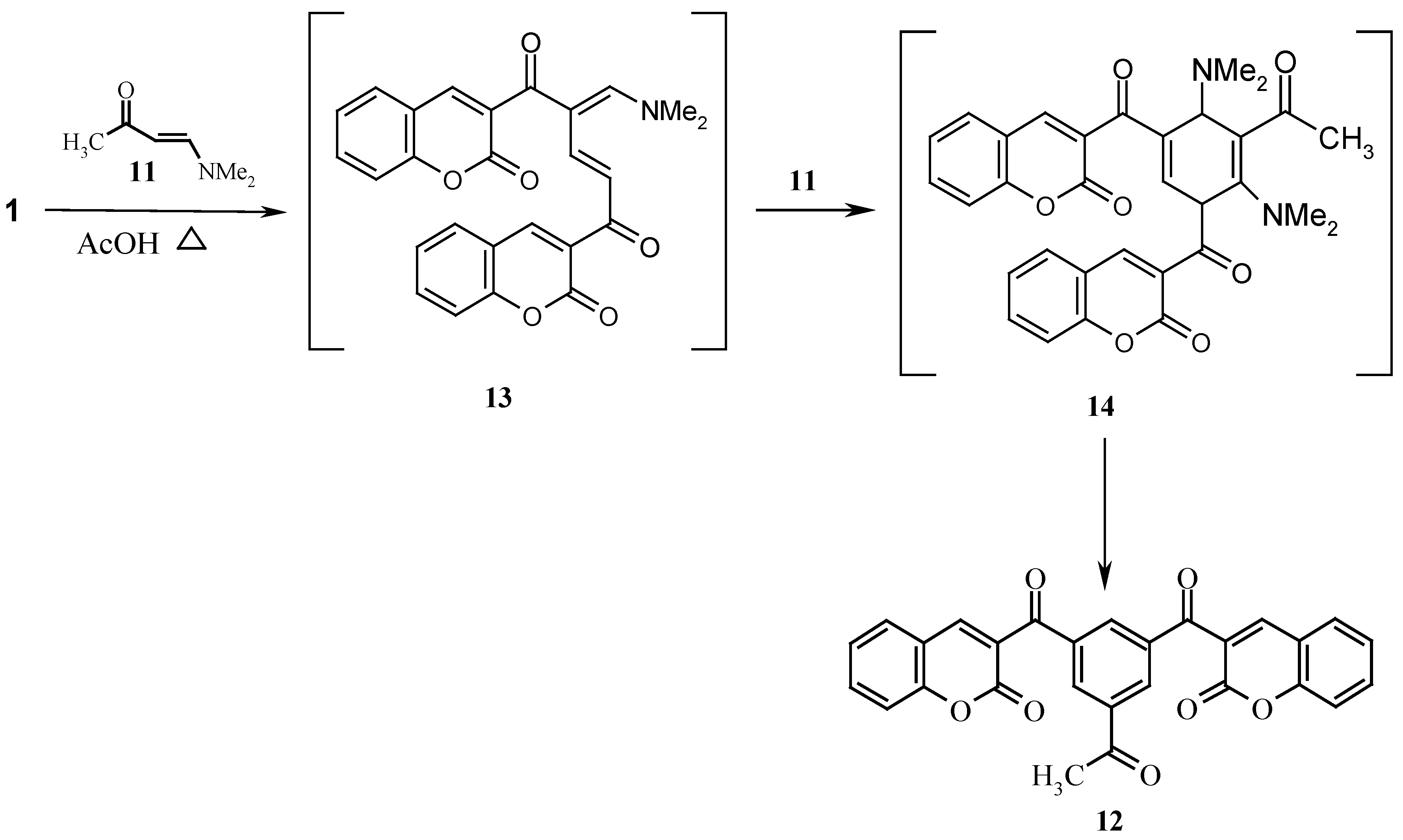
Thermal treatment of
1 with the enaminone
11 in acetic acid (
Scheme 3) gave
12, which may be a product of a thermal 2+2+2 cycloaddition or a product of initial dimerization of
1 into
13 that reacts with the enaminone
11 to yield
14, that then aromatises to give the final isolated product
12 [
10].
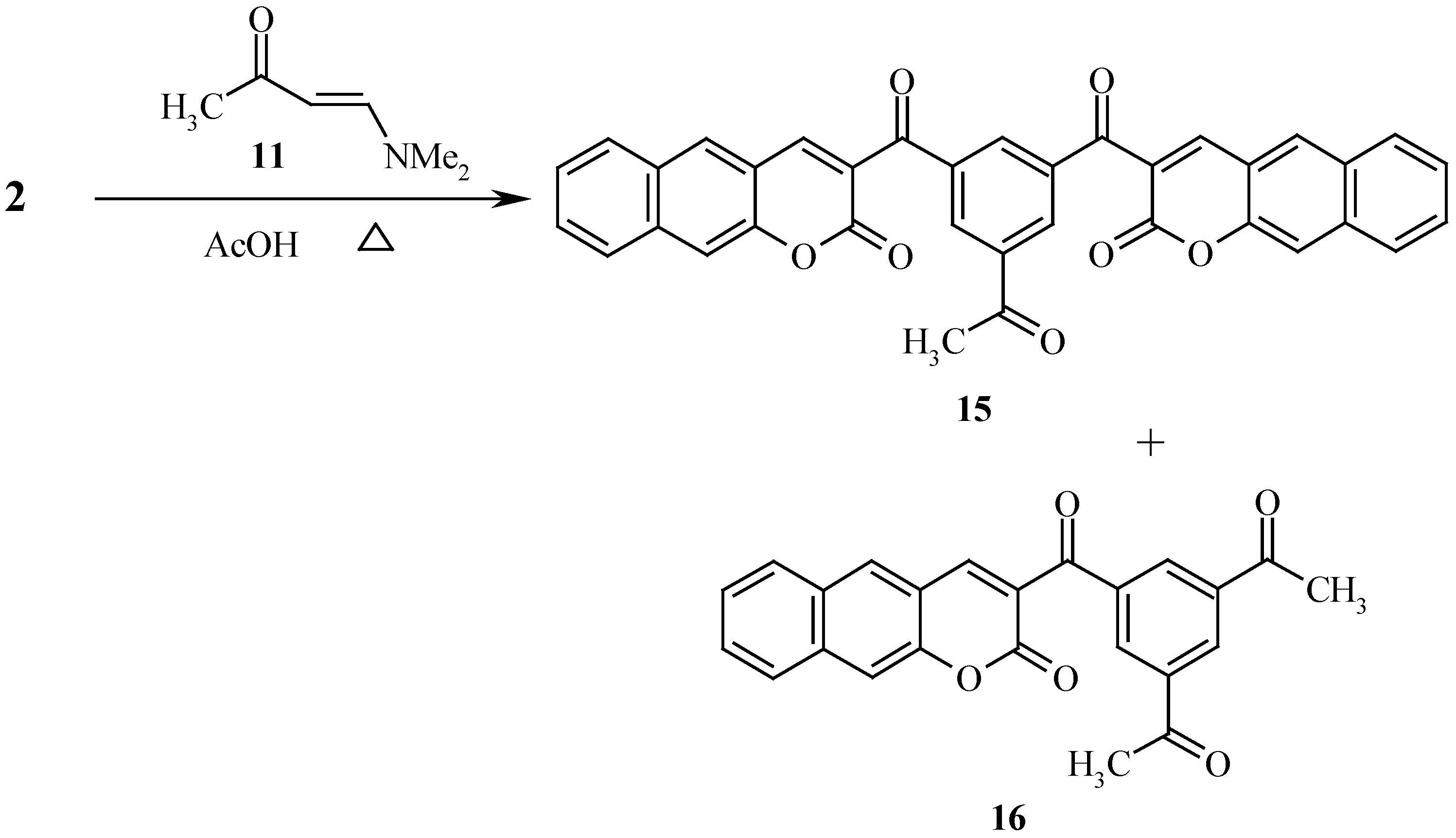
Under the same reaction conditions, compound
2 afforded a mixture of
15 and
16 (
Scheme 4)
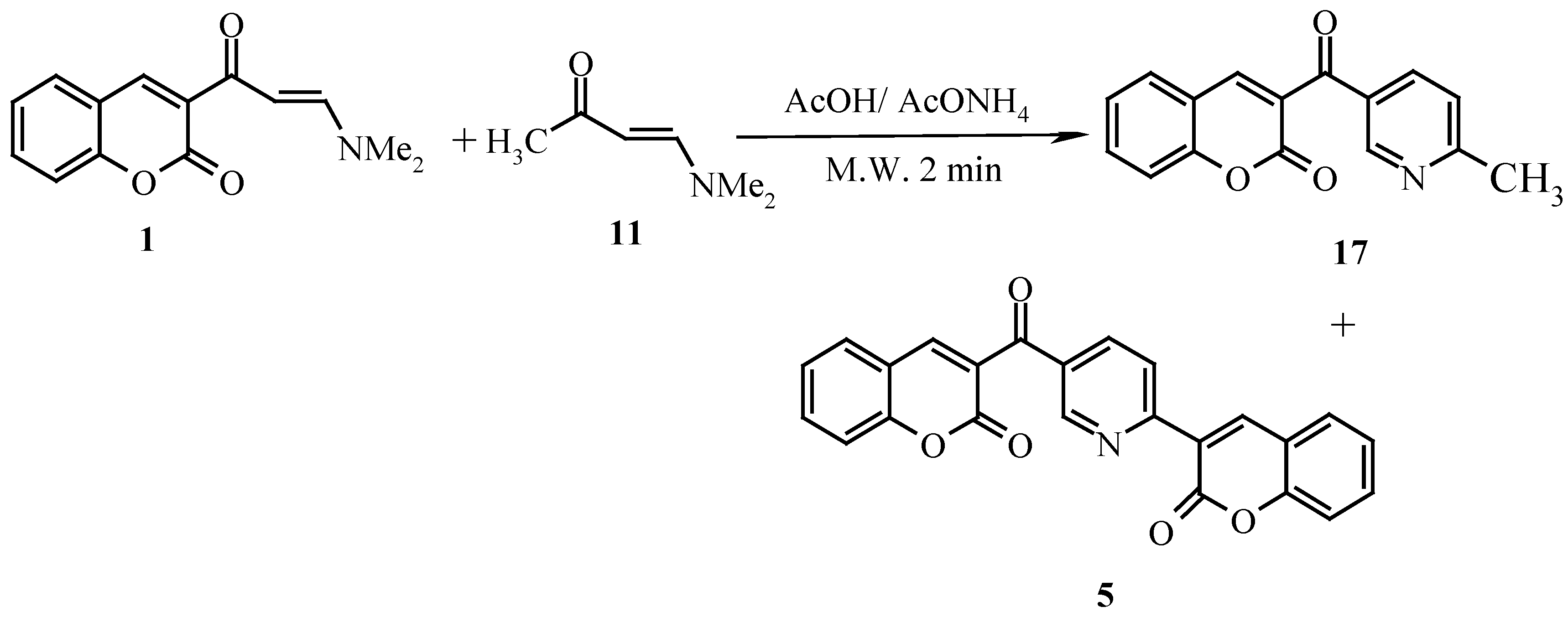
The reaction of
1 with
11 in a microwave oven had afforded a mixture of
17 and
5 (
Scheme 5). Product
5 has been obtained earlier by Elnagid
et al. via dimerization of
1 in presence of ammonium acetate [
8].
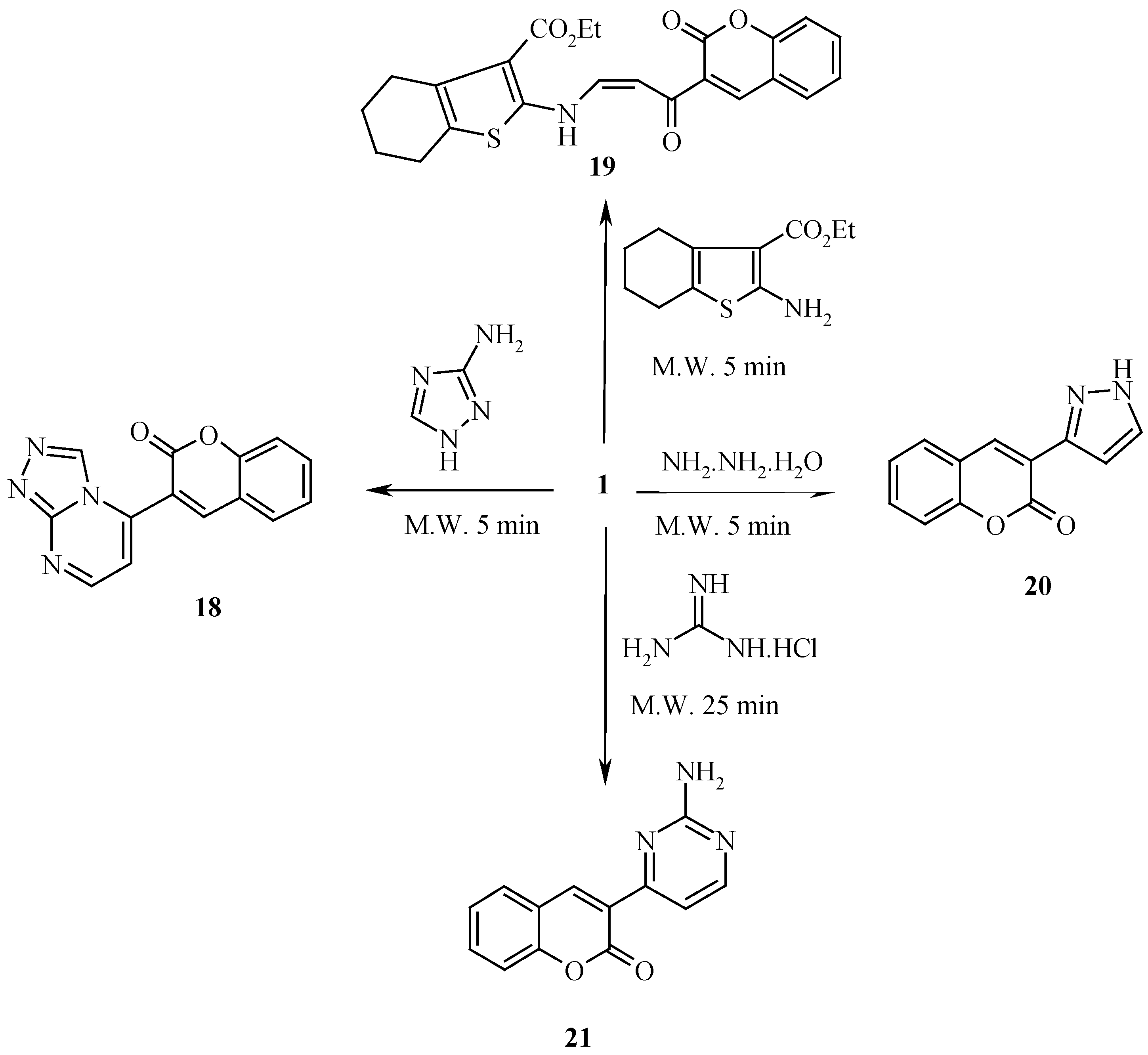
Compound
1 reacts readily with nitrogen nucleophiles in a microwave oven yielding products of addition, dimethylamine elimination and, in some cases, further cyclization affording a variety of 3-substituted heteroaromatic coumarin derivatives. For example, with 3(5)1,2,4-aminotriazole, the coumarinyl triazolopyrimidine derivative
18 has been obtained. The reaction of ethyl 2-aminotetrahydrobenzo[b]thiophene-3-caroboxylate with
1 afforded
19, which was found to exist totally in
cis form, as revealed by the
1H-NMR spectrum which displayed olefinic protons at δ 5.69 and δ 6.61 ppm with
J = 9Hz, typical for
cis protons. It is likely that hydrogen bonding makes the
cis form more stable than the
trans one. Reaction of
1 with hydrazine hydrate afforded the pyrazolyl coumarin
20. The reaction of
1 with guanidine hydrochloride in a microwave oven has afforded coumarinyl pyrimidine
21 in a very high yield (
Scheme 6).
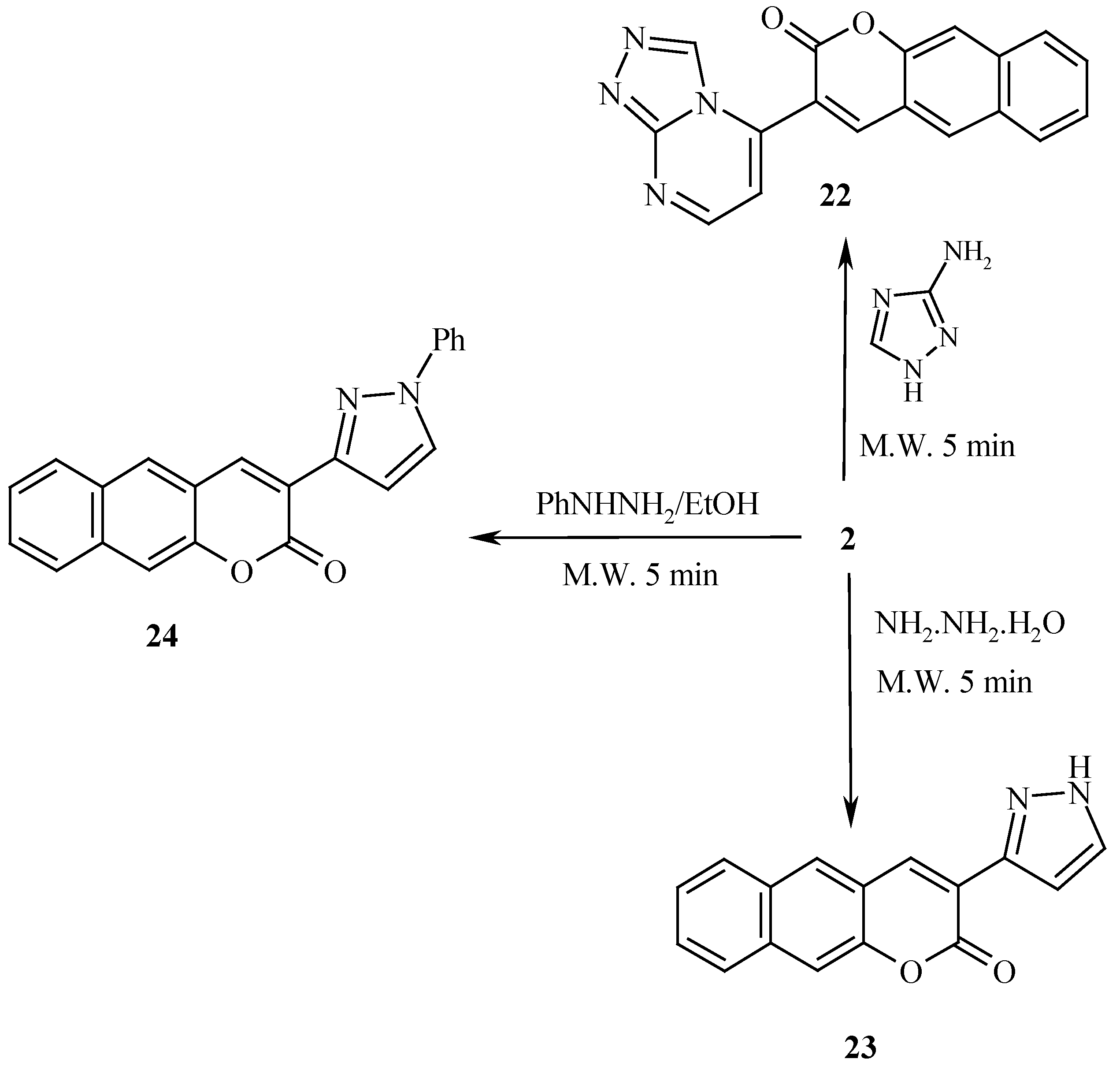
Similar to the behavior of
1, compound
2 reacted with 3(5)1,2,4-aminotriazole to yield the benzocoumarinyl triazolopyrimidine derivative
22, while with hydrazine hydrate and phenylhydrazine the pyrazolyl benzocoumarin derivatives
23 and
24 were formed, respectively (
Scheme 7). Compound
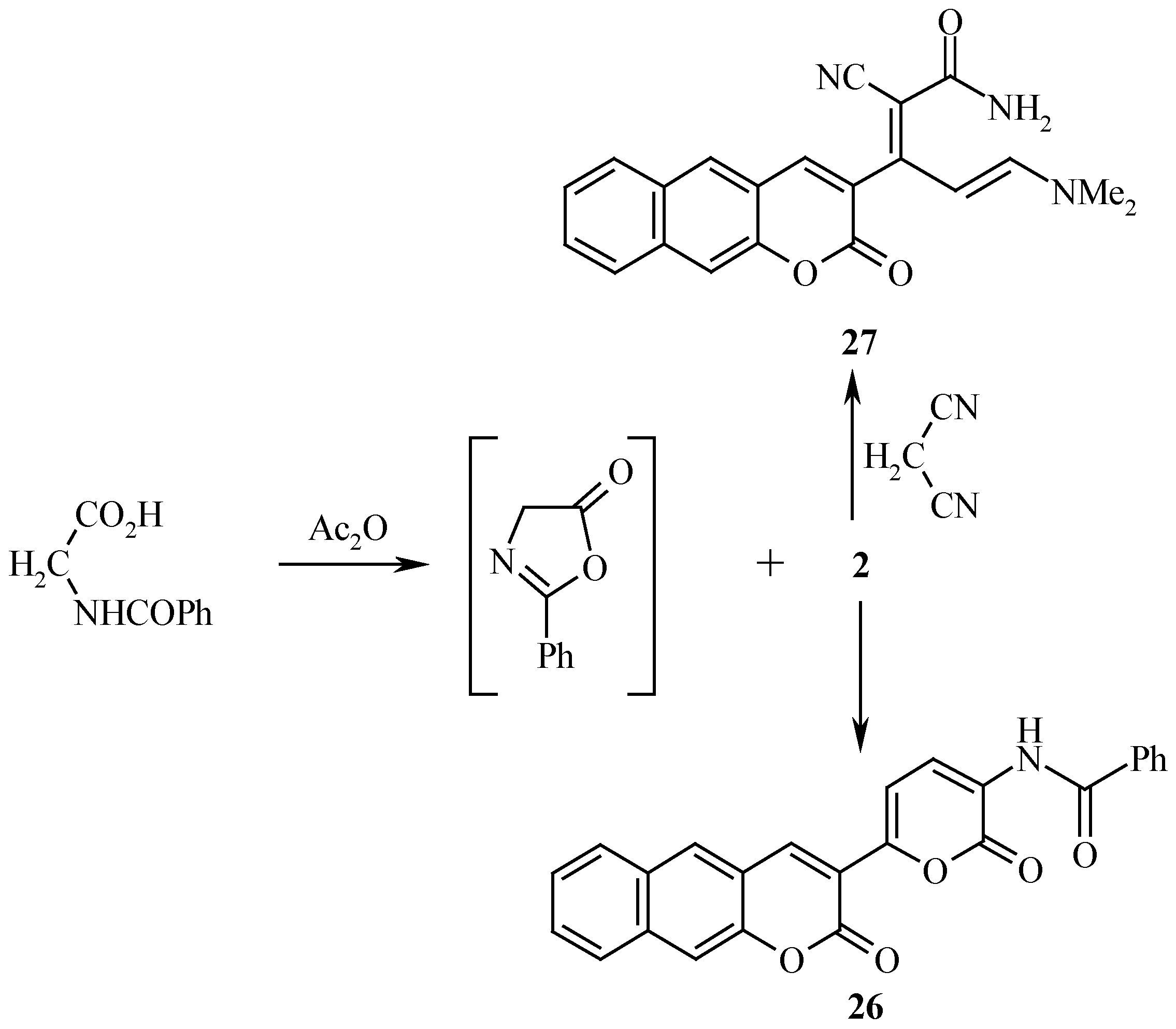
2 also reacted with hippuric acid to yield the benzocoumarinyl α-pyrone
26. It is believed that hippuric acid is first cyclized into isoxazolone
25 which then condense with
2 to yield
26, while reaction of
2 with malononitrile afforded
27 (
Scheme 8).
Structure 27 was assigned for this product based on 1H-NMR data, that revealed two trans olefin doublets at δ= 5.83 and 6.88 ppm with J = 13Hz .
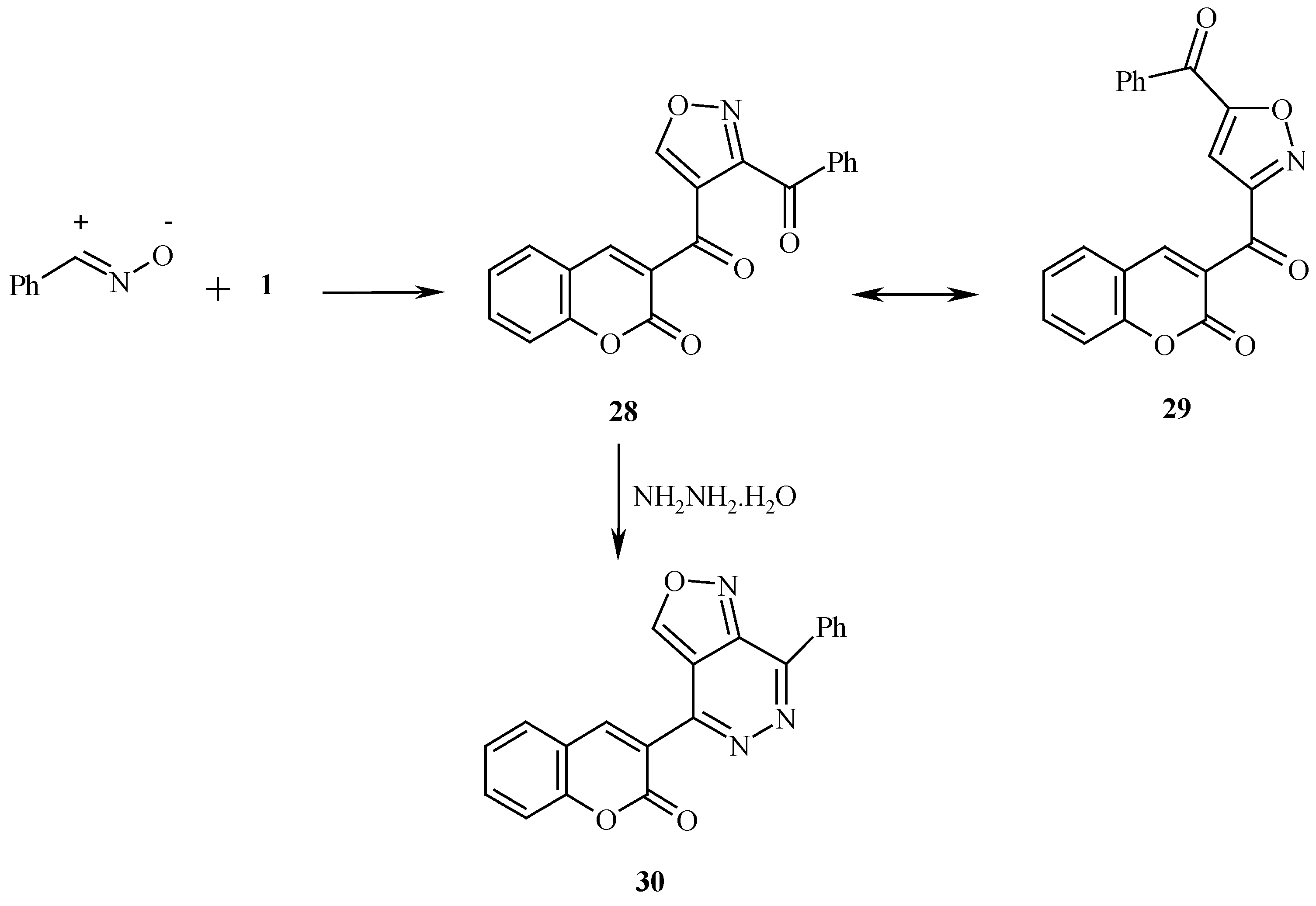
Reaction of
1 with nitrile oxide afforded product that was considered to be the isoxazole derivative
28 rather than potential isomeric product
29, based on the fact that this reaction product readily reacted with hydrazine hydrate to yield the coumarinyl isoxazolopyridazine
30 [
11]. Also, reaction of compound
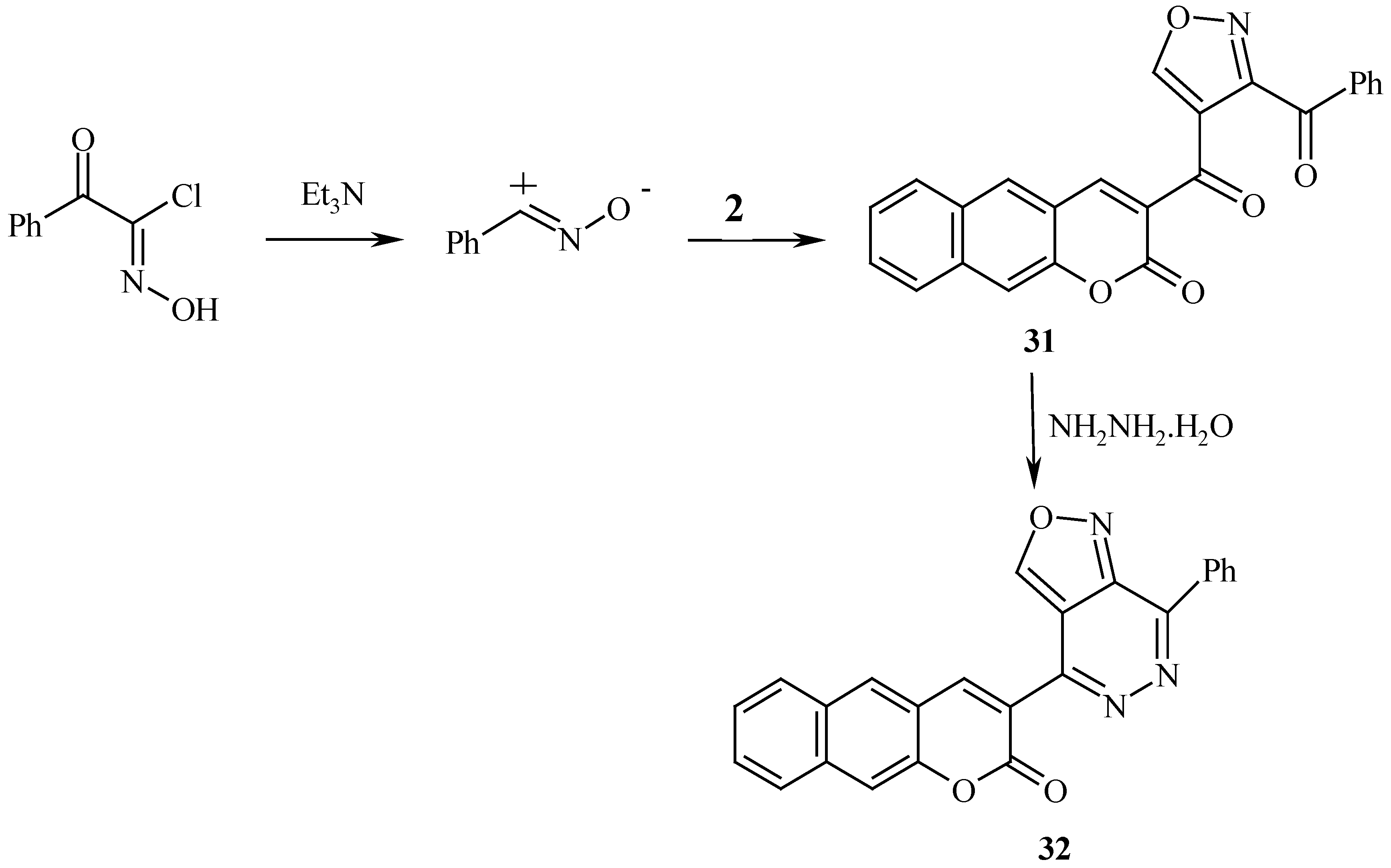
2 with nitrile oxide yielding the isoxazole derivatives
31 (
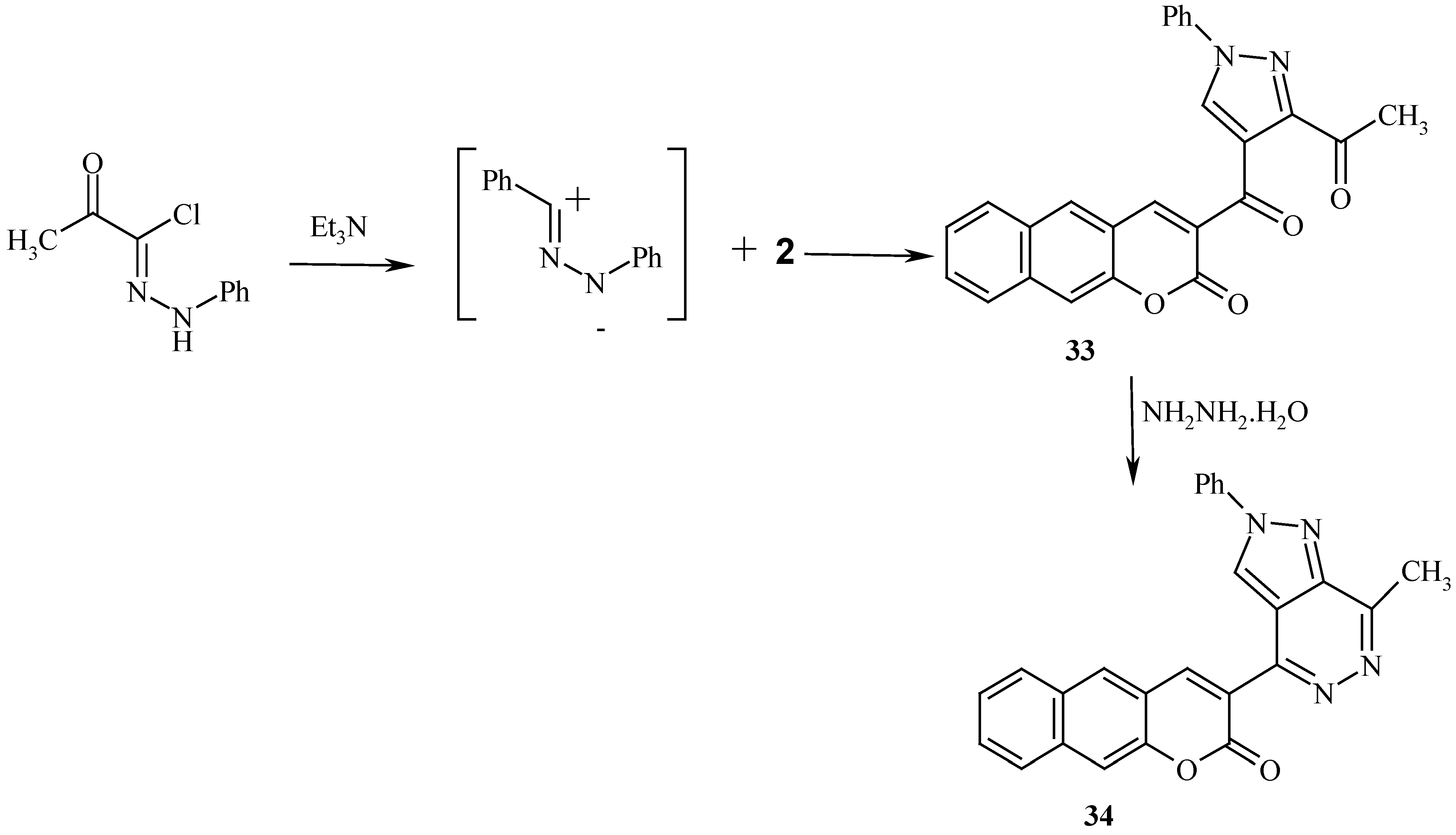
Scheme 10), while reaction with nitrile imine afforded the pyrazole derivative
33 (
Scheme 11).
Experimental
General
All melting points were measured on Gallenkamp electrothermal melting point apparatus and are uncorrected. IR spectra were recorded as KBr disks on a Pye Unicam SP 3-300 Spectrophotometer. 1H-NMR spectra were recorded in deuterated dimethylsulfoxide (DMSO-d6) at 200 MHz on a Varian Gemini NMR spectrometer using tetramethylsilane (TMS) as an internal reference and results are expressed as δ values. Mass spectra were performed on a Shimadzu GCMS-QP 1000 Ex mass spectrometer at 70 eV. Microwave irradiation was carried out using the commercial microwave oven (SGO 390 W). Elemental analyses were carried out at the Microanalytical Center of Cairo University, Egypt.
General Procedures for the Preparation of 1-(3-Coumarinyl)-3-dimethylamino-2-propen-1-one (1) and 1-(3-Benzocoumarinyl)-3-dimethylamino-2-propen-1-one (2) [8]:
Method A: Dimethylformamide dimethylacetal (DMFDMA, 0.1 mol) was added to 3-acetylbenzo-coumarin 3 (0.1 mol) in dioxane (50 mL), and the reaction mixture was refluxed for 6 hours. Removal of the solvent under reduced pressure yielded the crude product which was recrystallized from ethanol.
Method B: Compound 3 or 4 (0.1 mol) and DMFDMA (0.1mol) were placed in the microwave oven and irradiated at full power for 5 min., left to cool to room temperature and the solid formed was collected and recrystallized from ethanol.
Compound 2 was obtained as orange crystals (Method A: 85%; Method B: 95%), mp 157°C; IR: 1730 cm-1 (ring CO); 1H-NMR: δ = 2.95 (s, 3H, NCH3), 3.12 (s, 3H, CH3), 6.11 (d, J=15 Hz, olefinic-H), 7.41-8.21 (m, 7H, arom-H), 8.60 ppm (d, J=15 Hz, olefinic-H); MS: M+ (293); Anal. Calcd. for C18H15NO3 (293.33): C, 73.71; H, 5.15; N, 4.78; Found: C, 73.52; H, 5.24; N, 4.85.
General Procedure for the Preparation of 3,7-Bis-(2-hydroxyphenyl)-4H-dicyclopenta[b,d]pyrrole-1,5-dione (7) and 3,7-Bis-(3-hydroxynaphthalen-4-yl)-4H-dicyclopenta[b,d]pyrrole-1,5-dione (10).
Compound 1 or 2 (0.01mol), ammonium acetate (0.01mol) and a few drops of glacial acetic acid were placed in the microwave oven and irradiated at full power for 3-10 min., left to cool to room temperature, and the solid product was then collected and crystallized from ethanol.
Compound 7 was obtained as dark yellow crystals (85%), mp 245°C; IR cm-1: 3300 (OH), 3280 (NH); 1H-NMR: δ = 6.81 (s, 2H, H-2 and H-6), 6.85-7.97 (m, 8H, arom-H), 8.97 (s, 1H, OH), 9.14 ppm (s, 1H, NH); MS: M+ (355); Anal. Calcd. for C22H13NO4 (355.35): C, 74.36; H, 3.69; N, 3.94; Found: C, 74.52; H, 3.64; N, 3.95.
Compound 10 was obtained as buff crystals (96%), mp 200°C; IR cm-1: 3320 (NH), 3290 (OH); 1H-NMR: δ = 6.82 (s, 2H, H-2 and H-6), 7.40-8.10 (m, 12H, arom-H ), 8.97 (s, 1H, OH), 10.16 ppm (s,1H, NH); MS: M+ (455); Anal. Calcd. for C30H17NO4 (455.47): C, 79.11; H, 3.76; N, 3.08; Found: C, 79.22; H, 3.75; N, 3.10.
Procedure for the Preparation of 2-(Benzocoumarin-3/-yl)-5-(3-benzocoumarinoyl)pyirdine (8).
Compound 2 (0.01mol) and ammonium acetate (0.01mol) were refluxed in glacial acetic acid (30 mL) for 2 hr, then left to cool to room temperature. The solvent was then removed under vacuum and the residue cooled to deposit a solid, which was recrystallized from ethanol to give 8 as buff crystals (64%), mp 220°C; IR cm-1: 1725 (ring C=O), 1681 (C=O); 1H-NMR: δ = 7.31-7.79 (m, 12H, arom-H), 6.97-8.03 (d, 1H, pyridineH-3), 8.52 (s, 1H, pyridine H-6), 9.26 (d, 1H, pyridine H-4), 9.35, 9.61 ppm (2s, 2H, benzocoumarinyl H-4); MS: M+ (495); Anal. Calcd. for C32H17NO5 (495.50): C, 77.57; H, 3.46; N, 2.8; Found: C, 77.50; H, 3.50; N, 2.86.
General Procedure for the Preparation of 1-Acetyl-3,5-di(3-coumarinoyl)benzene (12), 1-Acetyl-3,5-di(3-benzocoumarinoyl)benzene (15) and (1,3-Diacetyl-5-(3-benzocoumarinoyl)benzene (16):
A mixture of either compound 1 or 2 (0.01 mol) and enaminone 11 (0.01mol) was refluxed in acetic acid for 2h, then left to cool to r.t. The target compounds separated as crystals that were collected by filtration and recrystallized from ethanol.
Compound 12 was obtained as buff crystals (66%), mp 178°C; IR cm-1: 1720 (ring CO), 1689 (acetyl CO), 1660 (aroyl CO); 1H-NMR: δ = 2.78 (s, 3H, CH3), 7.54-7.97 (m, 8H, arom-H), 8.65 (s, 2H, H-4), 8.70 (s, 2H, H-2 and H-6), 9.12 ppm (s, 2H, coumarinyl H-4); MS: M+ (464); Anal. Calcd. for C28H16O7 (464.44): C, 72.41; H, 3.47; Found: C, 72.22; H, 3.55.
Compound 15 was obtained as buff crystals (49%), mp >300°C; IR cm-1: 1700 (ring CO), 1685 (acetyl CO), 1658 (aroyl CO); 1H-NMR: δ = 3.33 (s, 3H, CH3), 7.54-8.31 (m, 12H, arom-H), 8.61 (s, 2H, H-4), 8.74 (s, 2H, H-2 and H-6), 9.22 ppm (s, 2H, benzocoumarinyl H-4); MS: M+ (564); Anal. Calcd. for C36H20O7 (564.56): C, 76.59; H, 3.57; Found: C, 76.60; H, 3.56.
Compound 16 was obtained as buff crystals (45%), mp >300°C; IR cm-1: 1710 (ring CO), 1680 (acetyl CO); 1650 (aroyl CO); 1H-NMR: δ = 2.72, 3.33 (2s, 3H, CH3), 8.67 (s, 2H, H-4 and H-6), 8.72 (s, 1H, H-2), 7.68-8.41 (m, 6H, arom-H), 9.32 ppm (s, 1H, benzocoumarinyl H-4); MS: M+ (384); Anal. Calcd. for C24H16O5 (384.39): C, 74.99; H, 4.20; Found: C, 74.96; H, 4.25.
Preparation of 5-(3-Coumarinoyl)-2-methylpyridine (17) and Compound 5 [8]:
A mixture of compound 1 (0.01 mol), enaminone 11, ammonium acetate (0.01mol) and a few drops of glacial acetic acid were placed in the microwave oven and irradiated at full power for 2 min., then left to cool to room temperature. The solid was collected and crystallized from ethanol.
Compound 17 was obtained as brown crystals (45%), mp 200°C; IR cm-1: 1710 (ring CO), 1670 (aroyl CO); 1H-NMR: δ = 2.55 (s, 3H, CH3), 7.68-8.41 (m, 6H, arom-H and H-3), 8.76 (d, 1H, H-4), 9.32 (s, 1H, H-6), 8.81 ppm (s, 1H, coumarinyl H-4); MS: M+ (265). Anal. Calcd. for C16H11NO3 (265.27): C, 72.45; H, 4.18; N, 5.28; Found: C, 72.55; H, 4.25; N, 5.30.
Reaction of Compounds 1 or 2 with 3(5)1,2,4-Aminotrizole and Aminocyclohexenothiophene: Preparation of 5-(Coumarin-3/-yl)-1,2,4-triazolo[4,3-a]pyrimidine (18), 5-(Benzocoumarin-3/-yl)-[1,2,4]triazolo-[4,3-a] pyrimidine (22) and 2-[3-Oxo-3-(2-oxo-2H-chromen-3-yl)-propenylamino]-4,5,6,7-tetrahydrobenzo[b] thiophene-3-carboxylic acid ethyl ester (19):
Method A: To a solution of 1 or 2 (0.01 mol) either 3-amino-1H-1,2,4-trizole or aminocyclo- hexenothiophene (0.01 mol) in acetic acid/ethanol (1:1, 30 mL) were added. The reaction mixture was heated under reflux for 2hr, then left to cool. The solid was collected and recrystallized from ethanol.
Method B: Compound 1 or 2 (0.01mol) and 3-amino-1H-1,2,4-trizole or aminocyclohexenothiophene (0.01 mol) and a few drops of glacial acetic acid were placed in the microwave oven and irradiated at full power for 5 min., then left to cool to room temperature and the solid was collected and recrystallized from ethanol.
Compound 18 was obtained as pale brown crystals (65%), mp 252°C; IR cm-1: 1730 (ring CO), 1660 (C=N); 1H-NMR: δ = 7.38-7.73 (m, 4H, arom-H), 7.8 (d, 1H, pyrimidine H-6), 8.50 (s, 1H, coumarinyl H-4), 8.9 (s, 1H, triazole H-3), 9.21ppm (d, 1H, pyrimidine H-5); MS: M+ (264). Anal. Calcd. for C14H8N4O2 (264.24): C, 63.64; H, 3.05; N, 21.20; Found : C, 63.74; H, 3.10; N, 21.26.
Compound 22 was obtained as yellow crystals (74%), mp 273 °C, microwave (90%). IR cm-1: 1730 (ring CO), 1660 (C=N); 1H-NMR: δ = 7.54-8.03 (m, 6H, arom-H), 8.07 (d, 1H, pyrimidine H-6), 8.50 (s, 1H, benzocoumarinyl H-4), 8.83 (s, 1H, triazole H-3), 9.23 ppm (d, 1H, pyrimidine H-5); MS: M+ (314); Anal. Calcd. for C18H10N4O2 (314.30): C, 68.79; H, 3.21; N, 17.83; Found: C, 68.74; H, 3.10; N, 17.76.
Compound 19 was obtained as light red crystals (45%) mp 202°C; microwave (69%) mp 202°C; IR cm-1: 3200 (NH), 1730 (ester CO), 1680 (ketone CO) 1648 cm-1 (ring CO); 1H-NMR: δ = 1.39 (t, 3H, CH3), 1.67-2.52 (m, 8H, cyclohexane-H), 4.32 (q, 3H, CH2), 5.69, 6.61 (d, J=9Hz, 2H, olefin), 7.25-8.22 (m, 6H, arom-H), 8.78 (s, 1H, coumarin H-4), 13.29 (s,1H, NH); MS: M+ (423); Anal. Calcd. for C23H21NO5S (423.49): C, 65.23; H, 5.00; N, 3.31; Found: C, 65.25; H, 4.80; N, 3.46.
Reaction of Compounds 1, 2 with Hydrazines: 3-(Coumarin-3/-yl)-pyrazole (20), 3-(Benzocumarin-3/-yl)-pyrazole (23) and 3-(Benzocumarin-3/-yl)-1-phenylpyrazole (24):
Method A: To a solution of 2 (0.01 mol) in ethanol (30 mL), either hydrazine hydrate or phenylhydrazine (0.01 mol) were added. The reaction mixture was heated under reflux for 4hr., then left to cool. The solid was collected and recrystallized from ethanol.
Method B: Compound 1 or 2 (0.01mol) and hydrazine hydrate or phenylhydrazine (0.01 mol) was placed in the microwave oven and irradiated at full power for 5-10 min., left to cool to room temperature and the solid was collected and recrystallized from ethanol.
Compound 20 was obtained as light brown crystals (60%), mp 235°C; IR cm-1: 3200 (NH), 1732 (CO), 1640 (C=N); 1H-NMR: δ = 6.60 (d, 1H, pyrazole H-4), 7.25-7.82 (m, 4H, arom-H), 8.58 (m, 1H, pyrazole H-5), 9.09 (s, 1H, coumarin H-4), 11.27 ppm (s, 1H, NH); MS: M+ (212); Anal. Calcd. for C12H8N2O2 (212.21): C, 67.92; H, 3.80; N, 13.20; Found: C, 67.82; H, 3.60; N, 13.13.
Compound 25 was obtained as yellow crystals (65%), mp 256°C, microwave (69%), mp 254°C; IR cm-1: 3200 (NH), 1730 (CO), 1630 (C=N); 1H-NMR: δ = 7.06 (d, 1H, pyrazole H-4), 7.25-8.22 (m, 6H, arom-H), 8.58 (m, 1H, pyrazole H-5), 9.29 (s, 1H, benzocoumarin H-4), 13.27 ppm (s, 1H, NH); MS: M+ (262); Anal. Calcd. for C16H10N2O2 (262.27): C, 73.27; H, 3.84; N, 10.68; Found: C, 73.25; H, 3.80; N, 10.66.
Compound 24 was obtained as dark yellow crystals (59%), mp 230°C, microwave (61%), mp 231°C; 1H-NMR: δ = 7.21 (d, 1H, pyrazole H-4), 7.35-8.32 (m, 11H, arom-H), 8.59 (m, 1H, pyrazole H-5), 9.29 ppm (s, 1H, benzocoumarin H-4); MS: M+ (338); Anal. Calcd. for C22H14N2O2 (338.37): C, 78.09; H, 4.17; N, 8.28; Found: C, 78.12; H, 4.18; N, 8.30.
Reaction of Compound 1 with Guanidine Hydrochloride: General Procedure for the Preparation of 2-Amino- 4-(coumarin-3\-yl) pyrimidine (21):
Method A: To a solution of 1 (0.01 mol) in dry pyridine (20 mL) and (0.01 mol) of guanidine hydrochloride were refluxed for 3 hr, the solvent was reduced under vacuum to half its volume and the solid was collected and recrystallized from ethanol.
Method B: Compound 1 (0.01mol) and guanidine hydrochloride (0.01 mol) was placed in the microwave oven and irradiated at full power for 25 min., then left to cool to room temperature and the solid was collected and recrystallized from ethanol.
Compound 21 was obtained as dark yellow crystals (45%), mp 194°C, microwave (60%), mp 194°C; IR cm-1: 3200 (NH2), 1703 (ring CO); 1H-NMR: δ = 7.48-7.90 (m, 4 H, arom-H), 7.26-8.03 (m, 6 H, arom-H), 8.23 (s, 1H, coumarin H-4), 8.40 (s, 1 H, NH2), 8.81 (d, 1H, pyrimidine H-6), 9.02 (d, 1H, pyrimidine H-5); MS: M+ (239); Anal. Calcd. for C13H9N3O2(239.24): C, 65.27; H, 3.79; N, 17.56; Found : C, 65.25; H, 3.80; N, 17.46.
3-Benzamido-6-(benzocoumarin-3/-yl) pyran-2-one (26):
Method A: Compound 2 (0.1mol) and hippuric acid (0.1mol) were refluxed in acetic anhydride (20 mL) for 1h, then left to cool at room temperature and poured into ice-cold water. The solid product thus formed was collected by filtration and recrystallized from ethanol.
Method B: Compound 2 (0.01mol), hippuric acid (0.01mol) and a few drops of acetic anhydride were placed in the microwave oven and irradiated at full power for 5 min., then left to cool to room temperature and the solid was collected and recrystallized from ethanol.
Compound 26 was obtained as red crystals (60%), mp 254 °C, and microwave (90%) IR cm-1: 3309 (NH), 1690 (ring C=O), 1685 (ring C=O), 1660 (amide C=O); 1H-NMR: δ = 7.38-8.09 (m, 11 H, arom-H), 8.21(s, 1H, pyran H-5). 8.62 (s, 1H, pyran H-4), 8.83 (s, 1H, benzocoumarin H-4) and 9.93 ppm (s, 1H, NH); MS: M+ (409); Anal. Calcd. for C25H15NO5 (409.40): C, 73.35; H, 3.69; N, 3.42; Found: C, 73.44; H, 3.72; N, 3.51.
3-(Benzocoumarin-3’-yl)-2-cyano-5-dimethylamino-2,4-pentadienoic amide (27):
Method A: To compound 2 (0.1 mol) and malononitrile (0.12 mol) in ethanol (30 mL), a few drops of piperidine were added .The reaction mixture was refluxed for 2h, then left to cool at room temperature and treatment with ethanol. The solid product, so formed, was collected by filtration and recrystallized from ethanol.
Method B: Compound 2 (0.1 mol) and malononitrile (0.12 mol) and a few drops of piperidine were added was placed in the microwave oven and irradiated at full power for 1 min., then left to cool to room temperature and the solid was collected and recrystallized from ethanol.
Compound 27 was obtained as dark red crystals (45%), mp 250 °C, and microwave (60%); IR cm-1: 3370, (NH2), 1670 (ring C=O), 1660 (C=O); 1H-NMR: δ = 3.67, 3.69(2s, 6H, N(CH3)2), 5.83, 6.88 (d,1H,olefinic H), 7.18-8.03 (m, 6H, arom-H), 8.05 (s, 1H, benzocoumarin H-4) and 11.82 ppm (s, 1H, NH); MS: M+ (359); Anal. Calcd. for C21H17N3O3(359.39): C,70.18; H,4.77; N,11.69; Found : C, 70.24; H, 4.72; N, 11.51.
General Procedures for the Preparation of 6-Benzoyl-3-(coumarin-3/-yl) isoxazole (28), 6-Benzoyl- 3-(benzocoumarin-3/-yl) isoxazole (31) and 3-Acetyl-4-(benzocoumarin-3/-yl) -1-phenylpyrazole (33):
Method A: To a stirred solution of the appropriate hydroximoyl chloride (0.01mol), or hydrazonyl halide (0.01mol) and the appropriate enaminone 1 or 2 (0.01mol) in dry benzene (20 mL), triethylamine (0.2 mL) was added portionwise over a period of 30 min. The mixture was stirred at room temperature for 24-48 h and the precipitated triethylamine hydrochloride was filtered off, the filtrate was evaporated under reduced pressure and the residue was triturated with ethanol. The solid products were collected by filtration, washed with water and dried. Recrystallization from ethanol afforded the corresponding isoxazoles 28, 31 and the pyrazole 33, respectively.
Method B: Compound 1 or 2 (0.01mol) and the appropriate hydroximoyl chlorides (0.01mol), or hydrazonyl halides (0.01mol) and a few drops of triethylamine were placed in the microwave oven and irradiated at full power for 25 min., then left to cool to room temperature and the solid was collected and recrystallized from ethanol.
Compound 28 was obtained as dark yellow crystals (41%), mp 165°C, microwave (54%); 1H-NMR: δ = 7.61-8.31 (m, 9H, arom-H) 8.91 (s, 1H, coumarin H-4) and 9.36 ppm (s, 1H, H-5); MS: M+ (345); Anal. Calcd. for C20H11NO5 (345.31): C, 69.57; H, 3.21; N, 4.06; Found: C, 69.89; H, 3.17; N, 4.12.
Compound 31 was obtained as yellowish green crystals (45%), mp 190°C, microwave (54%); 1H-NMR: δ = 7.61-8.71 (m, 11H, arom-H), 9.42 (s, 1H, benzocoumarin H-4) and 10.03 (s, 1H, H-5); MS: M+ (395); Anal. Calcd. for C24H13NO5 (345.37): C, 72.91; H, 3.31; N, 3.54; Found: C, 72.89; H, 3.32; N, 3.62.
Compound 33 was obtained as dark yellow crystals (43%), mp 228 °C, microwave (55%); 1H-NMR: δ= 2.92 (s, 3H, CH3), 7.58-8.59 (m, 11H, arom-H) 9.17 (s, 1H, benzocoumarin H-4) and 9.27 ppm (s, 1H, H-5); MS: M+ (408); Anal. Calcd. for C25H16N2O4 (408.42): C, 73.52; H, 3.95; N,6.86; Found: C, 73.54; H, 4.00; N, 6.91.
Reaction of the Isoxazoles 28, 31 and the Pyrazole 33 with Hydrazine Hydrate: General Procedure for the Preparation of 4-(Coumarin-3/-yl)-7-phenylisoxazolo[3,4-d]pyridazine (30), 4-(Benzocoumarin-3/-yl)-7-phenylisoxazolo[3,4-d]pyridazine (32) and 4-(Benzocoumarin-3/-yl)-7-methyl-2-phenyl-pyrazolo[3,4-d] pyridazine (34):
A mixture of the appropiate isoxazole 28, 31 or the appropiate pyrazole 33 (0.01mol) and hydrazine hydrate (80%, 0.01 mL) in absolute ethanol (20 mL) was heated under reflux for 3-4h. The solvent was evaporated under reduced pressure and the residue was left to cool to room temperature. The solid products were filtered off, washed with ethanol and dried. Recrystallization from dimethylformamide afforded the the isoxazolo[3,4-d]-pyridazines 30, 32 and pyrazolo[3,4-d]pyridazine 34, respectively.
Compound 30 was obtained as dark yellow crystals (71%), mp 245 °C; 1H-NMR: δ = 7.65-8.31 (m, 9H, arom-H), 9.11 (s, 1H, coumarin H-4) and 9.31ppm (s, 1H, H-5); MS: M+ (341); Anal. Calcd. for C20H11N3O3 (341.33): C, 70.38; H, 3.25; N, 12.31; Found: C, 70.40; H, 3.18; N, 12.12 .
Compound 32 was obtained as yellow crystals (74%), mp 189 °C; 1H-NMR: δ= 7.65-8.61 (m, 11H, arom-H), 9.14 (s, 1H, benzocoumarin H-4) and 9.41ppm (s, 1H, H-3); MS: M+ (391); Anal. Calcd. for C24H13N3O3 (391.39): C, 73.65; H, 3.35; N, 10.74; Found: C, 73.70; H, 3.40; N, 10.78.
Compound 34 was obtained as orange crystals (73%), mp 230°C; 1H-NMR: δ = 2.52 (s, 3H, CH3), 7.45-8.49 (m, 11H, arom-H), 9.47(s, 1H, benzocoumarin H-4) and 9.07 ppm (s, 1H, H-3); MS: M+ (408); Anal. Calcd. for C25H16N4O2 (404.13): C, 74.25; H, 3.99; N,13.85; Found: C, 74.34; H, 4.01; N, 13.90.
Table 1.
Comparison between microwave and solution reactions
Table 1.
Comparison between microwave and solution reactions
| Product | Time/min | Yield % | m.p °C |
|---|
| Solvent ∗ | MW # | Solvent ∗ | MW # | Solvent ∗ | MW # |
|---|
| 1 | 360 | 5 | 75 | 96 | 165 | 165 |
| 2 | 360 | 5 | 73 | 95 | 157 | 157 |
| 5 | 30 | 2 | 65 | 55 | 265 | 267 |
| 7 | - | 5 | - | 85 | - | 245 |
| 8 | 120 | - | 64 | - | 220 | - |
| 10 | - | 3 | - | 96 | - | 200 |
| 12 | 120 | - | 66 | - | 178 | - |
| 15 | 120 | - | 49 | - | >300 | - |
| 16 | 120 | - | 45 | - | >300 | - |
| 17 | - | 2 | - | 45 | - | 200 |
| 18 | 360 | 5 | 70 | 65 | 250 [8] | 252 |
| 19 | 120 | 5 | 45 | 69 | 202 | 202 |
| 20 | 240 | 5 | 60 | 60 | 237 [8] | 235 |
| 21 | 180 | 25 | 45 | 60 | 194 | 194 |
| 22 | 120 | 5 | 74 | 90 | 273 | 273 |
| 23 | 240 | 5 | 65 | 69 | 256 | 254 |
| 24 | 240 | 5 | 59 | 61 | 230 | 231 |
| 26 | 60 | 5 | 60 | 90 | 254 | 254 |
| 27 | 120 | 1 | 45 | 60 | 250 | 250 |
| 29 | 1440 | 10 | 41 | 54 | 165 | 165 |
| 30 | 180 | - | 71 | - | 245 | - |
| 32 | 1440 | 10 | 45 | 54 | 190 | 190 |
| 33 | 180 | - | 74 | - | 189 | - |
| 34 | 1440 | 10 | 43 | 55 | 228 | 228 |
| 35 | 180 | - | 73 | - | 230 | - |