Application of Δ- and Λ-Isomerism of Octahedral Metal Complexes for Inducing Chiral Nematic Phases
Abstract
:1. Introduction
- The ΔΛ chirality has a more rigid nature than that of asymmetric carbon. Besides its size extends over 1 nm as a whole including a central metal ion and ligands. The scope under the influence of a chiral metal complex is even larger when it includes a bulky planar ligand such as aromatic rings.
- The molecular properties of a dopant can be changed systematically by changing the coordination structures such as the degree of ligand replacement, geometrical isomers and the linking of coordination units by bridging ligands. This character would be helpful to reveal the structure-function relations on this topic.
- Additional functions are attached to a dopant molecule by coupling the properties of metal ions such as photo-response, redox properties and asymmetric catalyses. This property may be utilized to construct a bi-functional liquid crystal system.
2. Application of β-Diketonato Complexes as Chiral Dopants
3. Molecular Mechanism in Induction of Chiral Nematic Phases
4. Control of Handedness in Chiral Nematic Phases on the Basis of Molecular Design
5. Design of Photoresponsive Dopant of ΔΛ-Isomerism
6. Application of New Spectroscopic Method to Observation of Structural Changes
7. Conclusions
Acknowledgments
References and Notes
- Kitzerow, H-S; Bahr, C. Chirality in Liquid Crystals; Springer-Verlag: New York, NY, USA, 2001. [Google Scholar]
- Solladie, G; Zimmermann, RG. Liquid crystals: A tool for studies on chirality. Angew. Chem. Int. Ed 1984, 23, 348–362. [Google Scholar]
- Goodby, JW. Chirality in liquid crystals. J. Mater. Chem 1991, 1, 307–318. [Google Scholar]
- Lee, S-E; Raynes, EP. Transmission and amplification of information and properties in nanostructured liquid crystals. Angew. Chem. Int. Ed 2008, 47, 2754–2787. [Google Scholar]
- Ernst, K-H. Amplification of chirality in two-dimensional molecular lattices. Curr. Opin. Colloid Interface Sci 2008, 13, 54–59. [Google Scholar]
- Pijper, D; Feringa, BL. Control of dynamic helicity at the macro- and supramolecular level. Soft Matter 2008, 7, 1349–1372. [Google Scholar]
- Takezoe, H; Takanishi, Y. Bent-core liquid crystals: Their mysterious and attractive world. Jpn. J. Appl. Phys. PT. 1 2006, 45, 597–625. [Google Scholar]
- Hofmeier, H; Schubert, US. Recent developments in the supramolecular chemistry of terpyridine-metal complexes. Chem. Soc. Rev 2004, 33, 373–399. [Google Scholar]
- Link, DR; Natale, G; Shao, R; Maclennan, JE; Clark, NA; Körblova, E; Walba, DM. Spontaneous formation of macroscopic chiral domains in a fluid smectic phase of achiral molecules. Science 1997, 278, 1924–1927. [Google Scholar]
- Di Matteo, A; Todd, SM; Gottarelli, G; Solladié, G; Williams, VE; Lemieux, RP; Ferrarini, A; Spada, GP. Correlation between molecular structure and helicity of induced chiral nematics in terms of short-range and electrostatic-induction interactions. The case of chiral biphenyls. J. Am. Chem. Soc 2001, 123, 7842–7851. [Google Scholar]
- Pieraccini, S; Ferrarini, A; Spada, GP. Chiral doping of nematic phases and its application to the determination of absolute configuration. Chirality 2008, 20, 749–759. [Google Scholar]
- Ferrarini, A; Moro, GJ; Nordio, PL. Simple molecular model for induced cholesteric phases. Phys. Rev. E 1996, 53, 681–688. [Google Scholar]
- Tang, K; Green, MM; Cheon, KS; Selinger, JV; Garetz, BA. Chiral conflict. The effect of temperature on the helical sense of a polymer controlled by the competition between structurally different enantiomers: From dilute solution to the lyotropic liquid crystal state. J. Am. Chem. Soc 2003, 125, 7313–7323. [Google Scholar]
- Eelkema, R; van Delden, RA; Feringa, BL. Direct visual detection of the stereoselectivity of a catalytic reaction. Angew. Chem. Int. Ed 2004, 43, 5013–5016. [Google Scholar]
- Eelkema, R; Feringa, BL. Amplification of chirality in liquid crystals. Org. Biomol. Chem 2006, 4, 3729–3745. [Google Scholar]
- Feringa, BL; Huck, NPM; van Doren, HA. Chiroptical switching between liquid crystalline phases. J. Am. Chem. Soc 1995, 117, 9929–9930. [Google Scholar]
- Ruslim, C; Ichimura, K. Conformational effect on macroscopic chirality modification of cholesteric mesophases by photochromic azobenzene dopants. J. Phys. Chem. B 2000, 104, 6529–6535. [Google Scholar]
- Mathews, M; Tamaoki, N. Planar chiral azobenzenophanes as chiroptic switches for photon mode reversible reflection color control in induced chiral nematic liquid crystals. J. Am. Chem. Soc 2008, 130, 11409–11416. [Google Scholar]
- Mathews, M; Tamaoki, N. Reversibly tunable helicity induction and inversion in liquid crystal self-assembly by a planar chiroptic trigger molecule. Chem Commun 2009, 3609–3611. [Google Scholar]
- Ichimura, K. Photoalignment of liquid-crystal systems. Chem. Rev 2000, 100, 1847–1873. [Google Scholar]
- Ikeda, T; Mamiya, J-I; Yu, Y. Photomechanics of liquid-crystalline elastomers and other polymers. Angew. Chem. Int. Ed 2007, 46, 506–528. [Google Scholar]
- Tejedor, RM; Oriol, L; Serrano, JL; Sierra, T. Chiral photochemical induction in liquid crystals. J. Mater. Chem 2008, 18, 2899–2908. [Google Scholar]
- Choi, S-W; Kawauchi, S; Ha, NY; Takezoe, H. Photoinduced chirality in azobenzene-containing polymer systems. Phys. Chem. Chem. Phys 2007, 9, 3671–3681. [Google Scholar]
- Matsuoka, Y; Sato, H; Yamagishi, A; Okamoto, K; Hoshino, N. ΔΛ-Isomerism of mixed 1,3-diketonate complexes of Ru(III)—A designed new source of chirality in nematic liquid crystals. Chem Mater 2005, 17, 4910–4917. [Google Scholar]
- Sato, H; Yamagishi, A; Yoshida, J; Nakano, H; Hoshino, N. A microscopic model for helical twisting power by the optical isomers of an octahedral metal complex. Jpn J App Phys 2005, 44, 4067–4072. [Google Scholar]
- Sato, H; Yamagishi, A. Application of the ΔΛ isomerism of octahedral metal complexes as a chiral source in photochemistry. J. Photochem. Photobiol. C: Photochem. Rev 2007, 8, 67–84. [Google Scholar]
- Yoshida, J; Sato, H; Yamagishi, A; Hoshino, N. On the parity in helical twisting power of Ru(III) 1,3-diketonates of C2 symmetry in nematic liquid crystals. J. Am. Chem. Soc 2005, 127, 8453–8456. [Google Scholar]
- Yoshida, J; Sato, H; Yamagishi, A; Hoshino, N. Induction and structural control of chiral nematic phases by the use of photoresponsive tris(β-diketonato) Co(III) and Ru(III) complexes. J. Phys. Chem. B 2008, 112, 9677–9683. [Google Scholar]
- Furuno, F; Sato, H; Yoshida, Y; Hoshino, H; Fukuda, F; Yamagishi, A. Linkage effects of chromium(III) acetylacetonato units on chiral induction of liquid crystal phases. J. Phys. Chem. B 2007, 111, 521–526. [Google Scholar]
- Mitsuoka, T; Sato, H; Yoshida, J; Yamagishi, A; Einaga, Y. Photomodulation of a chiral nematic liquid crystal by the use of a photoresponsive ruthenium(III) complex. Chem. Mater 2006, 18, 3442–3447. [Google Scholar]
- Taniguchi, T; Monde, K; Nishimura, S-I; Yoshida, J; Sato, H; Yamagishi, A. Rewinding of helical systems by use of the Cr(III) complex as a photoresponsive chiral dopant. Mol. Cryst. Liq. Cryst 2006, 460, 107–116. [Google Scholar]
- Drake, AF; Gian, GG; Spada, P. The twisting power of some chiral tris-(pentane-2,4-dionato)metal(III) complexes in nematic liquid crystals. Chem. Phys. Lett 1984, 110, 630–633. [Google Scholar]
- Hoshino, N; Matsuoka, Y; Okamoto, K; Yamagishi, A. Δ-[Ru(acac)L] (L = a mesogenic derivative of bpy) as a novel chiral dopant for nematic liquid crystals with large helical twisting power. J. Am. Chem. Soc 2003, 125, 1718–1719. [Google Scholar]
- Zheng, H; Lai, CK; Swager, TM. Controlling intermolecular interactions between metallomesogens: Side-chain effects in discotic copper, palladium, and vanadyl bis(β-diketonates). Chem. Mater 1995, 7, 2067–2077. [Google Scholar]
- Piao, G; Akagi, K; Shirakawa, H. Chiroptical titanium complexes as catalytically active chiral dopants available for asymmetric acetylene polymerization. Synth. Metals 1999, 101, 92–93. [Google Scholar]
- Hamakubo, K; Hama, S; Yagi, S; Nakazumi, H; Mizutani, T. Extraordinary doping effects of chiral helical linear tetrapyrrole–Zn(II) complexes on chiral nematic induction of MBBA liquid crystal. Chem. Lett 2005, 34, 1454–1455. [Google Scholar]
- Braun, M; Hahn, A; Engelmann, M; Fleischer, R; Frank, W; Kryschi, C; Haremza, S; Kürschner, K; Parker, R. Bis-chelated imine-alkoxytitanium complexes: Novel chiral dopants with high helical twisting power in liquid crystals. Chem. Eur. J 2005, 11, 3405–3412. [Google Scholar]
- Engelmann, M; Braun, M; Kuball, H-G. Helical twisting power of chiral titanium complexes in nematic compounds. Liq. Cryst 2007, 34, 73–77. [Google Scholar]
- Yamagishi, A; Taniguchi, M; Imamura, Y; Sato, H. Clay column chromatography for optical resolution: Selectivities of Λ-[Ru(phen)] and Λ-[Ru(bpy)] laponite columns towards 1,1′-binaphthol. Appl. Clay Sci 1996, 11, 1–10. [Google Scholar]
- Anzai, N; Machida, S; Horie, K. Chirooptical control of liquid crystalline textures containing chromium complex by irradiation of circular polarized light. Chem Lett 2001, 888–889. [Google Scholar]
- Anzai, N; Machida, S; Horie, K. Light-induced control of textures and cholesteric pitch in liquid crystals containing chromium complexes, by means of circular and linear polarized light. Liq. Cryst 2003, 30, 359–366. [Google Scholar]
- Stevenson, KL. Partial photoresolution. III. The tris(acetylacetonato)chromium(III) system. J. Am. Chem. Soc 1972, 94, 6652–6654. [Google Scholar]
- Choi, S-W; Kawauchi, S; Tanaka, S; Watanabe, J; Takezoe, H. Vibrational circular dichroism spectroscopic study on circularly polarized light-induced chiral domains in the B4 phase of a bent mesogen. Chem. Lett 2007, 36, 1018–1019. [Google Scholar]




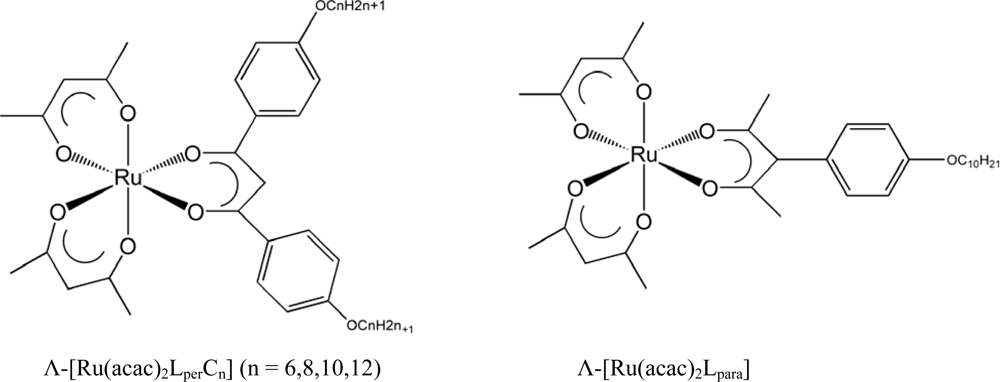
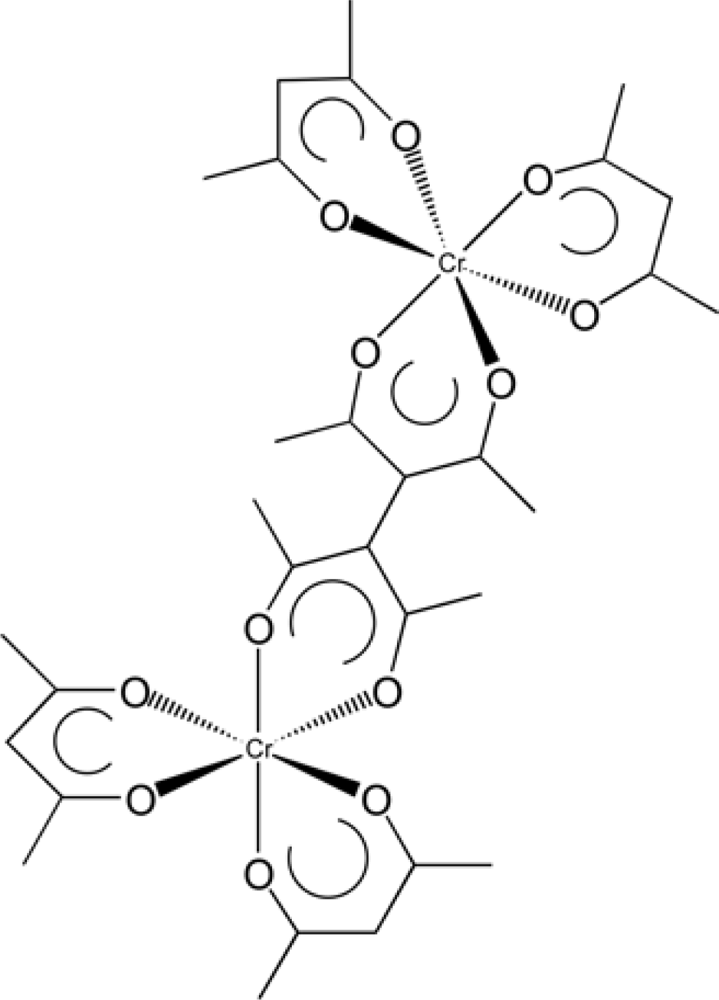




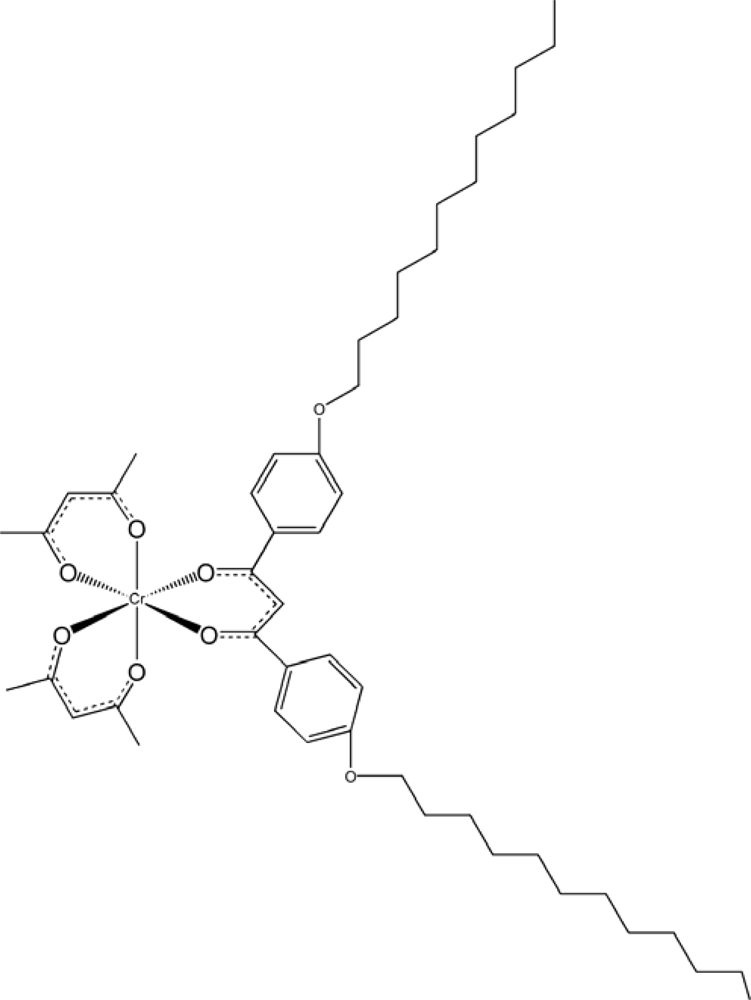

| n | βM/μm−1 | |
|---|---|---|
| Λ | Δ | |
| 0 | − | −48 |
| 2 | 176 | −175 |
| 3 | 131 | −133 |
| 4 | (84) | −95 |
| 5 | 70 | (−38) |
| (a) [Ru(acac)2LperCn] | ||||
|---|---|---|---|---|
| βM/μm−1 | n = 6 | 8 | 10 | 12 |
| Λ | 130 | 176 | 130 | 120 |
| Δ | −146 | −175 | −140 | −127 |
| (b) [Ru(acac)2Lpara] | |
|---|---|
| βM/μm−1 | n = 10 |
| Λ | −24 |
| Δ | 24 |
| Dopant | MBBA | EBBA | ZLI-1132 |
|---|---|---|---|
| Δ-Ru(acac)2(LC12)] | −127 | −66 | −55 |
| Λ-Ru(acac)2(LC12)] | 120 | 72 | 58 |
| Δ-[Co(acac)2(LC12)] | −144 | −64 | −69 |
| Λ-[Co(acac)2(LC12)] | 135 | 68 | 71 |
| Oligomer | Isomer | β(μm−1)/MBBA | β(μm−1)/ ZLI-1132 |
|---|---|---|---|
| Monomer | Λ | +99.5 | +23.0 |
| Δ | −91.0 | −25.3 | |
| Binuclear species | ΛΛ | +97.9 | +26.0 |
| ΔΔ | −88.9 | −29.1 | |
| Trinuclear species | ΛΛΔ or ΛΔΛ | +128.0 | − |
| ΔΔΛ or ΔΛΔ | −90.9 | − |
| Initial | Visible | UV | ||
|---|---|---|---|---|
| Λ | βM /μm−1 | +38 | +34 | +22 |
| Δ | βM /μm−1 | −50 | −44 | −27 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sato, H.; Yamagishi, A. Application of Δ- and Λ-Isomerism of Octahedral Metal Complexes for Inducing Chiral Nematic Phases. Int. J. Mol. Sci. 2009, 10, 4559-4574. https://doi.org/10.3390/ijms10104559
Sato H, Yamagishi A. Application of Δ- and Λ-Isomerism of Octahedral Metal Complexes for Inducing Chiral Nematic Phases. International Journal of Molecular Sciences. 2009; 10(10):4559-4574. https://doi.org/10.3390/ijms10104559
Chicago/Turabian StyleSato, Hisako, and Akihiko Yamagishi. 2009. "Application of Δ- and Λ-Isomerism of Octahedral Metal Complexes for Inducing Chiral Nematic Phases" International Journal of Molecular Sciences 10, no. 10: 4559-4574. https://doi.org/10.3390/ijms10104559
APA StyleSato, H., & Yamagishi, A. (2009). Application of Δ- and Λ-Isomerism of Octahedral Metal Complexes for Inducing Chiral Nematic Phases. International Journal of Molecular Sciences, 10(10), 4559-4574. https://doi.org/10.3390/ijms10104559




