1. Introduction
Recently, Tissue Engineering (TE) has become a well recognized technique, and many researchers with different areas of expertise are entering this medical field, thereby increasing its scientific implications. The objective of tissue engineering is to regenerate natural tissues from living cells to replace defective or lost tissues and organs [
1].
It is undoubtedly necessary for cell-based tissue regeneration to increase the number of cells that constitute the tissue, as well as reconstruct the structure to support the cells. In addition, growth factors are required to promote cell differentiation and proliferation and thus achieve tissue regeneration. However, few researchers have applied this Drug Delivery System (DDS) to the field of Regenerative Medicine (RM) [
2]. Drugs for RM include organic compounds and genes that are effective in promoting the proliferation and differentiation of cells for the induction of tissue and organ regeneration [
2].
RA, the main biologically-active derivative of Vitamin A or retinol [
3], is a potent regulator of gene transcriptions [
4], and play an important role in regulating cell growth, differentiation [
3], embryonic development, apoptosis, homeostasis [
5] and can reverse malignant cell growth
in vitro and
in vivo. All-
trans-retinoic acid (ATRA) is being increasingly included in antitumour therapeutical schemes for the treatment of various tumoral diseases such as acute promyelocytic leukaemia, Kaposi’s sarcoma, head and neck squamous cell carcinoma, ovarian carcinoma, bladder cancer and neuroblastoma [
6] and has shown antiangiogenic effects in several systems [
5]. As a result of good clinical responses in treatment of these malignancies, retinoids are being investigated for their therapeutic potential in an increasing range of diseases [
3]. However, due to its hydrophobicity [
6] (0.21 μM in physiological solution, pH 7.3) [
7], and short half-life in blood [
8], ATRA parenteral administration is very difficult and to date no commercial parenteral formulation is available. On the other hand the oral formulations in clinical use are characterized by uncertain drug bioavailability due to the variable absorption in the gastrointestinal tract and the hepatic first pass effect, implying metabolism by hepatocytes of ATRA to less active compounds. Furthermore its intestinal absorption is affected by the pH and fatty acid composition of intraluminal bile [
9]. Therefore, plasma concentration following oral administration of ATRA is highly variable [
6]. In addition to the poor solubility, combined with chemical instability of ATRA complicates its administration.
In spite of its pronounced therapeutical effect, several side effects such as teratogenicety [
10], retinoid acute resistance, hypertriglyceridemia, mucocutaneous dryness, headache [
11] hepatotoxicity, nausea, vomiting, and abdominal pain [
3] are reported and limit the clinical applications of ATRA [
8].
Different treatment modalities have been investigated in attempt to overcome the accelerated metabolism of ATRA that leads to a progressive decline in plasma ATRA concentrations during a chronic daily schedule. These modalities include the use of inhibitors of cytochrome p450 enzymes, administration of oral ATRA on an intermittent schedule, and encapsulation of ATRA into microspheres [
12], microemulsions [
13], nanodisks [
14], liposomes [
15], micelles [
16] and nanoparticles [
6,
8,
9,
17,
18]. The dimensions of the carrier have a critical role in the achievement of optimized therapeutic regimens. It has been shown that nanocarriers can penetrate through small capillaries, across numerous physiological barriers and can be taken up by cells, thus inducing efficient drug accomulation at the target site [
20].
There are a number of techniques available for the preparation of drug loaded nanoparticulate systems such as the emulsion solvent evaporation/extraction method, spray drying, phase separationcoacervation, interfacial deposition, and
in situ polymerization. Each method has its own advantages and disadvantages. The choice of a particular technique depends on polymer and drug features, site of action and therapy regimes [
21].
Although a number of different method exist for the preparation of nanoparticles the choice of a preparation method is an hard task and general procedures that works for all nanoparticles material seem to do not exist. Furthermore it is highly desirable to provide with alternative methods, aimed at producing polymeric particles, that do not entail the utilization of organic solvents that are undesiderable for their potential toxicity. Such a method is expected to receive a particular attention in biotechnological field [
21].
In this work an organic solvent-free method has been developed for the preparation of VAM 41 nanoparticles loaded with RA. VAM 41 is an amphiphilic polymeric material developed by hemiesterification of alternating copolymers of maleic anhydride (MAn) and alkyl vinyl ethers (RVE) Quite a large number of examples are reported in the literature in which hemiesters of copolymers of maleic anhydride and alkyl vinyl ethers have been utilized for the formulation of release systems. For instance, the
n–butyl hemiester of maleic anhydride–methyl vinyl ether alternating copolymer has been used in ophthalmology for the preparation of monolithic systems or as pH sensitive covers of microcapsules [
22]. Similarly, alkyl hemiesters of maleic anhydride–oligo(oxyethylene) vinyl ether copolymers were formulated as monolithic inserts for the controlled release of pilocarpine [
23], and γ-interferon [
22,
24].
The developed method is easy to be perform, effective for the preparation of relatively small nanoparticles with a narrow size distribution and is operator friendly, since it does not require handling any toxic organic solvents. The SK-N-SH neuroblastoma cell line was chosen as a model to verify the possibility of differentiating for a neuronal phenotype after exposure to bioactive principles and to examine whether the activity of RA was affected by incorporation in VAM41 nanoparticles by the developed method. The preparation process guarantee for the chemical stability and biological activity of the incorporated drug.
2. Results and Discussion
The novel nanoparticle preparation method was conceived by taking into account the characteristics of RA, VAM41 and HSA and the behaviour of their different mixtures in response to pH variations. RA is a hydrophobic compound with one polar, ionisable end group that confers amphiphilic character to the molecule. In non-polar hydrocarbon solvents, RA self associates by forming tail-to-tail dimers that are stabilized by hydrogen bonding between the carboxyl groups of two RA molecules. On the contrary, in water RA self associates in micelle-like structures by hydrophobic interaction among the rings of several molecules [
25]. RA at pH > 7.4 and at a 1.5 mM concentration is soluble in water, as evidenced by the clarity of the solution and the lack of any observed light scattering. When the pH is lowered to 7, RA self associates to micellar like structure with a diameter of 120 nm. This phenomenon is macroscopically evidenced by the appearence of turbidity and the size evaluated by granulometry measurements. A further decrease of the solution pH causes the formation of flakes of micelles that leads to RA precipitation. At a pHs below the critical micellar concentration which is about 2 x 10
−6 mol/L (pH 7) [
26], the negative surface charge which stabilize the RA micelles is gradually lost. Particles collide forming aggregates that precipitate.
In presence of HSA (nHSA/nRA 1:10) a stable yellowish milky-like particle suspension with a diameter size of 90 nm forms at pH 7. HSA hampers the precipitation of the drug up to pH 5. However, at pHs below 5 the colloidal suspension looses its stability and tends to agglomerate.
This behaviour could be explained by the so-called hydration forces [
27]. HSA, once its binding sites for RA are saturated, tends to be adsorbed on the RA colloid’s surface. It is well established that water molecules strongly bind to protein surfaces. An overlap of the solvent layers near to the two mutually approaching colloid surfaces creates repulsive forces [
28]. At pH 5, a pH lower than the isoelectric point (pI) of HSA, protein molecules have a compact coil structure and an electrostatic attraction force favours the protein (positively charged) surface union [
27] which leads to the loss of colloidal stability of the system. VAM41, due to the presence of the ionisable carboxylate group is soluble in water at pHs above 8 and it is insoluble at pHs below 7 (Figure
1). HSA, as previously reported, is able to complex RA and stabilize suspension colloidal particles, is soluble at all mentioned pHs, and due to the presence of many charged residues on the molecule, it has buffering properties. At physiological pH (7.4) it displays a net negative charge [
29].
It was observed that the addition of a drop of 1N chloride acid to a water solution of VAM41 and HSA at pH 8 led, as expected, to the formation of polymer flakes. Unexpectedly, after few seconds under energetic stirring, these flakes completely re-dissolved. HSA, thanks to its buffering properties, was able to deprotonate the carboxylic moieties of VAM41. The further addition of drops of acid led to a slow decrease of the pH of the solution, to a reduction of the ability of HSA to accept protons and a milk-like colloidal suspension of VAM41 formed at about pH 6. At pH 5.5 a colloidal suspension of VAM41 with average diameter size of the particles of 2.74 μm (polydispersity: 0.3) and a rather wide distribution of the diameters (Figure
2) was observed that became 4.64 μm at pH 2.5.
On the basis of this observation and keeping in mind the characteristics of the material employed, a water solution of VAM41, RA and HSA was prepared by adding NaOH to a water suspension of the materials. The solution pH was adjusted to 8 and subsequently drops of HCl 1 N and 0.1 N, as in a typical titration experiment, were added under energetic stirring in order to prepare nanoparticles. As already experienced, the addition of drops of acid to the solution led to the formation of flakes that rapidly re-dissolved. As hypothesized, at roughly pH 7.4 micelles of RA formed, while the polymer kept re-dissolving in the same fashion. The subsequent additions of acid caused the polymer to absorb and coat uniformly the surface of the formed colloidal suspension. The energetic stirring was essential for a uniform distribution of the polymer onto RA micelles. HCl 1 N was used in the first part of the experiment when the dissolution of the forming flakes was quite fast. Once the buffering properties of HSA were reduced, HCl 0.1 N was used to terminate the covering process. RA acted as nucleating agent during the preparation of nanoparticle suspensions.
The colloidal suspension was stable up to pH 5.3. Below this pH particles joined together forming a dense aggregate that could be uniformly redispersed just by adding drops of dilute NaOH. The formation of aggregates could be ascribed to the above mentioned loss of proteins stabilizing effect at pHs close or below to their pI. Particle formation and development was followed by pHmeter and light scattering (Table
1).
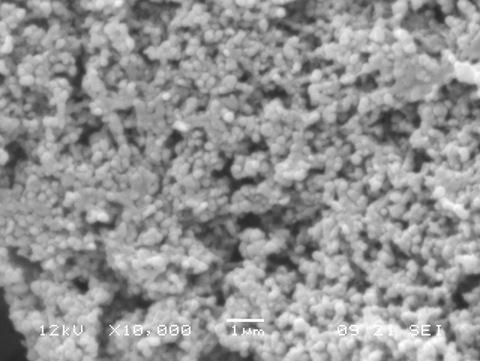
Nanoparticles with good morphology and an average diameter size of about 65 nm were prepared using this technique (Figure
3).
Moreover a drug loading content of 6.7% with a loading efficiency of 97% was obtained when the experiment was terminated at pH 5.3. Drug loading efficiency is very high and depends on the extent of acidification of the solution, since the lower is the pH reached, the higher is the amount of polymer that covers and forms nanoparticles. This formulation method is basically suitable for any material with water solubility influenced by the pH.
During the preparation of nanoparticles by Colloidal Coating, RA is subjected to pH variations, freezing and drying process. In order to examine whether the activity of RA was affected by incorporation in VAM41 nanoparticles, it was tested on SK-N-SH human neuroblastoma cell line that are known to undergo inhibition of proliferation and neuronal differentiation upon treatment with RA. SK-N-SH neuroblastoma cell line was chosen as a model to verify the possibility to differentiate towards a neuronal phenotype after exposure to bioactive principles.
Numerous studies have shown that RA induces differentiation, growth arrest, and apoptosis in neuroblastoma cell lines by inhibition of telomerase activity, and in increased endogenous ceramide levels [
30]. Results of the qualitative investigations of SK-N-SH cells treated with RA loaded nanoparticles appear to indicate a growth inhibition in respect to the untreated cells (negative control) (Figure
4), thus suggesting that the antiproliferative and differentiating activity of RA was not impaired by the nanoparticles incorporation and freeze-drying process. Observation of cells treated with free RA indicates a higher growth inhibition activity in respect to the RA loaded into nanoparticles. It is reasonable to speculate that this result is related to a controlled release of the bioactive agent RA from nanoparticles.
3. Experimental Section
3.1. Materials
The bioerodible polymer VAM41 [2-methoxyethanol hemiester of poly(maleic anhydride-alt-butyl vinyl ether)] was kindly provided by Polymer Laboratories Ltd (UK). Retinoic acid (RA) and human serum albumin (HSA, 96 – 99% powder) were purchased from Sigma (St. Louis, MO, USA). A 3% mannitol solution was prepared by dissolving 3.0 g of mannitol (Baker) in 100 mL distilled water (Phillipsburg, NJ, USA). Hydrochloric Acid 37 – 38% the commercial product (J.T. Baker, Philipsburg, NJ, USA) was used as received and diluted with deionised water to prepare 1 N and 0.1 N acid solutions. SK-N-SH human neuroblastoma cell line (HTB-11) was purchased from American Type Culture Collection (ATCC, Rockville, MD) and propagated as indicated by the supplier. Minimum Essential Medium (MEM), 0.01 M phosphate buffer saline (pH 7.4) free of Ca2+ and Mg2+ (PBS), fetal calf serum, glutamine and antibiotics (penicillin/streptomycin) were purchase from GIBCO Brl (Rockville, MD).
3.2. Preparation of Unloaded Nanoparticles
50 mg of VAM41 were dissolved in 5 mL of water by adding a drop 10 N NaOH and then 200 μL of 25 % HSA solution (corresponding to 50 mg HSA) were added. The solution was left under stirring conditions for 1 h and the pH was adjusted to 8. At room temperature and under rapid magnetic stirring (7,500 rpm) 1 N HCl was dropped into the solution until the formation of a suspension (~ pH 5). Each drop was added after the disappearing of the flakes formed by the addition of the previous drop.
3.3. Preparation of RA Loaded Nanoparticles
50 mg of VAM41 were dissolved in 5 mL of water by adding a drop 10 N NaOH and then 200 μL of 25 % HSA solution (corresponding to 50 mg HSA) were added and the solution was kept under stirring condition for 1 h. Subsequently 225 μL of a solution of retinoic acid in DMSO (10 mg/mL) were added and the pH was adjusted to 8. At room temperature and under rapid magnetic stirring 1 N HCl was dropped into the solution until the formation of a yellowish suspension (~ pH 7.4). Each drop was added after the disappearing of the flakes formed by the addition the previous drop. In the same fashion drops of 1 N and 0.1 N HCl were added up to pH 5.3.
3.4. Nanoparticles Purification
Nanoparticle suspensions were purified by centrifugation in an ALCPK121R centrifuge at 8,000 g for 30 minutes at room temperature in order to remove the excess of polymer, drug and protein. The samples were either placed in 50 mL falcon or in Eppendorf tubes and centrifuged by using AM-10 or AM-21 rotors, respectively. Harvested nanoparticles were freeze-dried in a 5Pascal Lio 5P lyophilizator.
3.5. Cryoprotection
Nanoparticle suspensions (6.0 mL) were centrifuged at 8,000 g for 30 minutes. The resulting pellets were dispersed in 3.0 mL of aqueous solutions containing 3 % mannitol. The resulting suspensions were frozen at −20 °C, freeze-dried in a 5Pascal Lio 5P lyophilizator. The recovered lyophilized powders were re-dispersed in 3.0 mL of deionised water.
3.6. Nanoparticle Characterization
Size Analysis: dimensional analyses were carried out by using a Coulter LS230 Laser Diffraction Particle Size Analyzer, equipped with small volume module plus. Nanoparticle suspensions were added into the cell until 30 – 50% obscuration of PIDS detector was reached. Deionised water was used as background and diameter distribution was processed using the Fraunhofer optical model. Three runs were performed on each sample.
Morphological Analysis: nanoparticles morphology was investigated by means of scanning electron microscopy (SEM), by using a JEOL LSM5600LV scanning electron microscope. Gold sputtering was performed before SEM analysis.
3.7. Evaluation of Retinoic Acid Incorporation
Samples of purified and lyophilised VAM41 nanoparticles were weighted properly and dissolved into 5 mL of water by adding 1 N NaOH. The final pH was adjusted to 8. Drug concentration was determined spectrophotometrically [
31] at 347 nm by using a standard curve obtained by plotting the average blank-corrected absorbance of each standard vs. its RA concentration (0.04 – 10 mg/L; y = 0.1823x + 0.0.0051; R
2 = 0.9994).
3.8. In Vitro Evaluation of Encapsulated Retinoic Acid Activity
SK-N-SH human neuroblastoma cells were seeded in 6 multi-well plates (1.5 × 104 cells/well) and maintained in Minimum Essential Medium (MEM) containing 10% fetal calf serum, 4 mM glutamine and 100 U/mL:100 μg/mL penicillin:streptomycin at 37 °C in 5% CO2 for 24 h. Cells were treated either with 40 μM free-RA or 40 μM encapsulated RA for 6 days. Encapsulated or free RA was added to the cells in fresh growth media at every 48 h and morphological differentiation assessed by phase contrast microscopy, by using a Nikon TE-2000 inverted microscope.