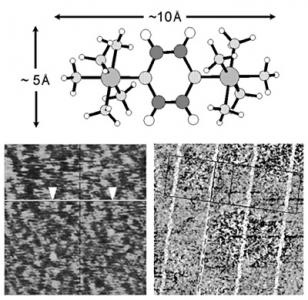
Selective Binding, Self-Assembly and Nanopatterning of the Creutz-Taube Ion on Surfaces
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Substrate Preparation
2.3. Surface Attachment of the Creutz-Taube ions on substrates
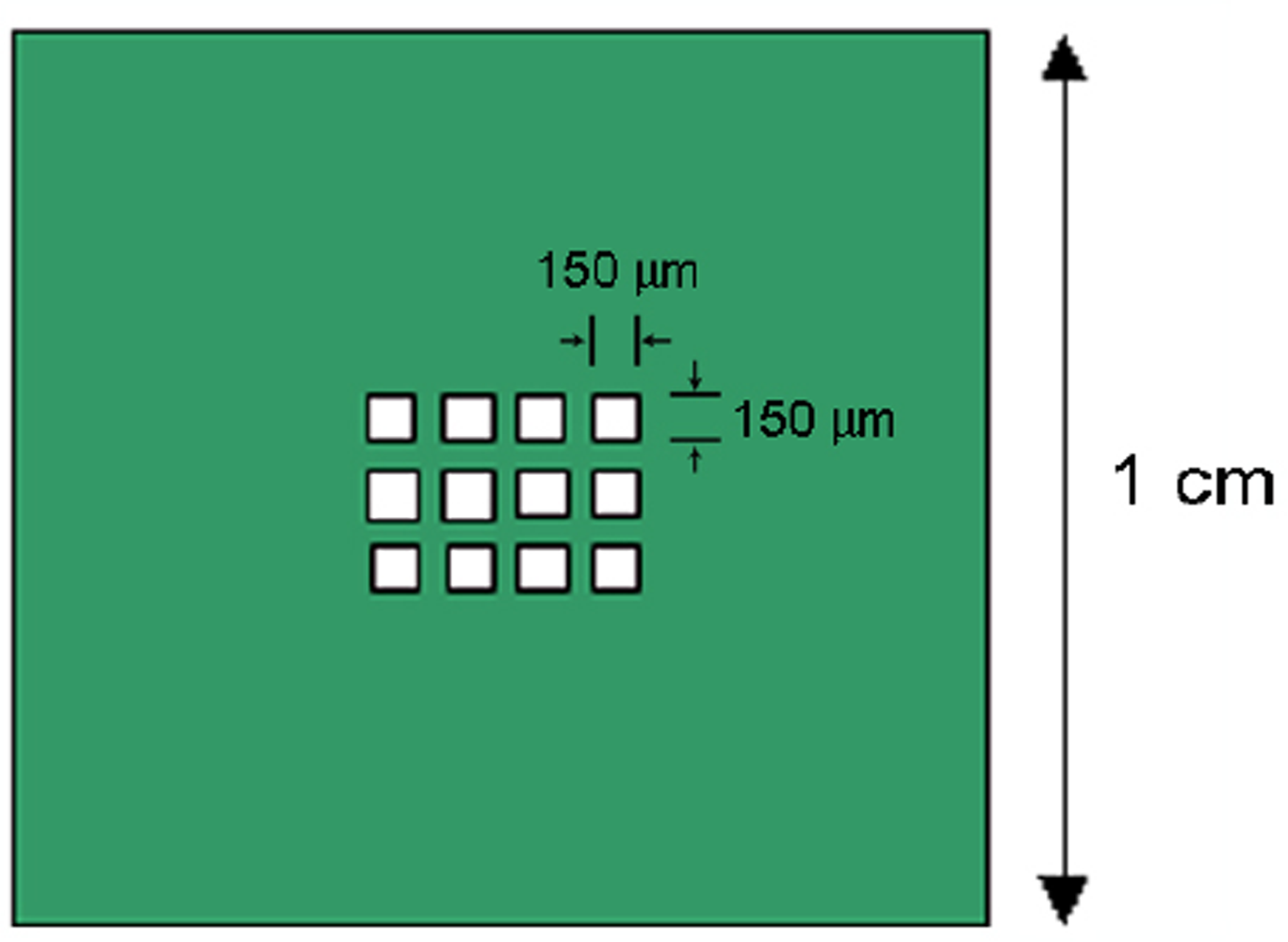
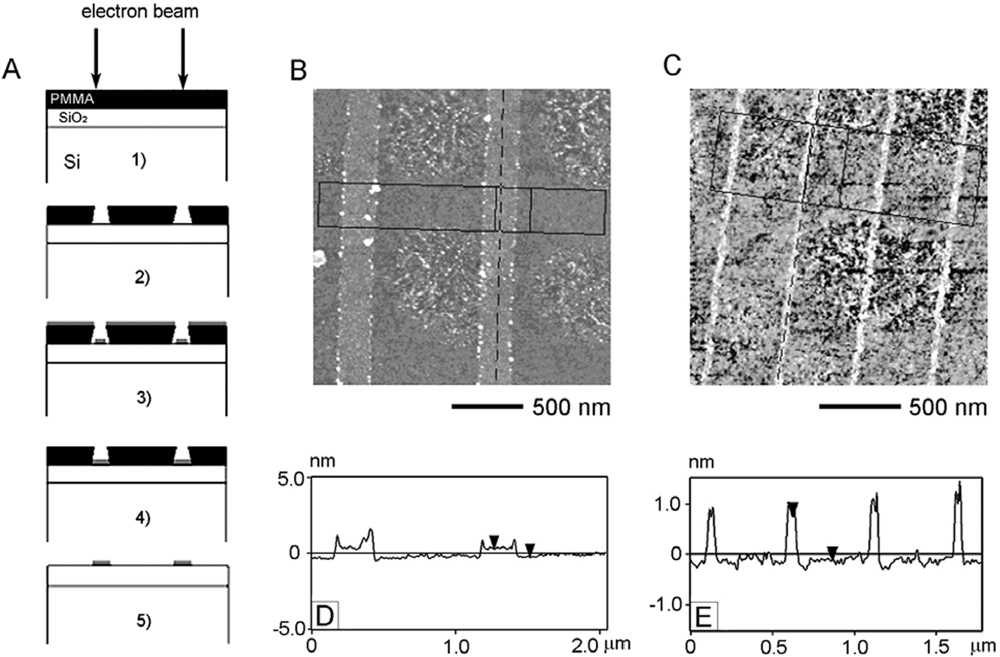
2.4. Electron Beam Lithography Patterning of the Creutz-Taube Ions
2.5. XPS measurement
2.6. Ellipsometric Measurement
2.7. Contact Angle Measurement
2.8. AFM Measurement
3. Results and Discussion
3.1. XPS Measurements on Bare Substrates and Bulk Powder Samples as Control Experiments
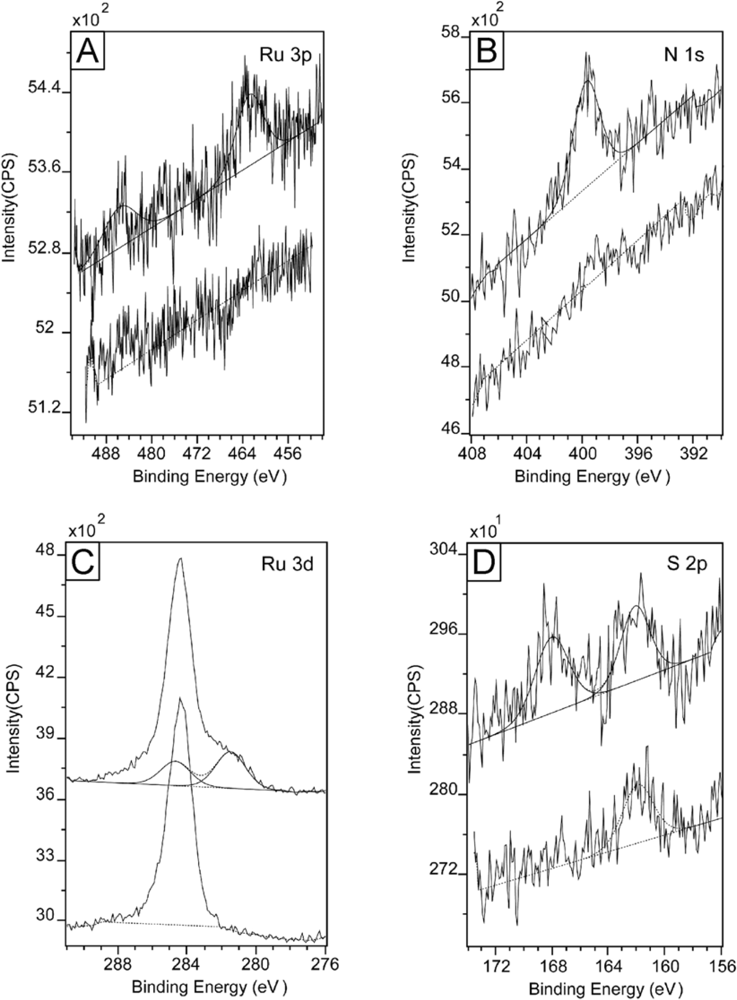
3.2. Selective Binding Properties on Surfaces
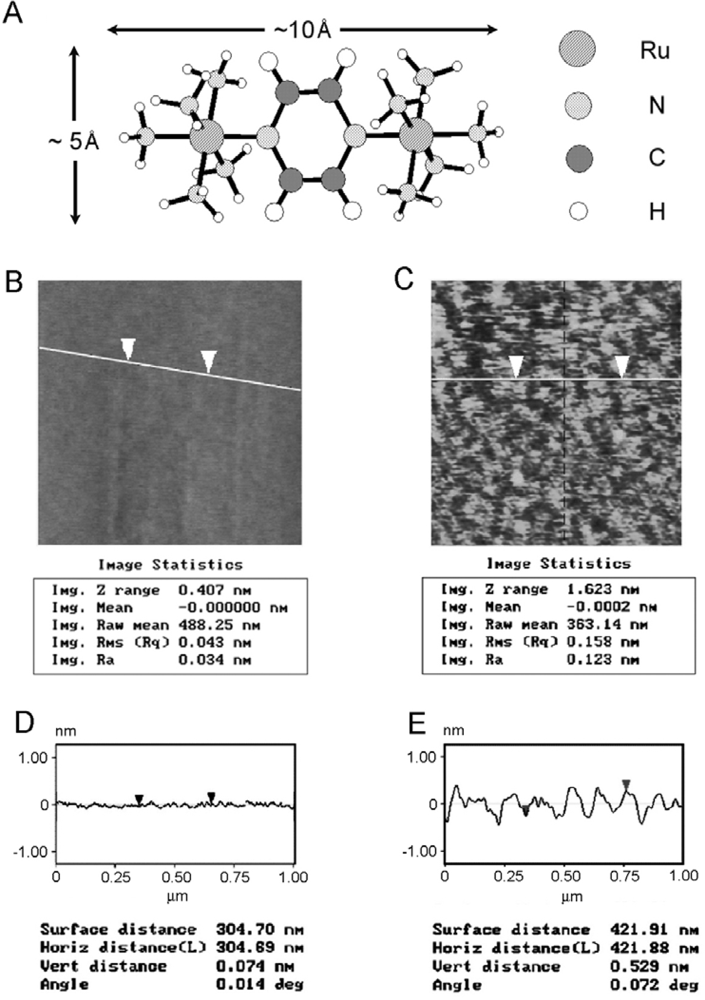
3.3. Inorganic SAMs: Characterization of the Creutz-Taube Ion on SiO2
3.4. Nanolines of the Creutz-Taube Ions Patterned through High-Resolution PMMA Masks
4. Conclusions
Acknowledgments
References and Notes
- Somorjai, GA. Chemistry in Two Dimensions: Surfaces; Cornell University Press: Ithaca, NY, USA, 1981. [Google Scholar]
- Porter, MD; Bright, TB; Allara, DL; Chidsey, CED. Spontaneously organized molecular assemblies. 4. Structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry. J. Am. Chem. Soc. 1987, 109, 3559–3568. [Google Scholar]
- Finklea, HO; Avery, S; Lynch, M; Furtsch, T. Blocking oriented monolayers of alkyl mercaptans on gold electrodes. Langmuir 1987, 3, 409–413. [Google Scholar]
- Finklea, HO; Robinson, LR; Blackburn, A; Richter, B; Allara, D; Bright, T. Formation of an organized monolayer by solution adsorption of octadecyltrichlorosilane on gold: Electrochemical properties and structural characterization. Langmuir 1986, 2, 239–244. [Google Scholar]
- Li, T; Weaver, MJ. Intramolecular electron transfer at metal surfaces. 4. Dependence of tunneling probability upon donor-acceptor separation distance. J. Am. Chem. Soc. 1984, 106, 6107–6108. [Google Scholar]
- Roberts, GG. An applied science perspective of Langmuir-Blodgett films. Advan. Phys. 1985, 34, 475–512. [Google Scholar]
- Broers, AN; Pomerantz, M. Rapid writing of fine lines in langmuir-blodgett films using electron beams. Thin Solid Films 1983, 99, 323–329. [Google Scholar]
- Fariss, G; Lando, J; Rickert, S. Electron beam resists produced from monomer-polymer Langmuir-Blodgett films. Thin Solid Films 1983, 99, 305–315. [Google Scholar]
- Wang, X; Hu, W; Ramasubramaniam, R; Bernstein, GH; Snider, G; Lieberman, M. Formation, characterization, and Sub-50-nm patterning of organosilane monolayers with embedded disulfide bonds: An engineered self-assembled monolayer resist for electron-beam lithography. Langmuir 2003, 19, 9748–9758. [Google Scholar]
- Denis, FA; Hanarp, P; Sutherland, DS; Gold, J; Mustin, C; Rouxhet, PG; Dufrene, YF. Protein adsorption on model surfaces with controlled nanotopography and chemistry. Langmuir 2002, 18, 819–828. [Google Scholar]
- Bourgoin, J-P; Doublet, F; Palacin, S; Vandevyver, M. High in-plane anisotropy in phthalocyanine LB films. Langmuir 1996, 12, 6473–6479. [Google Scholar]
- Sauer, T; Arndt, T; Batchelder, DN; Kalachev, AA; Wegner, G. The structure of Langmuir - Blodgett films from substituted phthalocyaninato-polysiloxanes. Thin Solid Films 1990, 187, 357–374. [Google Scholar]
- Cook, MJ; Mayes, DA; Poynter, RH. Spectroscopic monitoring of thermally induced molecular reorganisations within spin-coated and Langmuir - Blodgett films of mesogenic phthalocyanines. J. Mater. Chem. 1995, 5, 2233–2238. [Google Scholar]
- Fujiki, M; Tabei, H; Kurihara, T. In-plane dichroisms of phthalocyanine Langmuir-Blodgett films. Langmuir 1988, 4, 1123–1128. [Google Scholar]
- Lu, X; Hipps, KW; Wang, XD; Mazur, U. Scanning tunneling microscopy of metal phthalocyanines: d7 and d9 cases. J. Am. Chem. Soc. 1996, 118, 7197–7202. [Google Scholar]
- Vukusic, PS; Sambles, JR. Cobalt phthalocyanine as a basis for the optical sensing of nitrogen dioxide using surface plasmon resonance. Thin Solid Films 1992, 221, 311–317. [Google Scholar]
- Ulman, A. An Introduction to Ultrathin Organic Films: From Langmuir-Blodgett Films to Self-Assembly; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Whitesides, GM; Laibinis, PE. Wet chemical approaches to the characterization of organic surfaces: self-assembled monolayers, wetting, and the physical-organic chemistry of the solid-liquid interface. Langmuir 1990, 6, 87–96. [Google Scholar]
- Bain, CD; Troughton, EB; Tao, YT; Evall, J; Whitesides, GM; Nuzzo, RG. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 1989, 111, 321–335. [Google Scholar]
- Lee, H; Kepley, LJ; Hong, HG; Mallouk, TE. Inorganic analogs of Langmuir-Blodgett films: Adsorption of ordered zirconium 1,10-decanebisphosphonate multilayers on silicon surfaces. J. Am. Chem. Soc. 1988, 110, 618–620. [Google Scholar]
- Evans, SD; Ulman, A; Goppert-Berarducci, KE; Gerenser, LJ. Self-assembled multilayers of .omega.-mercaptoalkanoic acids: selective ionic interactions. J. Am. Chem. Soc. 1991, 113, 5866–5868. [Google Scholar]
- Wang, X; Lieberman, M. Zirconium phosphonate monolayers with embedded disulfide bonds. Langmuir 2003, 19, 7346–7353. [Google Scholar]
- Kim, Y-G; Kim, J; Ahn, H; Kang, B; Sung, C; Samuelson, LA; Kumar, J. Molecular assembly by sequential ionic adsorption of nanocrystalline TiO2 and a conjugated polymer. J. Macromol. Sci. Part A 2003, 40, 1307–1316. [Google Scholar]
- Rojas, OJ; Ernstsson, M; Neuman, RD; Claesson, PM. Effect of polyelectrolyte charge density on the adsorption and desorption behavior on mica. Langmuir 2002, 18, 1604–1612. [Google Scholar]
- Coupe, B; Evangelista, ME; Yeung, RM; Chen, W. Surface modification of poly(tetrafluoroethylene-co-hexafluoropropylene) by adsorption of functional polymers. Langmuir 2001, 17, 1956–1960. [Google Scholar]
- Pakula, M; Biniak, S; Swiatkowski, A. Chemical and electrochemical studies of interactions between iron(III) ions and an activated carbon surface. Langmuir 1998, 14, 3082–3089. [Google Scholar]
- Cheng, L; Niu, L; Gong, J; Dong, S. Electrochemical growth and characterization of polyoxometalate-containing monolayers and multilayers on alkanethiol monolayers self-assembled on gold electrodes. Chem. Mater. 1999, 11, 1465–1475. [Google Scholar]
- Dambies, L; Guimon, C; Yiacoumi, S; Guibal, E. Characterization of metal ion interactions with chitosan by X-ray photoelectron spectroscopy. Colloid Surf. A 2000, 177, 203–214. [Google Scholar]
- Meng, L; Hu, L; Chen, Y; Du, C; Wang, Y. Copolymeric network crown ether resins with pendent functional group: Synthesis and adsorption for metal ions. J. Appl. Polym. Sci. 2000, 76, 1457–1465. [Google Scholar]
- Brizzolara, RA. Adsorption of alginic acid to titanium investigated using x-ray photoelectron spectroscopy and atomic force microscopy. Surf. Interface Anal. 2002, 33, 351–360. [Google Scholar]
- Agasi, L; Berry, FJ; Carbucicchio, M; Marco, J; Mortimer, M; Vetel, F. X-Ray absorption and photoelectron spectroscopic investigation of vanadium interaction with NH4+- and Eu3+-exchanged zeolite-Y following calcination and steam treatment. J. Mater. Chem. 2002, 12, 3034–3038. [Google Scholar]
- Gier, S; Johns, WD. Heavy metal-adsorption on micas and clay minerals studied by X-ray photoelectron spectroscopy. Appl. Clay Sci. 2000, 16, 289–299. [Google Scholar]
- Rieke, PC; Tarasevich, BJ; Wood, LL; Engelhard, MH; Baer, DR; Fryxell, GE; John, CM; Laken, DA; Jaehnig, MC. Spatially resolved mineral deposition on patterned self-assembled monolayers. Langmuir 1994, 10, 619–622. [Google Scholar]
- Coda, ME; Du, H; Bhandarkar, S. Selected-area sol-gel deposition of barium strontium titanate thin films on thermally oxidized silicon through mediation of self-assembled monolayers. Supramol. Sci. 1997, 4, 43–50. [Google Scholar]
- Inumaru, K; Inoue, Y; Kakii, S; Nakano, T; Yamanaka, S. Organic - inorganic cooperative molecular recognition in nanostructure of alkyl-grafted MCM-41. Chem. Lett. 2003, 32, 1110–1112. [Google Scholar]
- Cauvel, A; Brunel, D; Garrone, FDR; Fubini, B. Hydrophobic and hydrophilic behavior of micelle-templated mesoporous silica. Langmuir 1997, 13, 2773–2778. [Google Scholar]
- Zhang, J; Xu, X; Chen, J; Kang, E-T. Covalent attachment of polymer thin layers to self-assembled monolayers on gold surface by graft polymerization. Thin Solid Films 2002, 413, 76–84. [Google Scholar]
- Fustin, C-A; Glasser, G; Spiess, HW; Jonas, U. Site-selective growth of colloidal crystals with photonic properties on chemically patterned surfaces. Adv. Mater. 2003, 15, 1025–1028. [Google Scholar]
- Huang, Z; Wang, P-C; MacDiarmid, AG; Xia, Y; Whitesides, G. Selective deposition of conducting polymers on hydroxyl-terminated surfaces with printed monolayers of alkylsiloxanes as templates. Langmuir 1997, 13, 6480–6484. [Google Scholar]
- Cant, NE; Critchley, K; Zhang, H-L; Evans, SD. Surface functionalisation for the self-assembly of nanoparticle/polymer multilayer films. Thin Solid Films 2003, 426, 32–39. [Google Scholar]
- Lee, I; Wool, RP. Controlling amine receptor group density on aluminum oxide surfaces by mixed silane self assembly. Thin Solid Films 2000, 379, 94–100. [Google Scholar]
- Bruinsma, GM; Mei, HC; Busscher, HJ. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials 2001, 22, 3217–3224. [Google Scholar]
- Deval, J; Umali, TA; Spencer, BL; Lan, EH; Dunn, B; Ho, C-M. Reconfigurable hydrophobic/hydrophilic surfaces based on self-assembled monolayers. Mater. Res. Soc. Sym. Proc. 2003, 774, 203. [Google Scholar]
- Papra, A; Bernard, A; Juncker, D; Larsen, NB; Michel, B; Delamarche, E. Microfluidic networks made of poly(dimethylsiloxane), Si, and Au coated with polyethylene glycol for patterning proteins onto surfaces. Langmuir 2001, 17, 4090–4095. [Google Scholar]
- Tangpasuthadol, V; Pongchaisirikula, N; Hove, VP. Surface modification of chitosan films: Effects of hydrophobicity on protein adsorption. Carbohydr. Res. 2003, 338, 937–942. [Google Scholar]
- Shirahata, N; Yonezawa, T; Miura, Y; Kobayashi, K; Koumoto, K. Patterned adsorption of protein onto a carbohydrate monolayer immobilized on Si. Langmuir 2003, 19, 9107–9109. [Google Scholar]
- More, SD; Hudecek, J; Urisu, T. Hydrophobic/hydrophilic interactions of cytochrome c with functionalized self-assembled monolayers on silicon. Surf. Sci. 2003, 532–535, 993–998. [Google Scholar]
- Clark, SL; Hammond, PT. The Role of secondary interactions in selective electrostatic multilayer deposition. Langmuir 2000, 16, 10206–10214. [Google Scholar]
- Hempenius, MA; Péter, M; Robins, NS; Kooij, ES; Vancso, GJ. Water-soluble poly(ferrocenylsilanes) for supramolecular assemblies by Layer-by-Layer deposition. Langmuir 2002, 18, 7629–7634. [Google Scholar]
- Sennerfors, T; Bogdanovic, G; Tiberg, F. Formation, chemical composition, and structure of polyelectrolyte nanoparticle multilayer films. Langmuir 2002, 18, 6410–6415. [Google Scholar]
- Lent, CS; Tougaw, PD; Porod, W; Bernstein, GH. Quantum cellular automata. Nanotechnology 1993, 4, 49–57. [Google Scholar]
- Lent, CS; Tougaw, PD. Lines of interacting quantum-dot cells: A binary wire. J. Appl. Phys. 1993, 74, 6227–6233. [Google Scholar]
- Lent, C; Tougaw, P; Porod, W. PhysComp 94: Proceedings of the Workshop on Physics and Computing; IEEE Computer Society Press, 1994. [Google Scholar]
- Orlov, AO; Amlani, I; Bernstein, GH; Lent, CS; Snider, GL. Realization of a functional cell for quantum-dot cellular automata. Science 1997, 277, 928–930. [Google Scholar]
- Creutz, C; Taube, H. Direct approach to measuring the Franck-Condon barrier to electron transfer between metal ions. J. Am. Chem. Soc. 1969, 91, 3988–3989. [Google Scholar]
- Creutz, C; Taube, H. Preparation and studies of di-nitrogen decaaquodiruthenium(II) fluoroborate. Inor. Chem. 1971, 10, 2664–2667. [Google Scholar]
- Creutz, C; Taube, H. Binuclear complexes of ruthenium ammines. J. Am. Chem. Soc. 1973, 95, 991–1354. [Google Scholar]
- Tom, GM; Creutz, C; Taube, H. Mixed valence complexes of ruthenium ammines with 4,4′-bipyridine as bridging ligand. J. Am. Chem. Soc. 1974, 96, 7827–7829. [Google Scholar]
- Lent, CS. Bypassing the transistor paradigm. Science 2001, 288, 1597–1599. [Google Scholar]
- Lieberman, M; Chellamma, S; Varughese, B; Wang, Y; Lent, CS; Bernstein, GH; Snider, GL; Peiris, FC. Quantum-Dot Cellular Automata at a Molecular Scale. Molecular Electronics II, Ann. N.Y. Acad. Sci. 2002, 960, 225–239. [Google Scholar]
- Lent, CS; Isaksen, B; Lieberman, M. Molecular Quantum-Dot Cellular Automata. J. Am. Chem. Soc. 2003, 125, 1056–1063. [Google Scholar]
- Varughese, B; Chellamma, S; Lieberman, M. XPS study of self-assembly of ruthenium dimers [((acac)2Ru)2bptz]0,+ on hydrophobic and hydrophilic SAMs. Langmuir 2002, 18, 7964–7970. [Google Scholar]
- Wang, Z-S; Ebina, Y; Takada, K; Watanabe, M; Sasaki, T. Inorganic multilayer assembly of titania semiconductor nanosheets and Ru complexes. Langmuir 2003, 19, 9534–9537. [Google Scholar]
- Monjushiro, H; Harada, K; Haga, M-A. Characterization of Langmuir monolayers of the amphiphilic Ru complex at the air/water interface by ultraviolet photoelectron yield spectroscopy. Langmuir 2003, 19, 9226–9230. [Google Scholar]
- Cox, LC. Nanocomposite multilayer film of a ruthenium metallodendrimer and a Dawson-Type polyoxometalate as a bifunctional electrocatalyst. Chem. Mater. 2002, 14, 6–8. [Google Scholar]
- Cassagneau, T; Fendler, JH; Johnson, SA; Mallouk, TE. Self-assembled diode junctions prepared from a ruthenium tris(bipyridyl) polymer, n-Type TiO2 nanoparticles, and graphite oxide sheets. Adv. Mater. 2000, 12, 1363–1366. [Google Scholar]
- Chidsey, CED; Bertozzi, CR; Putvinski, TM; Mujsce, AM. Coadsorption of ferrocene-terminated and unsubstituted alkanethiols on gold: Electroactive self-assembled monolayers. J. Am. Chem. Soc. 1990, 112, 4301–4306. [Google Scholar]
- Iler, R. The Chemistry of Silica; Wiley-Interscience Publications: NY, USA, 1979. [Google Scholar]
- Wang, Y; Lieberman, M. Growth of ultrasmooth octadecyltrichlorosilane self-assembled monolayers on SiO2. Langmuir 2003, 19, 1159–1167. [Google Scholar]
- Citrin, PH; Ginsbeg, AP. X-ray photoemission from the Creutz-Taube mixed valence complex: A reassessment. J. Am. Chem. Soc. 1981, 103, 3673–3679. [Google Scholar]
- Shepherd, RE; Proctor, A; Henderson, WW; Myser, TK. Assessment of the .pi.-acceptor capability of selected ligands based on the photoelectron spectra of ruthenium ammine complexes. Inorg. Chem. 1987, 26, 2440–2444. [Google Scholar]
- Auburn, PR; Dodsworth, ES; Haga, M; Liu, W; Nevin, WA; Lever, ABP. Bis(dioxolene)bis(pyridine)ruthenium redox series. Inorg. Chem. 1991, 30, 3502–3512. [Google Scholar]
- Gassman, PG; Winter, CH. Preparation, electrochemical oxidation, and XPS studies of unsymmetrical ruthenocenes bearing the pentamethylcyclopentadienyl ligand. J. Am. Chem. Soc. 1988, 110, 6130–6135. [Google Scholar]
- Moulder, J; Stickle, W; Sobol, P; Bomben, K. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Division, Perkin-Elmer: Eden Prairie, MN, USA, 1992–1995. [Google Scholar]
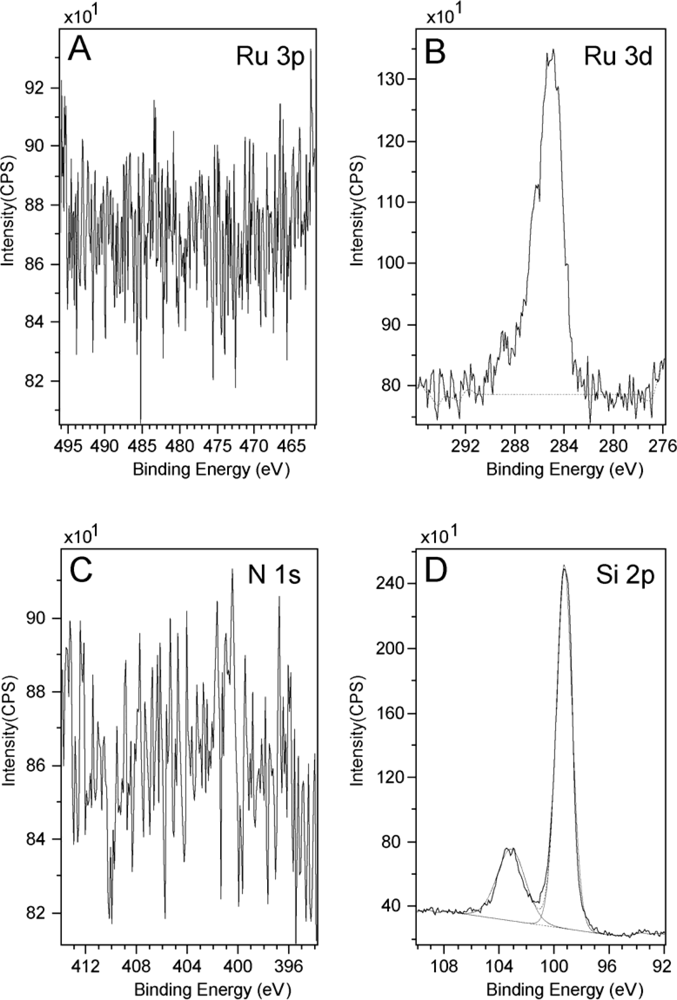



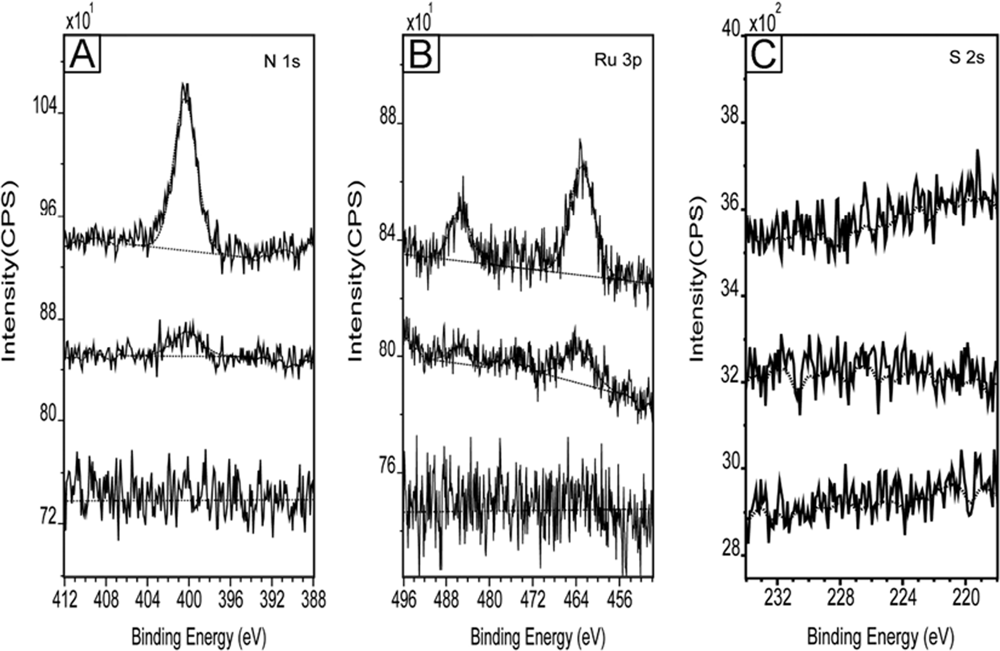


| Ru 3p | N 1s | S 2p | |
|---|---|---|---|
| Binding Energy (eV) | 461.8 (3p3/2)
484.7(3p1/2) | 399.7 | 167.9 |
| Rel. Conc. (%) | 10.2 | 62.3 | 27.5 |
| Ru | N | S | P | F | ||
|---|---|---|---|---|---|---|
| CT5(TOS)5 on SiO2 | B. E. (eV) | 462.4 (3p3/2)
485.7 (3p1/2) | 400.0 (1s) | No binding | -- | -- |
| Rel. Conc. (/Ru) | 1 | 5.78 | ||||
| CT5(TOS)5 on OTS/SiO2 | B. E. (eV) | No binding | No binding | No Binding | -- | -- |
| Rel. Conc. (/Ru) | ||||||
| CT5(TOS)5 on PMMA/SiO2 | B. E. (eV) | No binding | No binding | No Binding | -- | -- |
| Rel. Conc. (/Ru) | ||||||
| CT5(TOS)5 on HUT/Au | B. E. (eV) | 462.4 (3p3/2)
484.9 (3p1/2) | 399.8 (1s) | 168.1 (2p TOS)
162.0 (2p Thiol) | -- | -- |
| Rel. Conc. (/Ru) | 1 | 5.41 | 1.70 (2p TOS)
1.66 (2p Thiol) | |||
| CT5(TOS)5 on ODT/Au | B. E. (eV) | No binding | No binding | No binding | -- | -- |
| Rel. Conc. (/Ru) | ||||||
| CT5(PF6)5 Powder | B. E. (eV) | 462.7 (3p3/2)
484.5 (3p1/2) | 399.7 (1s) | -- | 135.9 (2p) | 686.5 (1s) |
| Rel. Conc. (/Ru) | 1 | 6.08 | 2.42 | 14.8 | ||
| CT5(PF6)5on SiO2 | B. E. (eV) | 461.5 (3p3/2)
484.5 (3p1/2) | 399.8 (1s) | -- | No binding | Bo binding |
| Rel. Conc. (/Ru) | 1 | 6.40 |
| Native SiO2 thickness before attachment of CT5 (Å) | 11.3 | 11.8 | 11.4 | 11.3 | 11.6 | 11.4 | 11.6 | Average 11.5±0.2 |
| Total Native SiO2 and CT5 thickness after attachment (Å) | 18.0 | 18.6 | 17.6 | 17.9 | 17.8 | 18.1 | 17.6 | Average 17.9±0.5 |
| Calculated CT5 monolayer thickness (Å) | 6.7 | 6.8 | 6.2 | 6.6 | 6.2 | 6.7 | 6.0 | Average 6.4±0.4 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.; Lieberman, M.; Hang, Q.; Bernstein, G. Selective Binding, Self-Assembly and Nanopatterning of the Creutz-Taube Ion on Surfaces. Int. J. Mol. Sci. 2009, 10, 533-558. https://doi.org/10.3390/ijms10020533
Wang Y, Lieberman M, Hang Q, Bernstein G. Selective Binding, Self-Assembly and Nanopatterning of the Creutz-Taube Ion on Surfaces. International Journal of Molecular Sciences. 2009; 10(2):533-558. https://doi.org/10.3390/ijms10020533
Chicago/Turabian StyleWang, Yuliang, Marya Lieberman, Qingling Hang, and Gary Bernstein. 2009. "Selective Binding, Self-Assembly and Nanopatterning of the Creutz-Taube Ion on Surfaces" International Journal of Molecular Sciences 10, no. 2: 533-558. https://doi.org/10.3390/ijms10020533