Paradoxical Acceleration of Dithiothreitol-Induced Aggregation of Insulin in the Presence of a Chaperone
Abstract
:1. Introduction
2. Results
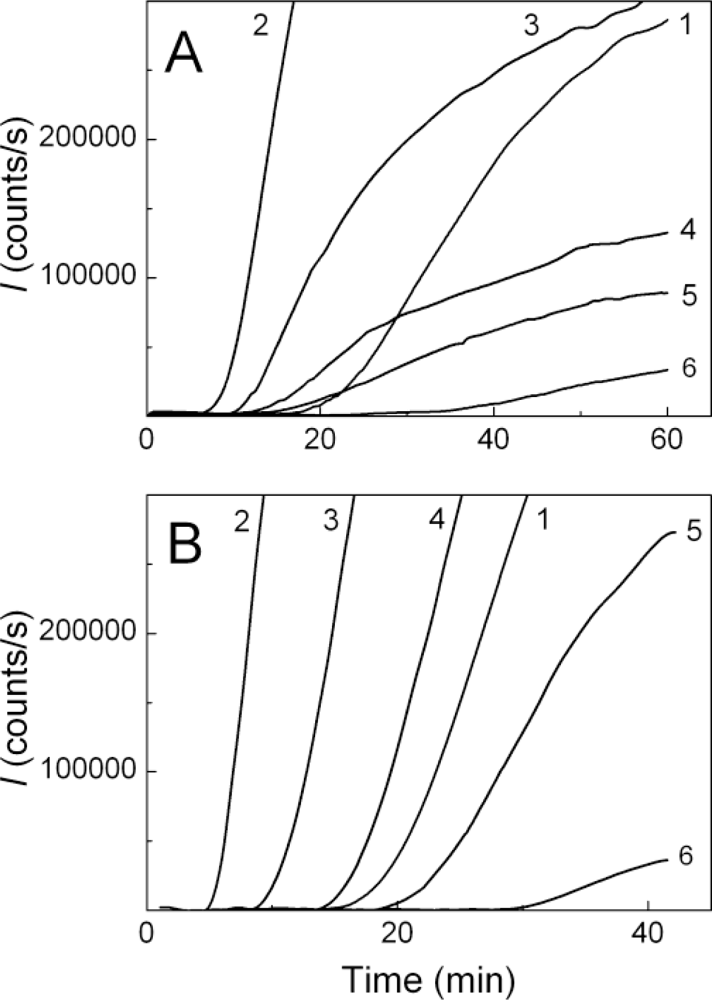
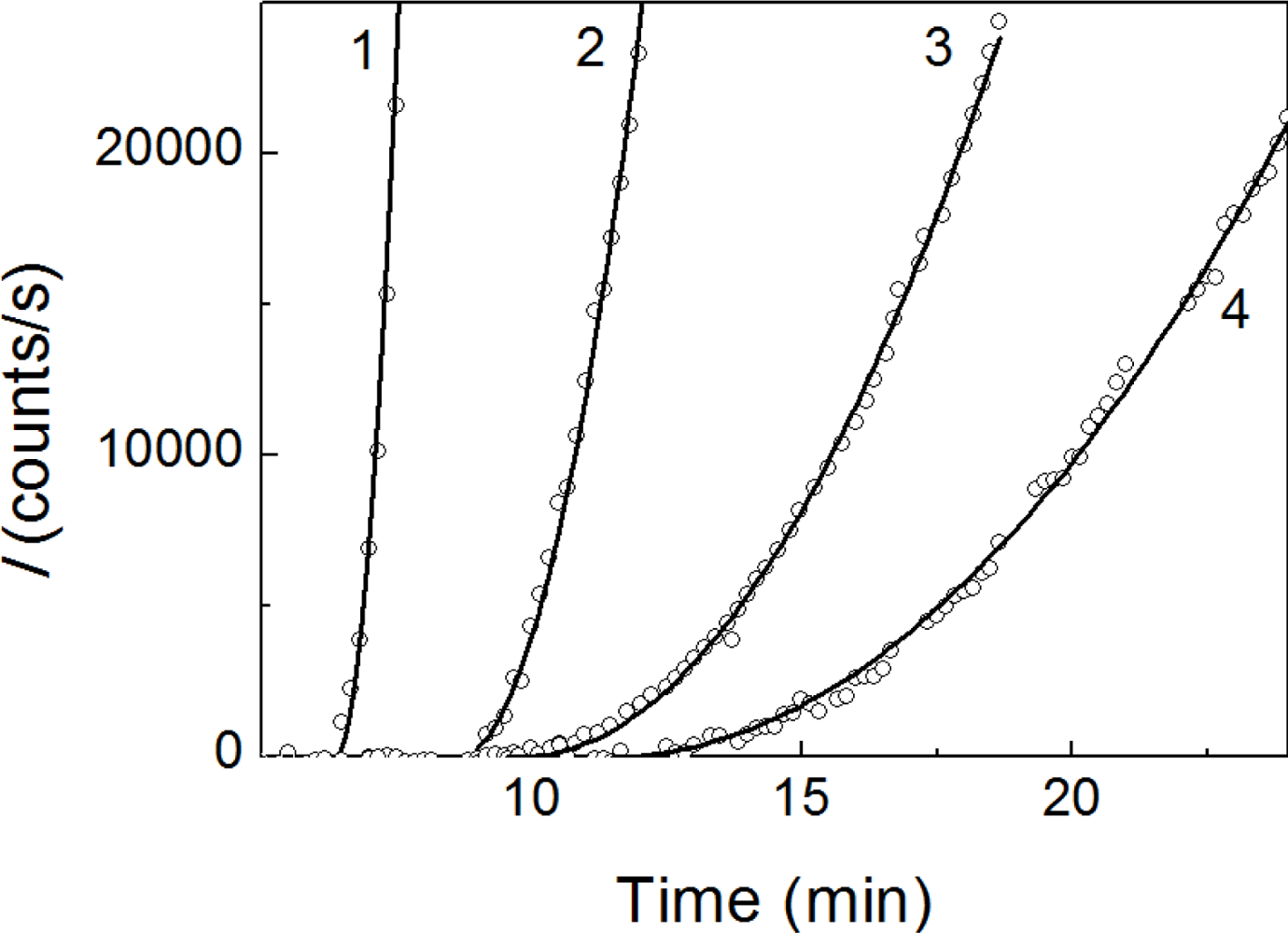
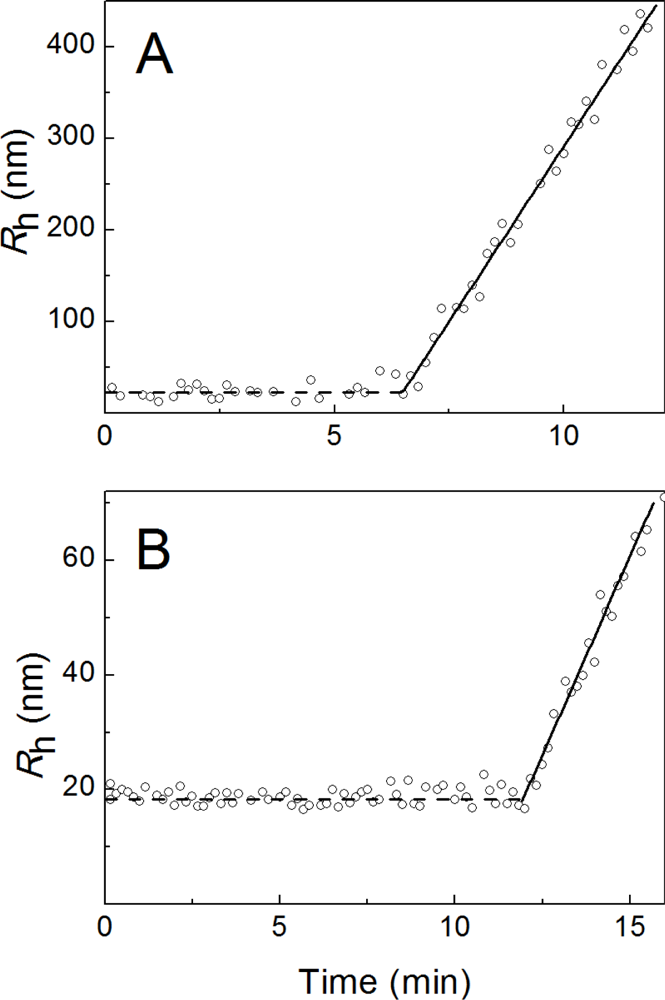
2.1. Kinetics of Dithiothreitol-Induced Aggregation of Insulin
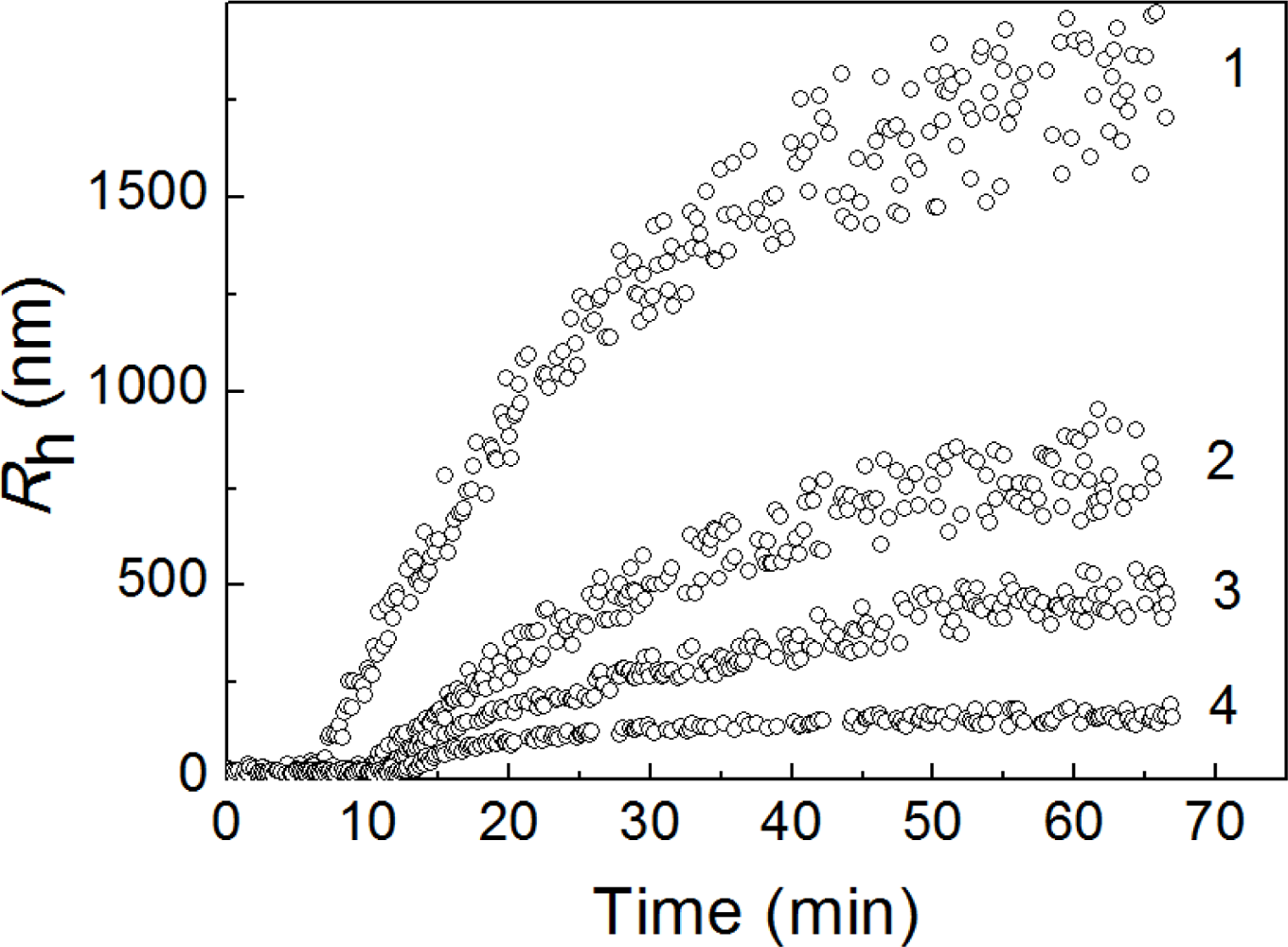
2.2. The Effect of α-Crystallin on the Kinetics of Dithiothreitol-Induced Aggregation of Insulin
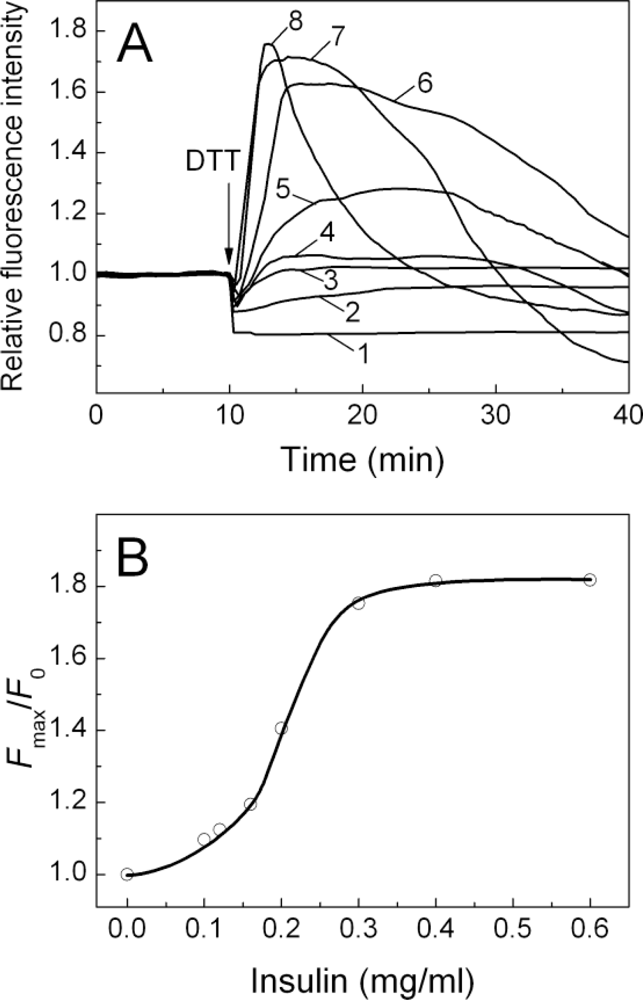
2.3. Fluorescence Studies of α-Crystallin Interaction with Insulin
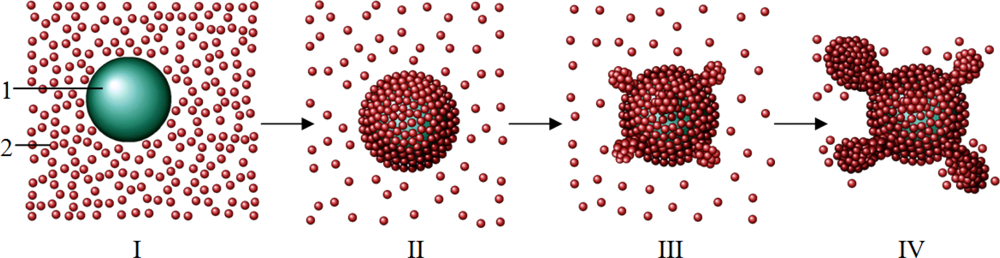
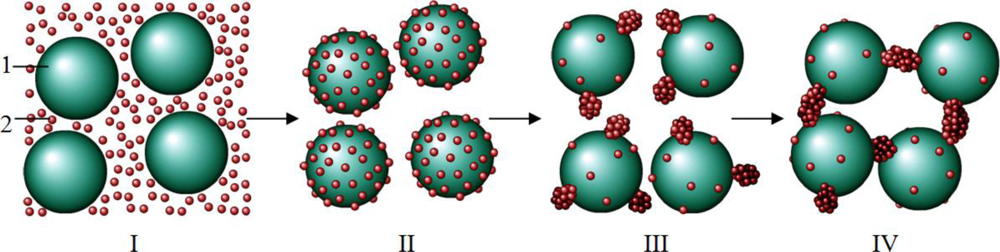
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Isolation and Purification of α-Crystallin
4.3. DTT-Induced Aggregation of Insulin
4.4. Dynamic Light Scattering Studies
4.5. Fluorescence Spectroscopy
5. Conclusions
Acknowledgments
References
- Horwitz, J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 1992, 89, 10449–10453. [Google Scholar]
- Peschek, J; Braun, N; Franzmann, TM; Georgalis, Y; Haslbeck, M; Weinkauf, S; Buchner, J. The eye lens chaperone α-crystallin forms defined globular assemblies. Proc. Natl. Acad. Sci. USA 2009, 106, 13272–13277. [Google Scholar]
- Bumagina, ZM; Gurvits, BY; Artemova, NV; Muranov, KO; Yudin, IK; Kurganov, BI. Mechanism of suppression of dithiothreitol-induced aggregation of bovine α-lactalbumin by α-crystallin. Biophys. Chem 2010, 146, 108–117. [Google Scholar]
- Chiti, F; Dobson, CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem 2006, 75, 333–366. [Google Scholar]
- Plum, L; Schubert, M; Bruning, J. The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab 2005, 16, 59–65. [Google Scholar]
- Brange, J; Whittingham, J; Edwards, D; Zhang, Y-S; Wollmer, A; Brandenburg, D; Dodson, G; Finch, J. Insulin structure and diabetes treatment. Curr. Sci 1997, 72, 470–476. [Google Scholar]
- Nettleton, EJ; Tito, P; Sunde, M; Bouchard, M; Dobson, CM; Robinson, CV. Characterization of the oligomeric states of insulin in self-assembly and amyloid fibril formation by mass spectrometry. Biophys. J 2000, 79, 1053–1065. [Google Scholar]
- Rasmussen, T; Kasimova, MR; Jiskoot, W; van de Weert, M. The chaperone-like protein α-crystallin dissociates insulin dimers and hexamers. Biochemistry 2009, 48, 9313–9320. [Google Scholar]
- Brange, J; Andersen, L; Laursen, ED; Meyn, G; Rasmussen, E. Toward understanding insulin fibrillation. J. Pharm. Sci 1997, 86, 517–525. [Google Scholar]
- Nilsson, MR; Dobson, CM. Chemical modification of insulin in amyloid fibrils. Protein Sci 2003, 12, 2637–2641. [Google Scholar]
- Hua, Q-X; Weiss, MA. Mechanism of insulin fibrillation. The structure of insulin under amyloidogenic conditions resembles a protein-folding intermediate. J. Biol. Chem 2005, 280, 21726–21730. [Google Scholar]
- Das, KR; Surewicz, WK. Temperature-induced exposure of hydrophobic surfaces and its effect on the chaperone activity of α-crystallin. FEBS Lett 1995, 369, 321–325. [Google Scholar]
- Farahbakhsh, ZT; Huang, Q-L; Ding, L-L; Altenbach, C; Steinhoff, H-J; Horwitz, J; Hubbell, WL. Interaction of α-crystallin with spin-labeled peptides. Biochemistry 1995, 34, 509–516. [Google Scholar]
- Linder, RA; Kapur, A; Mariani, M; Titmuss, SJ; Carver, JA. Structural alterations of α-crystallin during its chaperone action. Eur. J. Biochem 1998, 258, 170–183. [Google Scholar]
- Reddya, GB; Narayananb, S; Reddya, PY; Suroliab, I. Suppresion of DTT-induced aggregation of arbin by aA- and aB-crystallins: A model aggregation assay for a-crystallin chaperone activity in vitro. FEBS Lett 2002, 522, 59–64. [Google Scholar]
- Bhattacharyya, J; Srinivas, V; Sharma, KK. Evaluation of hydrophobicity versus chaperonelike activity of bovine αA- and αB-crystallin. J. Prot. Chem 2002, 21, 65–71. [Google Scholar]
- Vekshin, NL. How alpha-crystallin prevents the aggregation of insulin. Biochemistry (Moscow) 2008, 73, 458–462. [Google Scholar]
- Kumar, MS; Kapoor, M; Sinha, S; Reddy, GB. Insights into hydrophobicity and the chaperone-like function of αA- and αB-crystallins: An isothermal titration calorimetric study. J. Biol. Chem 2005, 280, 21726–21730. [Google Scholar]
- Ahmad, MF; Raman, B; Ramakrishna, T; Rao, CM. Effect of phosphorylation on αB-crystallin: Differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of αB-crystallin and its phosphorylation-mimicking mutant. J. Mol. Biol 2008, 375, 1040–1051. [Google Scholar]
- Markossian, KA; Yudin, IK; Kurganov, BI. Mechanism of suppression of protein aggregation by α-crystallin. Int. J. Mol. Sci 2009, 10, 1314–1345. [Google Scholar]
- Rasmussen, T; Tantipolphan, R; van de Weert, M; Jiskoot, W. The molecular chaperone α-crystallin as an excipient in an insulin formulation. Pharm. Res 2010, 27, 1337–1347. [Google Scholar]
- Lakowicz, JR. Principles of Fluorescence Spectroscopy, 3rd ed; Springer: Singapore, 2006; p. 954. [Google Scholar]
- Kurganov, BI. Kinetics of heat aggregation of proteins. Biochemistry (Moscow) 1998, 63, 364–366. [Google Scholar]
- Kornilaev, BA; Kurganov, BI; Eronina, TB; Chebotareva, NA; Livanova, NB; Orlov, VN; Cherniak, VY. Mechanism of heat denaturation of muscle glycogen phosphorylase b. Mol. Biol. (Moscow) 1997, 31, 98–107. [Google Scholar]
- Kurganov, BI; Kornilaev, BA; Chebotareva, NA; Malikov, VP; Orlov, VN; Lyubarev, AE; Livanova, NB. Dissociative mechanism of thermal denaturation of rabbit skeletal muscle glycogen phosphorylase b. Biochemistry 2000, 39, 13144–13152. [Google Scholar]
- De Young, LR; Fink, AL; Dill, KA. Aggregation of globular proteins. Acc. Chem. Res 1993, 26, 614–620. [Google Scholar]
- Speed, MA; King, J; Wang, DIC. Polymerization mechanism of polypeptide chain aggregation. Biotechnol. Bioeng 1997, 54, 333–343. [Google Scholar]
- Ferrone, F. Analysis of protein aggregation kinetics. Methods Enzymol 1999, 309, 256–274. [Google Scholar]
- Wang, K; Kurganov, BI. Kinetics of heat- and acidification-induced aggregation of firefly luciferase. Biophys. Chem 2003, 106, 97–109. [Google Scholar]
- Kodaka, M. Requirements for generating sigmoidal time-course aggregation in nucleation-dependent polymerization model. Biophys. Chem 2004, 107, 243–253. [Google Scholar]
- Kodaka, M. Interpretation of concentration-dependence in aggregation kinetics. Biophys. Chem 2004, 109, 325–332. [Google Scholar]
- Kurganov, BI. Protein aggregation kinetics. In Chemical and Biological Kinetics; Burlakova, EB, Varfolomeev, SD, Eds.; Koninklijke Brill NV: Leiden, The Netherlands, 2005; Volume 2, pp. 251–279. [Google Scholar]
- Philo, JS; Arakawa, T. Mechanisms of protein aggregation. Curr. Pharmacol. Biotechnol 2009, 10, 348–351. [Google Scholar]
- Golub, NV; Meremyanin, AV; Markossian, KA; Eronina, TB; Chebotareva, NA; Asryants, RA; Muronetz, VK; Kurganov, BI. Evidence for the formation of start aggregates as an initial stage of protein aggregation. FEBS Lett 2007, 581, 4223–4227. [Google Scholar]
- Ferrone, FA; Ivanova, M; Jasuja, R. Heterogeneous nucleation and crowding in sickle hemoglobin: An analytic approach. Biophys. J 2007, 82, 399–406. [Google Scholar]
- Kopito, RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 2000, 10, 524–530. [Google Scholar]
- Markossian, KA; Kurganov, BI. Protein folding, misfolding, and aggregation. Formation of inclusion bodies and aggresomes. Biochemistry (Moscow) 2004, 69, 971–984. [Google Scholar]
- Chiou, SH; Azari, P; Himmel, ME; Squire, PG. Isolation and physical characterization of bovine lens crystallins. Int. J. Pept. Protein Res 1979, 13, 409–417. [Google Scholar]
- Putilina, T; Skouri-Panet, F; Prat, K; Lubsen, NH; Tardieu, A. Subunit exchange demonstrates a differential chaperone activity of calf α-crystallin toward βLOW- and individual α-crystallins. J. Biol. Chem 2003, 278, 13747–13756. [Google Scholar]
- Khanova, HA; Markossian, KA; Kurganov, BI; Samoilov, AM; Kleimenov, SY; Levitsky, DI; Yudin, IK; Timofeeva, AC; Muranov, KO; Ostrovsky, MA. Mechanism of chaperone-like activity. Suppression of thermal aggregation of βL-crystallin by α-crystallin. Biochemistry 2005, 44, 15480–15487. [Google Scholar]
- Maloletkina, OI; Markossian, KA; Belousova, LV; Kleimenov, SY; Orlov, VN; Makeeva, VF; Kurganov, BI. Thermal stability and aggregation of creatine kinase from rabbit skeletal muscle. Effect of 2-hydroxypropyl-β-cyclodextrin. Biophys. Chem 2010, 148, 121–130. [Google Scholar]
- Chebotareva, NA; Makeeva, VF; Bazhina, SG; Eronina, TB; Gusev, NB; Kurganov, BI. Interaction of Hsp27 with native phosphorylase kinase under crowding conditions. Macromol. Biosci 2010, 10, 783–789. [Google Scholar]
- Markossian, KA; Khanova, HA; Kleimenov, SY; Levitsky, DI; Chebotareva, NA; Asryants, RA; Muronetz, VI; Saso, L; Yudin, IK; Kurganov, BI. Mechanism of thermal aggregation of rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Biochemistry 2006, 45, 3375–13384. [Google Scholar]
- Diez-Orrite, S; Stoll, S; Schurtenberger, P. Off-lattice Monte Carlo simulations of irreversible and reversible aggregation processes. Soft Matter 2005, 1, 364–371. [Google Scholar]
- Weitz, DA; Lin, MY. Dynamic scaling of cluster-mass distributions in kinetic colloid aggregation. Phys. Rev. Lett 1986, 57, 2037–2040. [Google Scholar]
- Weitz, DA; Huang, JS; Lin, MY; Sung, J. Limits of the fractal dimension for irreversible kinetic aggregation of gold colloids. Phys. Rev. Lett 1985, 54, 1416–1419. [Google Scholar]
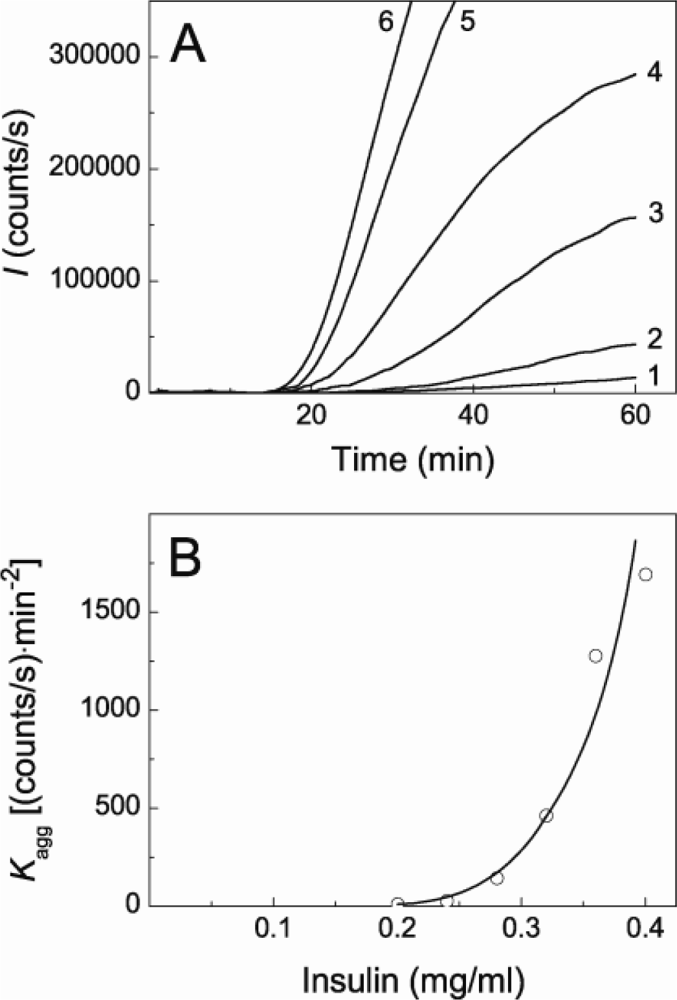
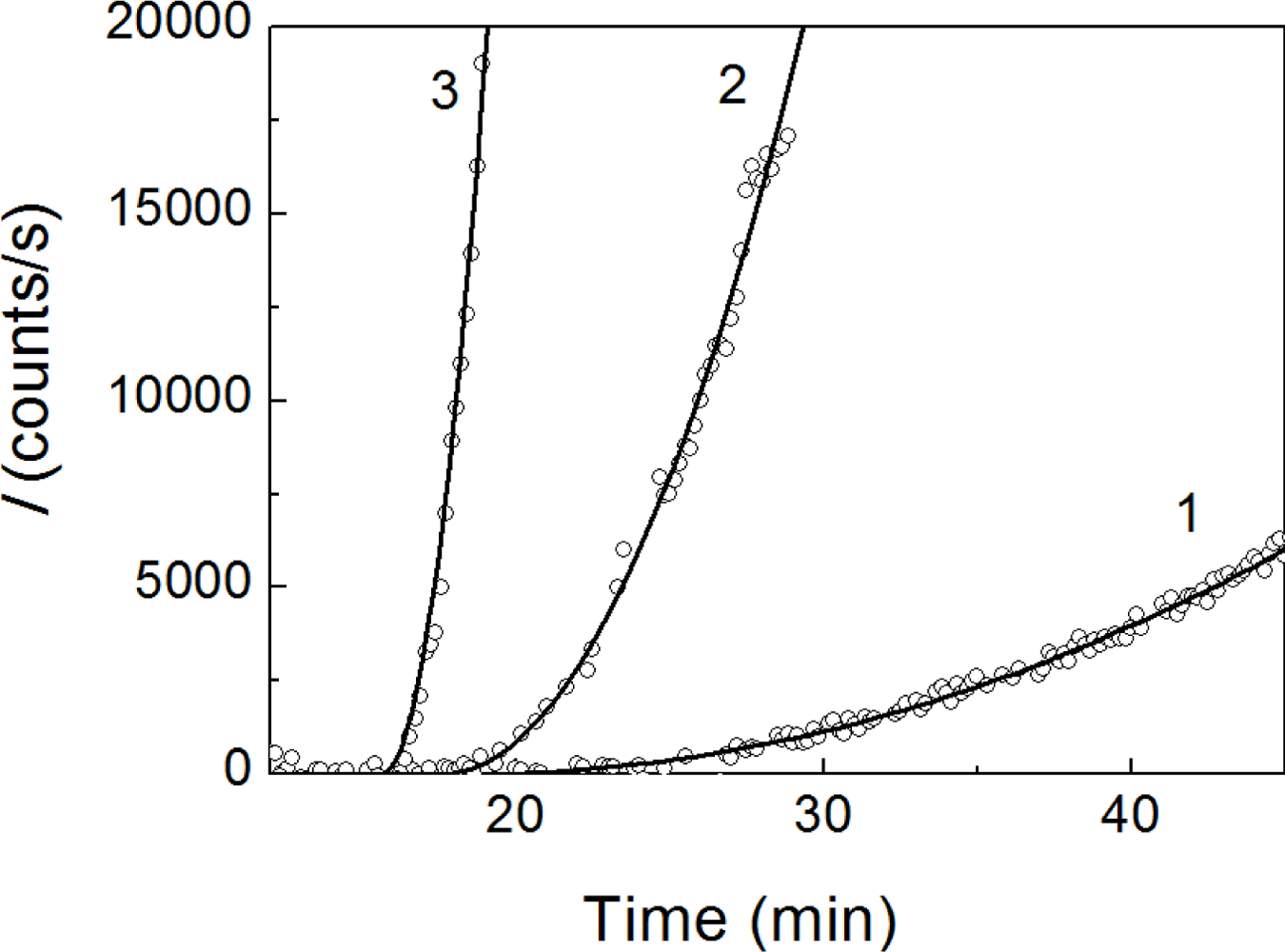
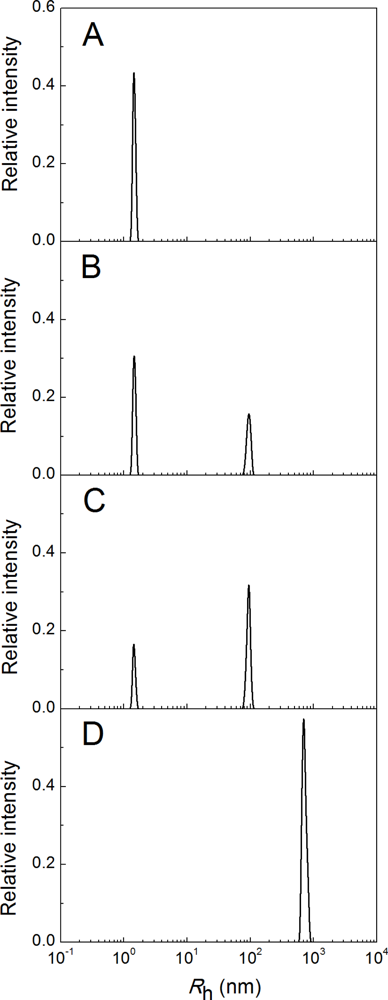
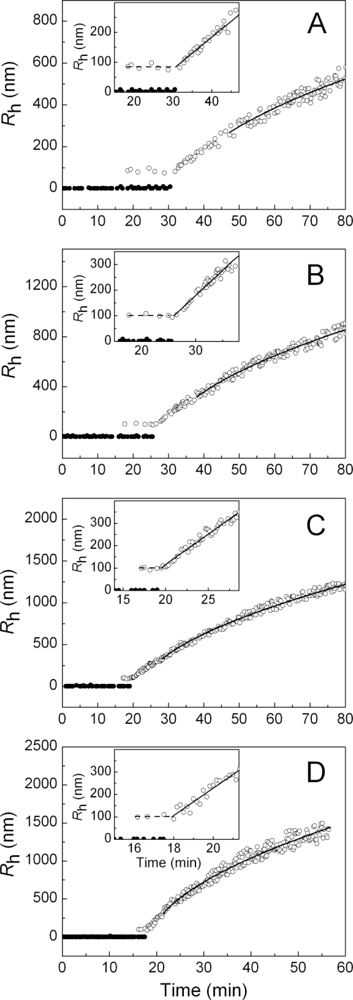
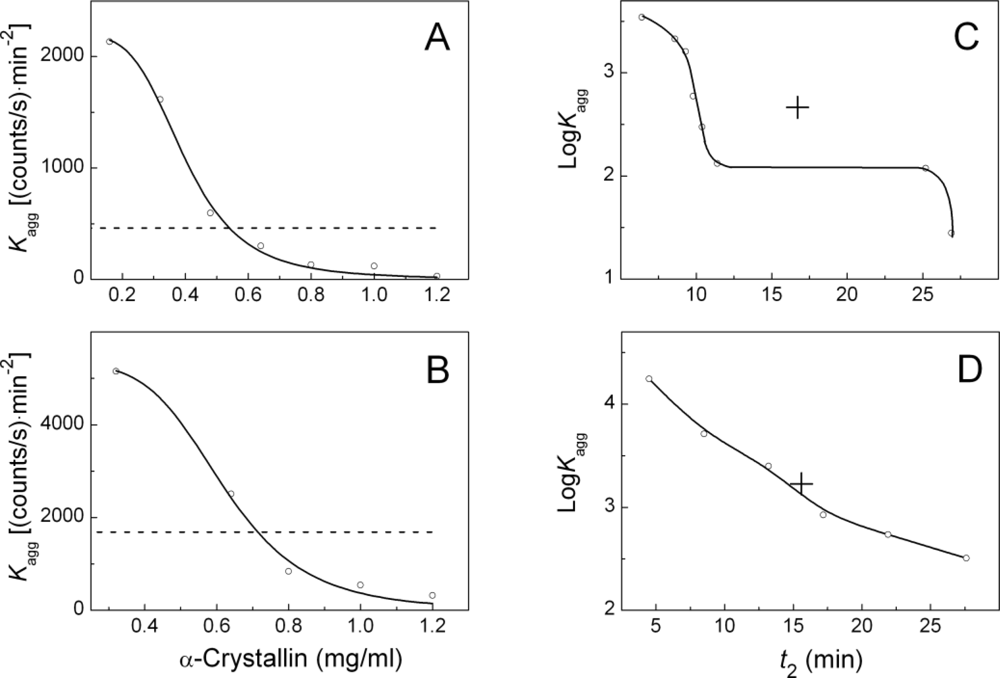
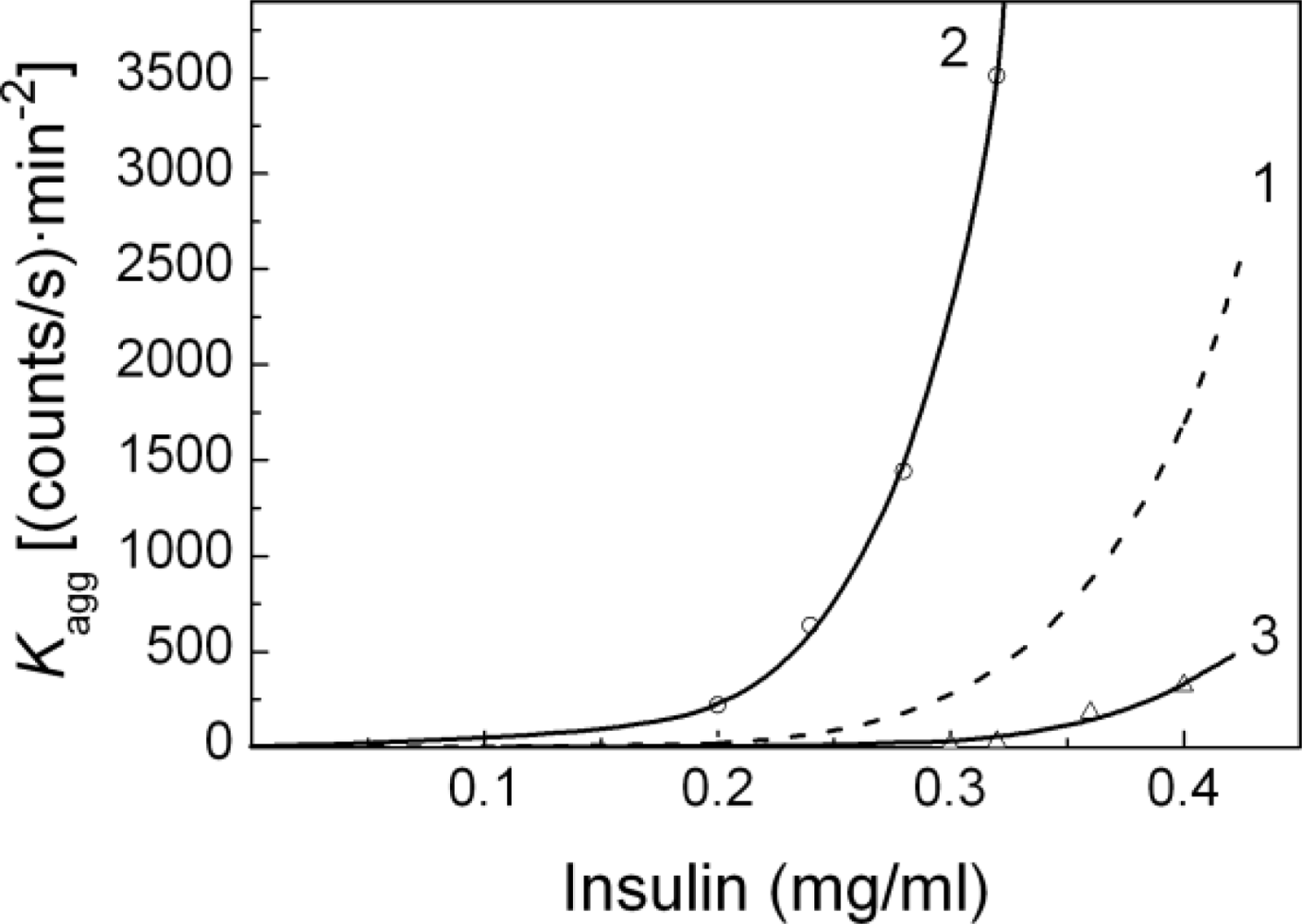
| [Insulin] (mg/mL) | t1 (min) | t2 (min) | Kagg, (counts/s min−2) | Rh,0 (nm) | t3 (min) | K1 (nm/min) | t* (min) | df |
|---|---|---|---|---|---|---|---|---|
| 0.20 | 18.5 | 18.4 ± 0.5 | 8.4 ± 0.6 | 84 ± 3 | 31.3 ± 0.4 | 11.9 ± 0.5 | 47 | 1.80 ± 0.01 |
| 0.24 | 18.3 | 18.3 ± 0.5 | 25 ± 2 | 81 ± 2 | 29.0 ± 0.3 | 13.6 ± 0.6 | 42 | 1.80 ± 0.01 |
| 0.28 | 17.7 | 17.5 ± 0.2 | 144 ± 3 | 101 ± 3 | 26.2 ± 0.3 | 20.3 ± 0.6 | 38 | 1.80 ± 0.01 |
| 0.32 | 17.2 | 16.7 ± 0.2 | 460 ± 10 | 100 ± 2 | 19.3 ± 0.2 | 26.6 ± 0.9 | 28 | 1.80 ± 0.01 |
| 0.36 | 16.8 | 16.2 ± 0.2 | 1270 ± 20 | 100 ± 2 | 18.1 ± 0.1 | 31.0 ± 0.8 | 24 | 1.79 ± 0.01 |
| 0.40 | 16.2 | 15.6 ± 0.2 | 1690 ± 30 | 99 ± 3 | 17.7 ± 0.2 | 60 ± 2 | 22 | 1.79 ± 0.01 |
| [α-Crystallin] (mg/mL) | t2 (min) | Kagg (counts/s min−2) | Rh,complex (nm) | t3 (min) | K1 (nm/min) | ||
|---|---|---|---|---|---|---|---|
| Concentration of insulin 0.32 mg/mL | |||||||
| 0.00 | 16.7 ± 0.1 | 460 ± 10 | |||||
| 0.08 | 6.4 ± 0.1 | 3460 ± 50 | 24.6 ± 1.2 | 6.5 ± 0.2 | 76.0 ± 5.0 | ||
| 0.16 | 8.6 ± 0.1 | 2130 ± 50 | 24.6 ± 1.1 | 9.1 ± 0.3 | 56.0 ± 4.0 | ||
| 0.32 | 9.3 ± 0.1 | 1620 ± 50 | 20.6 ± 0.8 | 10.0 ± 0.1 | 35.0 ± 2.0 | ||
| 0.48 | 9.8 ± 0.2 | 600 ± 20 | 22.3 ± 1.2 | 10.4 ± 0.1 | 23.5 ± 1.9 | ||
| 0.64 | 10.4 ± 0.1 | 300 ± 6 | 21.0 ± 0.8 | 11.1 ± 0.1 | 18.4 ± 0.8 | ||
| 0.80 | 11.4 ± 0.1 | 132 ± 3 | 18.4 ± 0.8 | 11.8 ± 0.2 | 13.0 ± 0.5 | ||
| 1.00 | 17.2 ± 0.3 | 119 ± 4 | 19.5 ± 0.8 | 17.7 ± 1.1 | 9.0 ± 0.5 | ||
| 1.20 | 18.9 ± 0.5 | 28 ± 3 | 18.9 ± 0.5 | 19.4 ± 1.4 | 5.6 ± 0.5 | ||
| Concentration of insulin 0.4 mg/mL | |||||||
| 0.00 | 15.6 ± 0.1 | 1690 ± 30 | |||||
| 0.08 | 4.5 ± 0.1 | 17600 ± 350 | 22.6 ± 1.2 | 5.4 ± 0.1 | 82 ± 6 | ||
| 0.32 | 8.5 ± 0.1 | 5100 ± 100 | 21.8 ± 0.8 | 9.1 ± 0.2 | 71 ± 5 | ||
| 0.64 | 13.2 ± 0.1 | 2500 ± 100 | 23.1 ± 1.1 | 13.8 ± 0.5 | 63 ± 2 | ||
| 0.80 | 17.2 ± 0.1 | 840 ± 10 | 21.6 ± 0.8 | 17.9 ± 1.1 | 48.4 ± 1.8 | ||
| 1.00 | 21.9 ± 0.1 | 540 ± 10 | 21.2 ± 1.0 | 22.3 ± 1.2 | 23.0 ± 1.0 | ||
| 1.20 | 27.6 ± 0.1 | 321 ± 8 | 21.4 ± 0.9 | 27.9 ± 1.1 | 11.0 ± 0.8 | ||
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bumagina, Z.; Gurvits, B.; Artemova, N.; Muranov, K.; Kurganov, B. Paradoxical Acceleration of Dithiothreitol-Induced Aggregation of Insulin in the Presence of a Chaperone. Int. J. Mol. Sci. 2010, 11, 4556-4579. https://doi.org/10.3390/ijms11114556
Bumagina Z, Gurvits B, Artemova N, Muranov K, Kurganov B. Paradoxical Acceleration of Dithiothreitol-Induced Aggregation of Insulin in the Presence of a Chaperone. International Journal of Molecular Sciences. 2010; 11(11):4556-4579. https://doi.org/10.3390/ijms11114556
Chicago/Turabian StyleBumagina, Zoya, Bella Gurvits, Natalya Artemova, Konstantin Muranov, and Boris Kurganov. 2010. "Paradoxical Acceleration of Dithiothreitol-Induced Aggregation of Insulin in the Presence of a Chaperone" International Journal of Molecular Sciences 11, no. 11: 4556-4579. https://doi.org/10.3390/ijms11114556




