Molecular Cloning and Expression Analysis of P-Selectin from Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Results
2.1. Cloning of Zebrafish P-Selectin
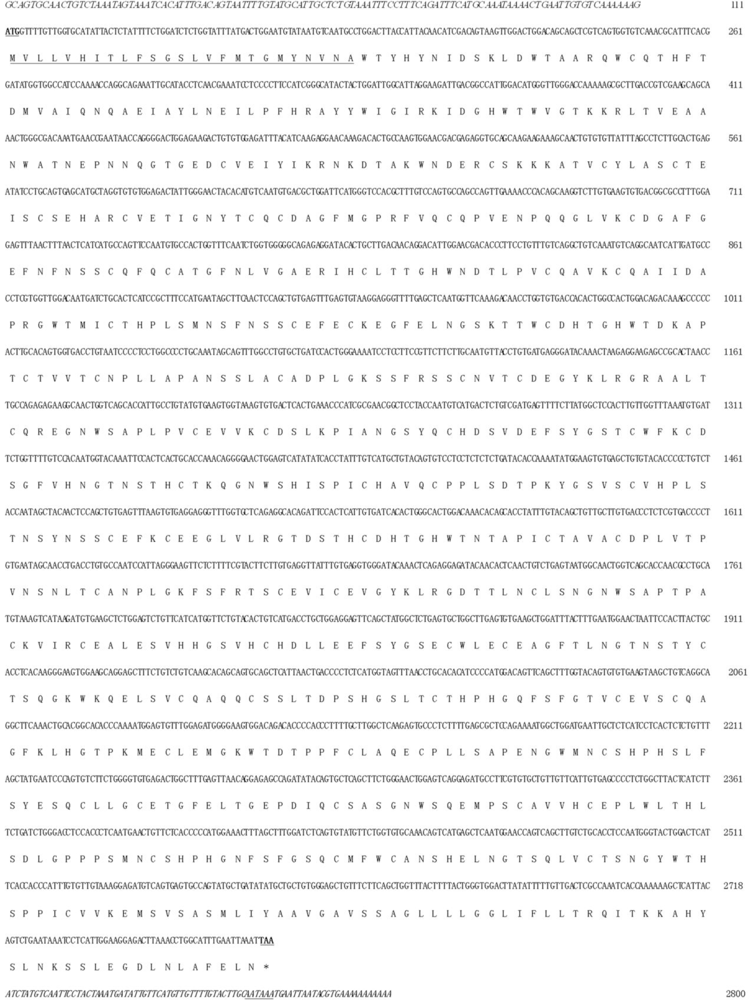
2.2. Nucleotide Sequence Analysis of Zebrafish P-Selectin
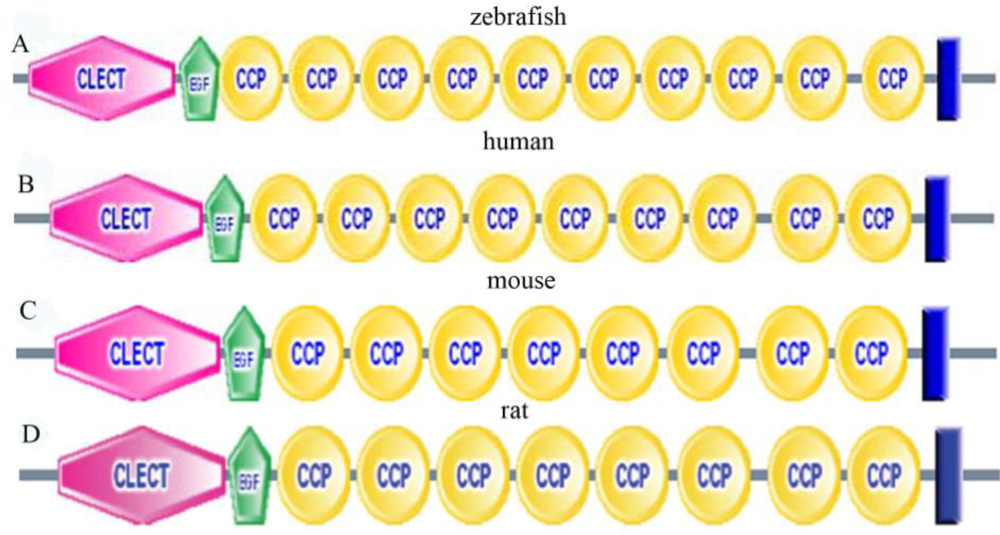
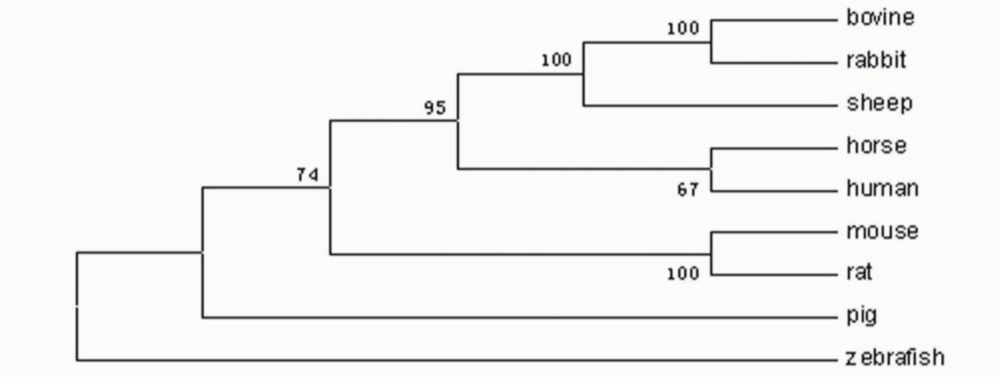
2.3. Amino Acid Sequence Analysis of Zebrafish P-Selectin
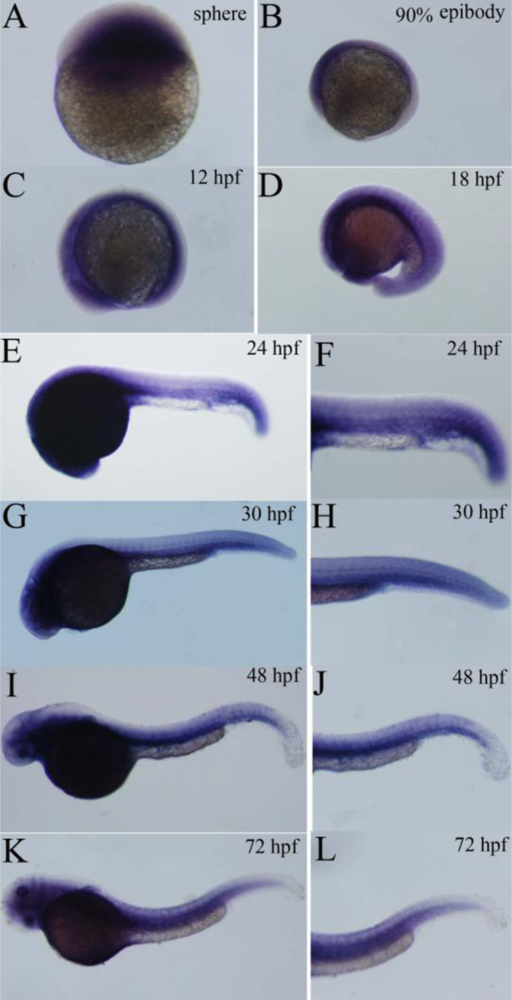
2.4. Expression Pattern of Zebrafish P-Selectin during Embryo Development
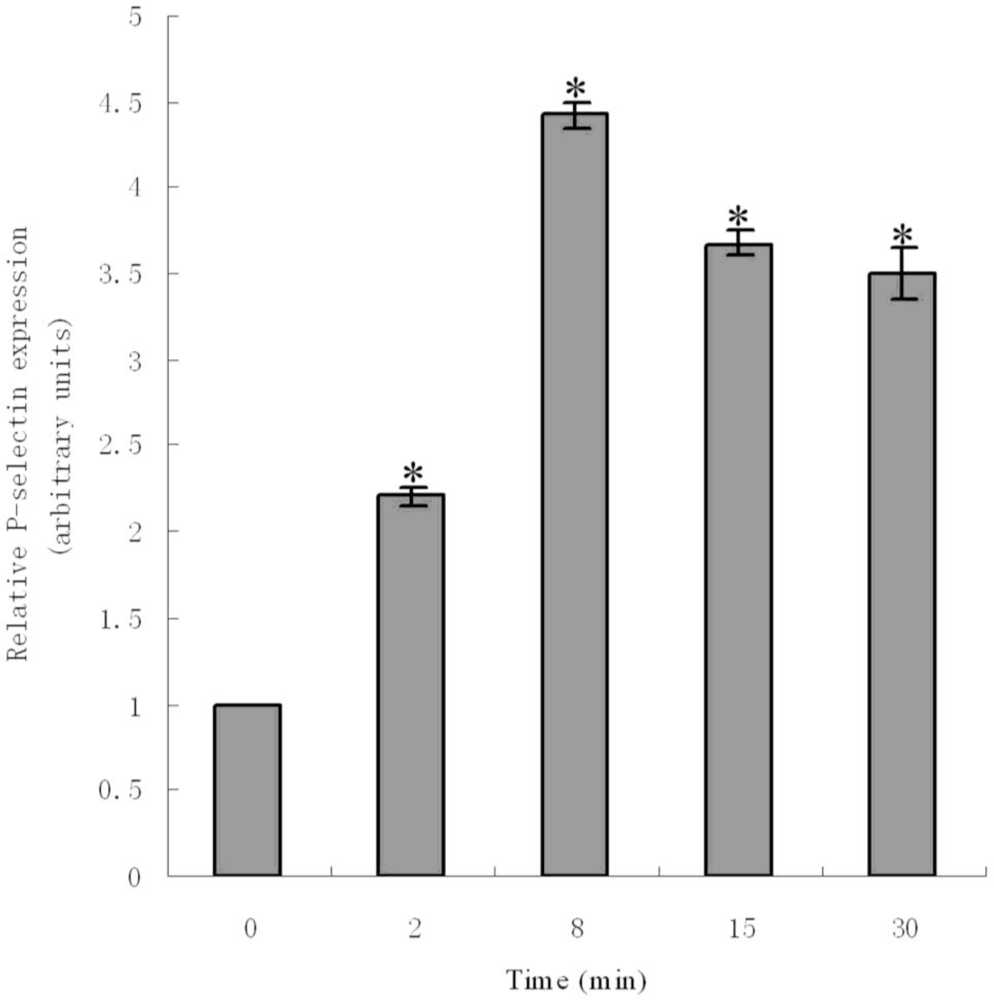
2.6. ADP-Induced Expression of P-Selectin
3. Experimental Section
3.1. Cloning of zebrafish P-selectin cDNA
3.2. Bioinformatics Analysis of Zebrafish P-Selectin
3.3. ADP Treatment
3.4. Real-Time Quantitative RT-PCR
3.5. Whole-Mount in Situ Hybridization
3.6. Statistical Analysis
4. Discussion
5. Conclusions
Acknowledgments
References
- Berman, CL; Yeo, EL; Wencel-Drake, JD; Furie, BC; Ginsberg, MH; Furie, BA. Platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J. Clin. Invest 1986, 78, 130–137. [Google Scholar]
- McEver, RP; Beckstead, JH; Moore, KL; Marshall-Carlson, L; Bainton, DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J. Clin. Invest 1989, 84, 92–99. [Google Scholar]
- Vestweber, D; Blanks, JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev 1999, 79, 181–213. [Google Scholar]
- Frenette, PS; Denis, CV; Weiss, L; Jurk, K; Subbarao, S; Kehrel, B; Hartwig, JH; Vestweber, D; Wagner, DD. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J. Exp. Med 2000, 191, 1413–1422. [Google Scholar]
- Wong, CS; Gamble, JR; Skinner, MP; Lucas, CM; Berndt, MC; Vadas, MA. Adhesion molecule GMP 140 inhibits superoxide anion release by humans neutrophils. Proc. Natl. Acad. Sci. USA 1991, 88, 2397–2401. [Google Scholar]
- Lorant, DE; Topham, MK; Whatley, RE; McEver, RP; McIntyre, TM; Prescott, SM; Zimmerman, GA. Inflammatory roles of P-selectin. J. Clin. Invest 1993, 92, 559–570. [Google Scholar]
- Nagata, K; Tsuji, T; Todoroki, N; Katagiri, Y; Tanoue, K; Yamazaki, HL; Hanai, NL; Irimura, T. Activated platelets induce superoxide anion release by monocytes and neutrophils through P-selectin (CD62). J. Immunol 1993, 151, 3267–3273. [Google Scholar]
- Khew-Goodall, Y; Butcher, MS; Litwin, CM; Newlands, S; Korpelainen, EI; Noack, LM; Berndt, MC; Lopez, AF; Gamble, JR; Vadas, MA. Chronic expression of P-selectin on endothelial cells stimulated by the T-cell cytokine, interleukin-3. Blood 1996, 87, 1432–1438. [Google Scholar]
- Palabrica, T; Lobb, R; Furie, BC; Aronovitz, M; Benjamin, C; Hsu, YM; Sajer, SA; Furie, B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature 1992, 359, 848–851. [Google Scholar]
- Chen, M; Geng, JG. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch. Immunol. Ther. Exp. (Warsz) 2006, 54, 75–84. [Google Scholar]
- Mayadas, TN; Johnson, RC; Rayburn, H; Hynes, RO; Wagner, DD. Leukocyte rolling and extravasation are severely compromised in P-selectin-deficient mice. Cell 1993, 74, 541–544. [Google Scholar]
- Subramaniam, M; Frenette, PS; Saffaripour, S; Johnson, RC; Hynes, RO; Wagner, DD. Defects in hemostasis in P-selectin-deficient mice. Blood 1996, 87, 1238–1242. [Google Scholar]
- Blann, AD; Nadar, SK; Lip, GY. The adhesion molecule P-selectin and cardiovascular disease. Eur. Heart J 2003, 24, 2166–2179. [Google Scholar]
- Woollard, KJ; Chin-Dusting, J. Therapeutic targeting of P-selectin in atherosclerosis. Inflamm. Allergy Drug Targets 2007, 6, 69–74. [Google Scholar]
- Dooley, K; Zon, L. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev 2000, 10, 252–256. [Google Scholar]
- Jagadeeswaran, P. Zebrafish: A tool to study hemostasis and thrombosis. Curr. Opin. Hematol 2005, 12, 149–152. [Google Scholar]
- Jagadeeswaran, P; Gregory, M; Day, K; Cykowski, M; Thattaliyath, B. Zebrafish: A genetic model for hemostasis and thrombosis. J. Thromb. Haemost 2005, 3, 46–53. [Google Scholar]
- Johnston, GI; Cook, RG; McEver, RP. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: Sequence similarity to proteins involved in cell adhesion and inflammation. Cell 1989, 56, 1033–1044. [Google Scholar]
- Sayasith, K; Bouchard, N; Boerboom, D; Brown, KA; Dore, M; Sirois, J. Molecular characterization of equine P-selectin (CD62P) and its regulation in ovarian follicles during the ovulatory process. Biol. Reprod 2005, 72, 736–744. [Google Scholar]
- Sanders, W; Wilson, RW; Ballantyne, CM; Beaudet, AL. Molecular cloning and analysis of in vivo expression of murine P-selectin. Blood 1992, 80, 795–800. [Google Scholar]
- Auchampach, JA; Oliver, MG; Anderson, DC; Manning, AM. Cloning, sequence comparison and in vivo expression of the gene encoding rat P-selectin. Gene 1994, 145, 251–255. [Google Scholar]
- Strubel, NA; Nguyen, M; Kansas, GS; Tedder, TF; Bischoff, J. Isolation and characterization of a bovine cDNA encoding a functional homolog of human P-selectin. Biochem. Biophys. Res. Commun 1993, 192, 338–344. [Google Scholar]
- Rollins, SA; Johnson, KK; Li, L; Birks, C; Matis, LA; Rother, RP. Role of porcine P-selectin in complement-dependent adhesion of human leukocytes to porcine endothelial cells. Transplantation 2000, 69, 1659–1667. [Google Scholar]
- Geng, JG; Heavner, GA; McEver, RP. Lectin domain peptides from selectins interact with both cell surface ligands and Ca2+ ions. J. Biol. Chem 1992, 267, 19846–19852. [Google Scholar]
- McEver, RP. Novel receptors that mediate leukocyte adhesion during inflammation. Thromb. Haemost 1991, 65, 223–228. [Google Scholar]
- Tedder, TF; Steeber, DA; Chen, A; Engel, P. The selectins: Vascularadhesion molecules. FASEB J 1995, 9, 866–873. [Google Scholar]
- Gotsch, U; Jager, U; Dominis, M; Vestweber, D. Expression of P-selectin on endothelial cells is up-regulated by LPS and TNF-a in vivo. Cell Adhes. Commun 1994, 2, 7–14. [Google Scholar]
- Yao, L; Pan, J; Setiadi, H; Patel, KD; McEver, RP. Interleukin-4 or oncostatin M induces a prolonged increase in P-selectin mRNA and protein in human endothelial cells. J. Exp. Med 1996, 184, 81–92. [Google Scholar]
- Merten, M; Thiagarajan, P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation 2000, 102, 1931–1936. [Google Scholar]
- Gaarder, A; Jonsen, A; Laland, S; Hellem, AJ; Owren, P. Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature 1961, 192, 531–532. [Google Scholar]
- Puri, RN; Colman, R. ADP-induced platelet activation. Crit. Rev. Biochem. Mol. Biol 1997, 32, 437–502. [Google Scholar]
- Wagner, DD; Burger, PC. Platelets in inflammation and thrombosis. Arterioscler Thromb. Vasc. Biol 2003, 23, 2131–2137. [Google Scholar]
- Semenov, AV; Romanov, YA; Loktionova, SA; Tikhomirov, OY; Khachikian, MV; Vasil’ev, SA; Mazurov, AV. Production of soluble P-selectin by platelets and endothelial cells. Biochemistry (Mosc.) 1999, 64, 1326–1335. [Google Scholar]
- Matzdorff, AC; Kemkes-Matthes, B; Voss, R; Pralle, H. Comparison of BTG, flow cytometry and platelet aggregometry to study platelet activation. Haemostasis 1996, 26, 98–106. [Google Scholar]
- Livak, KJ; Schmittgen, TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar]
- Thisse, C; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc 2008, 3, 59–69. [Google Scholar]

© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, G.; Pan, J.; Liu, K.; Wang, X.; Wang, S. Molecular Cloning and Expression Analysis of P-Selectin from Zebrafish (Danio rerio). Int. J. Mol. Sci. 2010, 11, 4618-4630. https://doi.org/10.3390/ijms11114618
Sun G, Pan J, Liu K, Wang X, Wang S. Molecular Cloning and Expression Analysis of P-Selectin from Zebrafish (Danio rerio). International Journal of Molecular Sciences. 2010; 11(11):4618-4630. https://doi.org/10.3390/ijms11114618
Chicago/Turabian StyleSun, Guijin, Jie Pan, Kechun Liu, Xue Wang, and Sifeng Wang. 2010. "Molecular Cloning and Expression Analysis of P-Selectin from Zebrafish (Danio rerio)" International Journal of Molecular Sciences 11, no. 11: 4618-4630. https://doi.org/10.3390/ijms11114618



