Structure-Activity Relationships for the 9-(Pyridin-2’-yl)- aminoacridines
Abstract
:Introduction

Results and Discussion
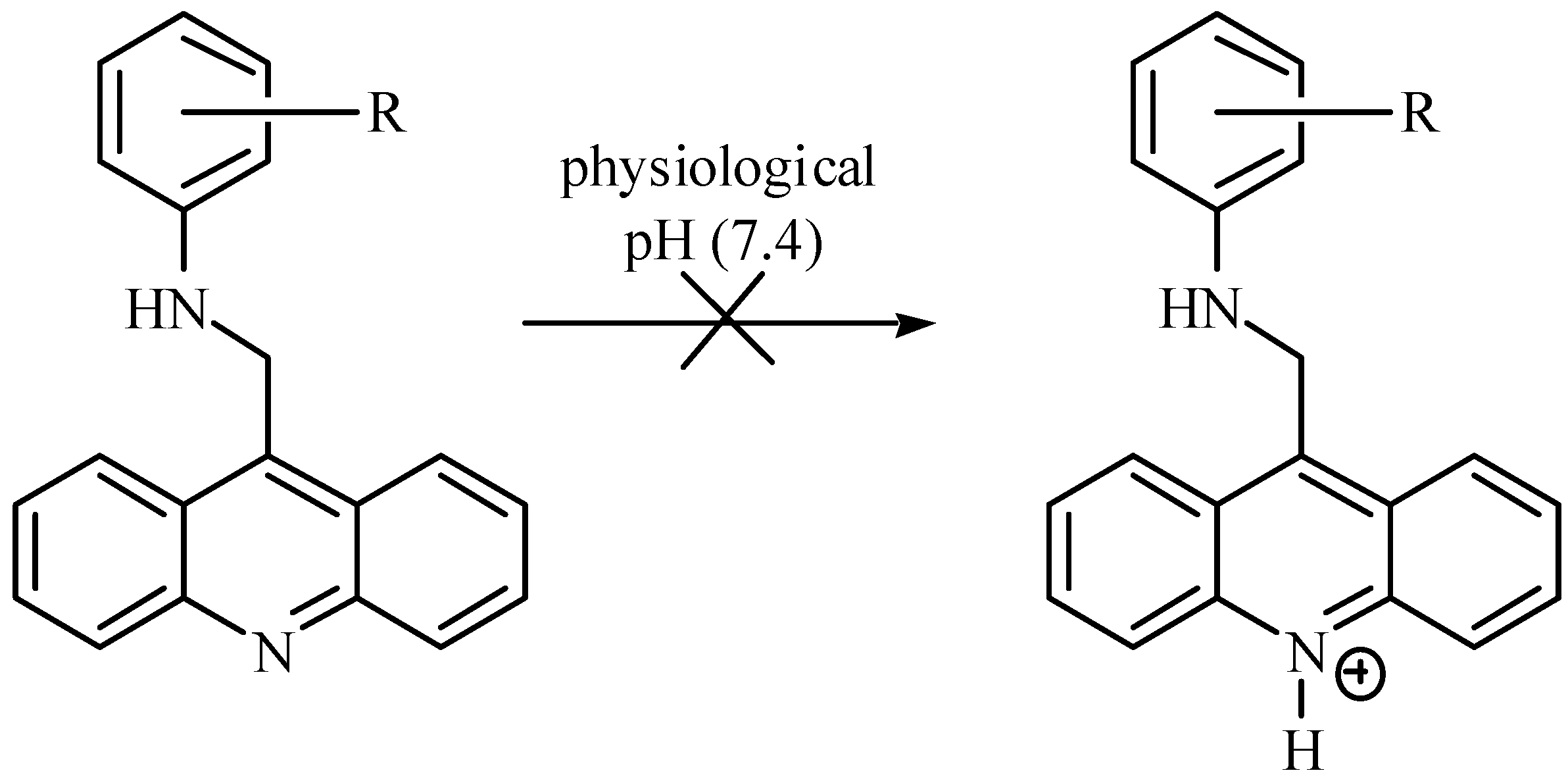
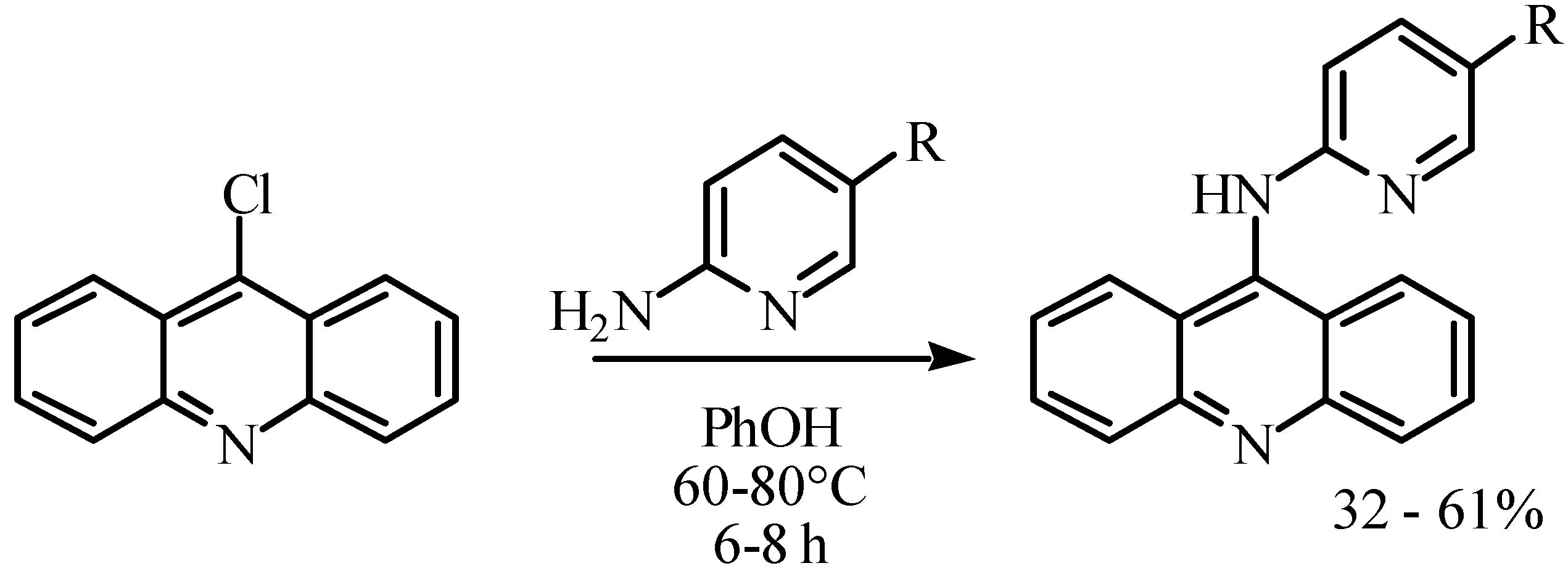
 | Compound | R | ∆Tm (90% confidence limits) |
| 2a | H | 15.3 ± 0.6 °C | |
| 2b | CH3 | 6.79 ± 0.8 °C | |
| 2c | NO2 | Not detnd. | |
| 2d | Cl | 18.3 ± 1.1°C | |
| 2e | Br | 19.4 ± 1.4°C |
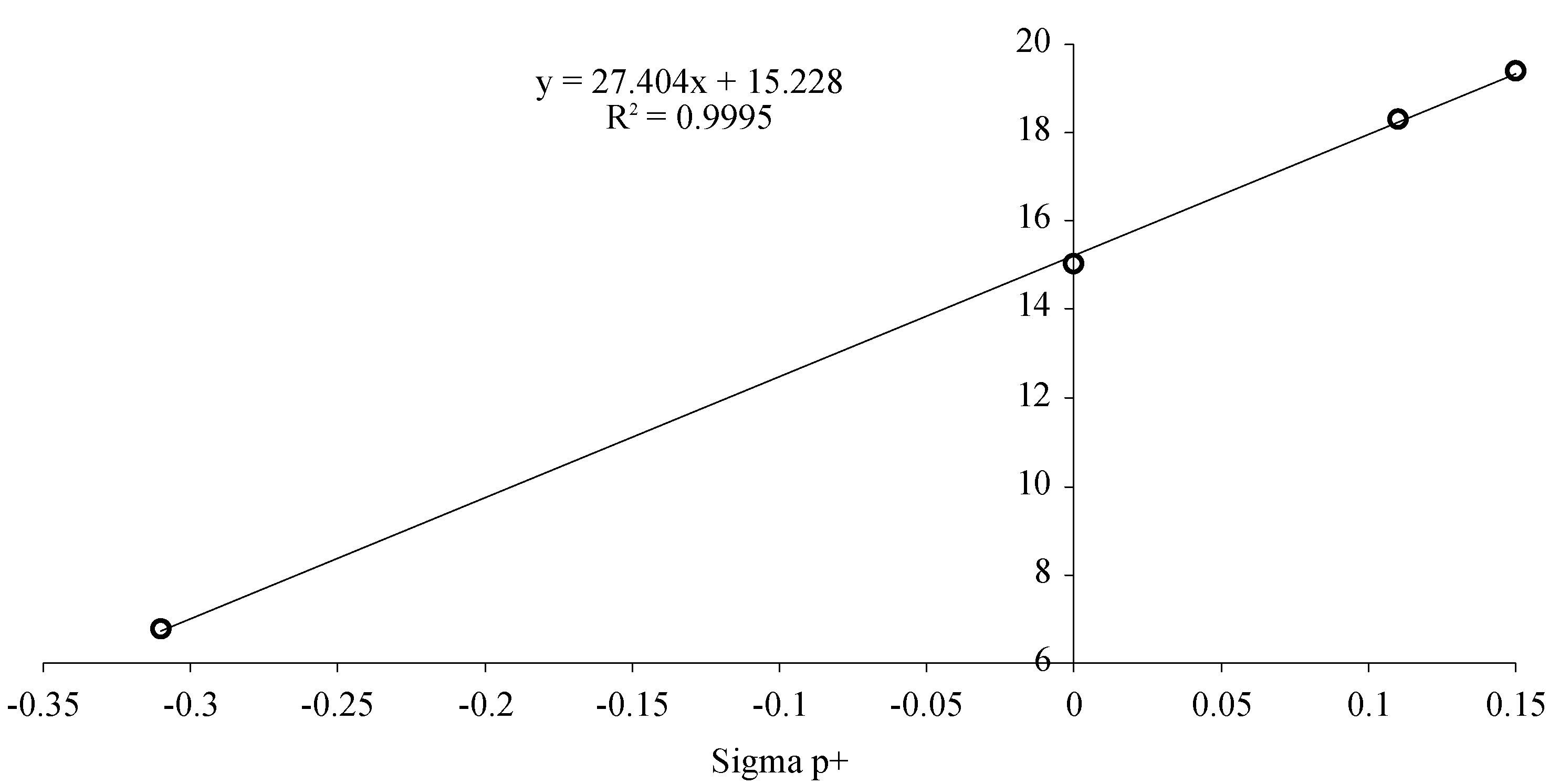
Conclusions
Experimental
General
General synthetic procedure
References and Notes
- Cancer Facts and Figures. American Cancer Society, 2004; p. 60.
- Centers for Disease Control. Morbid. Mortal. Rep. 2002, 51, 49.
- Zittoun, R. Eur. J. Cancer Clin. Oncol. 1985, 21, 649.Atwell, G.J.; Cain, B.F.; Seelye, R.N. J. Med. Chem. 1972, 15, 611.
- Denny, W.A.; Atwell, G.J.; Cain, B.F. J. Med. Chem. 1979, 22, 1453.
- Rose, F.L. J. Chem. Soc. 1951, 2770.
- Chourpa, I.; Manfait, M. J. Raman Spec. 1995, 26, 813.Rowe, T.C.; Chen, G.L.; Hsiang, Y.H.; Liu, L.F. Cancer Res. 1986, 46, 2022.Nelson, E.M.; Tewey, K.M.; Liu, L.F. Proc. Natl. Acad. Sci. 1984, 81, 1361.
- Jaycox, G.D.; Gribble, G.W.; Hacker, M.P. J. Heterocycl. Chem. 1987, 24, 1405.Wilson, W.R.; Cain, B.F.; Baguley, B.C. Chem Biol. Interactions 1977, 18, 163.
- Mosher, M.D.; Johnson, E. Heterocycl. Chem. 2003, 9. in press.
- Allen, C.F.H.; McKee, G.H.W. Org. Syntheses 1943, Coll. Vol 2, 15.
- Albert, A.; Ritchie, B. Org. Syntheses 1955, Coll. Vol 3, 53.
- Kuhlmann, K.F.; Charbeneau, N.J.; Mosher, C.W. Nuc. Acids Res. 1978, 5, 2629.
- Values for σp and σp+ were taken from: Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR: Hydrophobic, Electronic, and Steric Constants. American Chemical Society: Washington, DC, 1995. [Google Scholar]
- Sample Availability: Available from the authors.
© 2004 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mosher, M.D.; Holmes, K.L.; Frost, K.S. Structure-Activity Relationships for the 9-(Pyridin-2’-yl)- aminoacridines. Molecules 2004, 9, 102-108. https://doi.org/10.3390/90300102
Mosher MD, Holmes KL, Frost KS. Structure-Activity Relationships for the 9-(Pyridin-2’-yl)- aminoacridines. Molecules. 2004; 9(3):102-108. https://doi.org/10.3390/90300102
Chicago/Turabian StyleMosher, Michael D., Kristi L. Holmes, and Katherine S. Frost. 2004. "Structure-Activity Relationships for the 9-(Pyridin-2’-yl)- aminoacridines" Molecules 9, no. 3: 102-108. https://doi.org/10.3390/90300102
APA StyleMosher, M. D., Holmes, K. L., & Frost, K. S. (2004). Structure-Activity Relationships for the 9-(Pyridin-2’-yl)- aminoacridines. Molecules, 9(3), 102-108. https://doi.org/10.3390/90300102




