Supercritical Carbon Dioxide Extraction of Bioactive Compounds from Ampelopsis grossedentata Stems: Process Optimization and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of SC-CO2 Extraction
2.2. Effects of Various Pressures
2.3. Effects of Various Temperatures
2.4. Effects of Various Dynamic Times
2.5. Effects of Various Modifiers
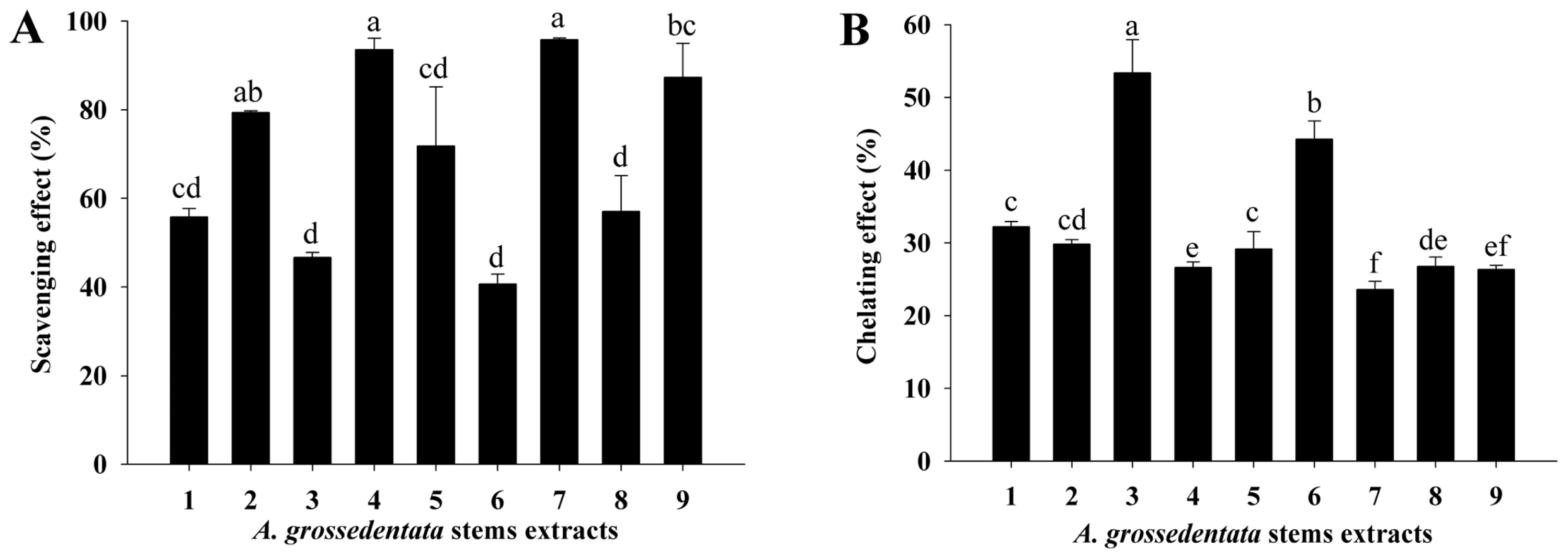
2.6. DPPH Radical Scavenging Activity
2.7. Ferrous Ion Chelating Activity
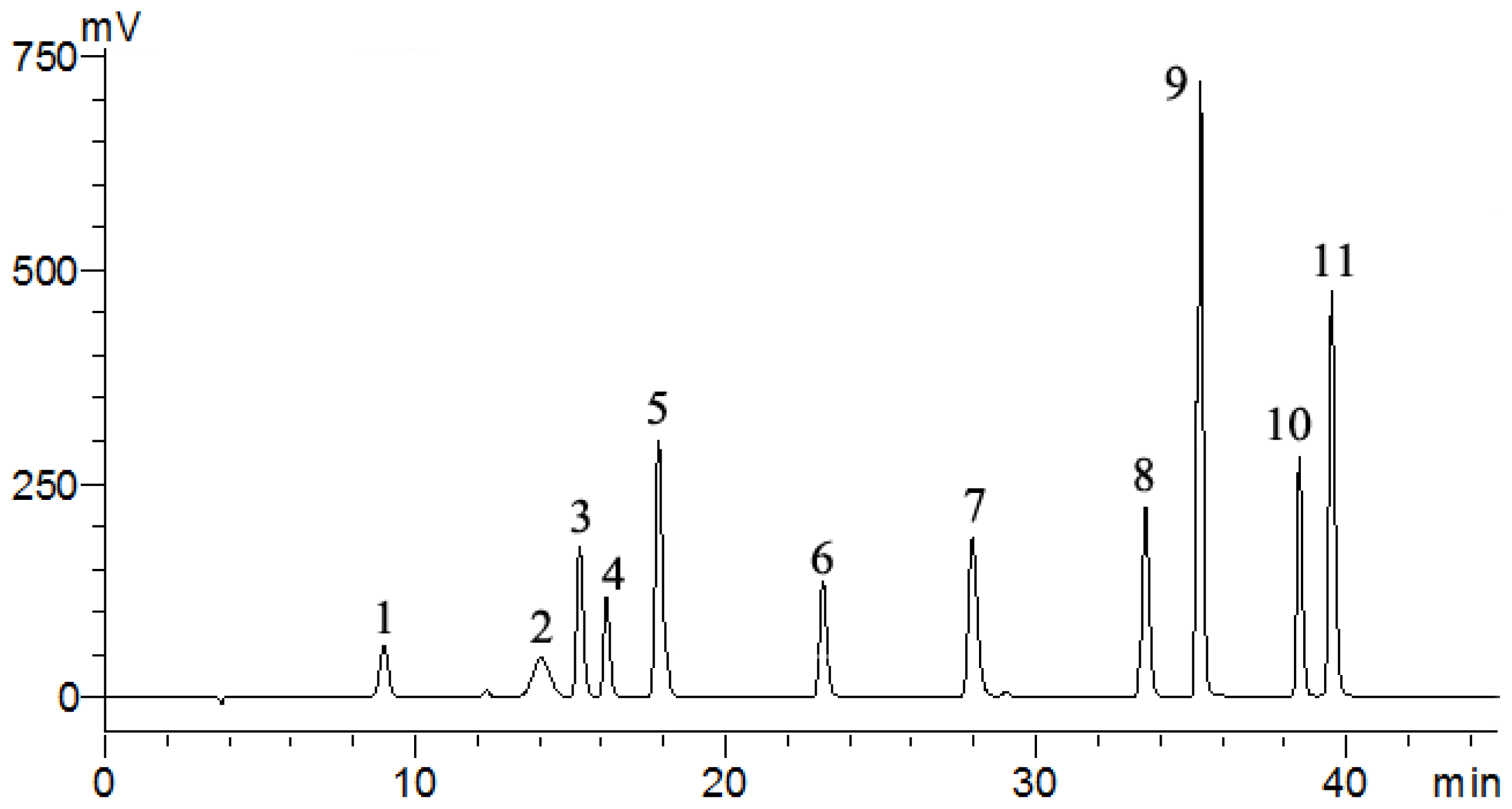
2.8. Quantification of the Main Flavonoids
3. Experimental Section
3.1. Materials
3.2. Chemicals
3.3. SC-CO2 Extraction
3.4. Determination of TFC of the Extracts
3.5. Determination of TPC of the Extracts
3.6. DPPH Free Radical Scavenging Activity
3.7. Chelating Effect on Ferrous Ion
3.8. High performance liquid chromatography (HPLC) analysis
3.9. Statistical Analysis
4. Conclusions
- Conflict of InterestThe authors declare no conflict of interest.
References
- Valko, M; Leibfritz, D; Moncol, J; Cronin, MTD; Mazur, M; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol 2007, 39, 44–84. [Google Scholar]
- Dalle-Donne, I; Rossi, R; Colombo, R; Giustarini, D; Milzani, A. Biomarkers of oxidative damage in human disease. Clin.Chem 2006, 52, 601–623. [Google Scholar]
- Gan, CY; Latiff, AA. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem 2010, 124, 1277–1283. [Google Scholar]
- Javanmardi, J; Stushnoff, C; Locke, E; Vivanco, J. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem 2003, 83, 547–550. [Google Scholar]
- Ozsoy, N; Can, A; Yanardag, R; Akev, N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem 2008, 110, 571–583. [Google Scholar]
- Gao, JH; Liu, BG; Ning, ZX; Zhao, RX; Zhang, AY; Wu, Q. Characterization and antioxdiant activity of flavonoid-rich extracts from leaves of Ampelopsis grossedentata. J. Food Biochem 2009, 33, 808–820. [Google Scholar]
- Du, Q; Chen, P; Jerz, G; Winterhalter, P. Preparative separation of flavonoid glycosides in leaves extract of Ampelopsis grossedentata using high-speed counter-current chromatography. J. Chromatogr. A 2004, 1040, 147–149. [Google Scholar]
- Du, Q; Cai, W; Xia, M; Ito, Y. Purification of (+)-dihydromyricetin from leaves extract of Ampelopsis grossedentata using high-speed countercurrent chromatograph with scale-up triple columns. J. Chromatogr. A 2002, 973, 217–220. [Google Scholar]
- He, G-X; Pei, G; Yang, WL; Li, B. Determination of dihydromyricetin in different parts of Ampelopsis grossedentata in different seasons by HPLC. Chin. Trad. Pat. Med 2004, 26, 210–212. [Google Scholar]
- Liza, MS; Abdul Rahman, RA; Mandana, B; Jinap, S; Rahmat, A; Zaidul, I; Hamid, A. Supercritical carbon dioxide extraction of bioactive flavonoid from Strobilanthes crispus (Pecah Kaca). Food Bioprod. Process 2010, 88, 319–326. [Google Scholar]
- Li, B; Xu, Y; Jin, YX; Wu, YY; Tu, YY. Response surface optimization of supercritical fluid extraction of kaempferol glycosides from tea seed cake. Ind. Crop. Prod 2010, 32, 123–128. [Google Scholar]
- Liu, W; Fu, YJ; Zu, YG; Tong, MH; Wu, N; Liu, XL; Zhang, S. Supercritical carbon dioxide extraction of seed oil from Opuntia dillenii Haw. and its antioxidant activity. Food Chem 2009, 114, 334–339. [Google Scholar]
- Wong, V; Wyllie, SG; Cornwell, CP; Tronson, D. Supercritical fluid extraction (SFE) of monoterpenes from the leaves of Melaleuca alternifolia (Tea Tree). Molecules 2001, 6, 92–103. [Google Scholar]
- Sajfrtová, M; Ličková, I; Wimmerová, M; Sovová, H; Wimmer, Z. β-sitosterol: supercritical carbon dioxide extraction from sea buckthorn (Hippopha rhamnoides L.) seeds. Int. J. Mol. Sci 2010, 11, 1842–1850. [Google Scholar]
- Bimakr, M; Rahman, RA; Taip, FS; Ganjloo, A; Salleh, LM; Selamat, J; Hamid, A; Zaidul, I. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process 2011, 89, 67–72. [Google Scholar]
- Lang, Q; Wai, CM. Supercritical fluid extraction in herbal and natural product studies-a practical review. Talanta 2001, 53, 771–782. [Google Scholar]
- Mantell, C; Rodriguez, M; Martinez de la Ossa, E. A screening analysis of the high-pressure extraction of anthocyanins from red grape pomace with carbon dioxide and cosolvent. Eng. Life Sci 2003, 3, 38–42. [Google Scholar]
- Vatai, T; Skerget, M; Knez, Z. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng 2009, 90, 246–254. [Google Scholar]
- Laroze, LE; Diaz-Reinoso, B; Moure, A; Zuniga, ME; Dominguez, H. Extraction of antioxidants from several berries pressing wastes using conventional and supercritical solvents. Eur. Food Res. Technol 2010, 231, 669–677. [Google Scholar]
- Liu, S; Yang, F; Zhang, C; Ji, H; Hong, P; Deng, C. Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology. J. Supercrit. Fluids 2009, 48, 9–14. [Google Scholar]
- Liu, B; Shen, B; Guo, F; Chang, Y. Optimization of supercritical fluid extraction of dl-tetrahydropalmatine from rhizome of Corydalis yanhusuo WT Wang with orthogonal array design. Sep. Purif. Technol 2008, 64, 242–246. [Google Scholar]
- Ak, T; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact 2008, 174, 27–37. [Google Scholar]
- Sharififar, F; Dehghn-Nudeh, G; Mirtajaldini, M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem 2009, 112, 885–888. [Google Scholar]
- AbouL-Enein, AM; El-Baz, FK; El-Baroty, GS; Youssef, A; El-Baky, HHA. Antioxidant activity of algal extracts on lipid peroxidation. J. Med. Sci 2003, 3, 87–98. [Google Scholar]
- Ebrahimzadeh, MA; Pourmorad, F; Bekhradnia, AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr. J. Biotechnol 2008, 7, 3188–3192. [Google Scholar]
- Radovanovic, A; Radovanovic, B; Jovancicevic, B. Free radical scavenging and antibacterial activities of southern Serbian red wines. Food Chem 2009, 117, 326–331. [Google Scholar]
- Mira, L; Tereza Fernandez, MT; Santos, M; Rocha, R; Helena Florêncio, M; Jennings, KR. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radical Res 2002, 36, 1199–1208. [Google Scholar]
- Ebrahimzadeh, MA; Nabavi, SM; Nabavi, SF. Correlation between the in vitro iron chelating activity and polyphenol and flavonoid contents of some medicinal plants. Pak. J. Biol. Sci 2009, 12, 934–938. [Google Scholar]
- Kim, IS; Yang, MR; Lee, OH; Kang, SN. Antioxidant activities of hot water extracts from various spices. Int. J. Mol. Sci 2011, 12, 4120–4131. [Google Scholar]
- Meda, A; Lamien, CE; Romito, M; Millogo, J; Nacoulma, OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 2005, 91, 571–577. [Google Scholar]
- Liu, W; Yu, Y; Yang, R; Wan, C; Xu, B; Cao, S. Optimization of total flavonoid compound extraction from Gynura medica leaf using response surface methodology and chemical composition analysis. Int. J. Mol. Sci 2010, 11, 4750–4763. [Google Scholar]
- Wang, J; Zhang, Q; Zhang, Z; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol 2008, 42, 127–132. [Google Scholar]


| Factors | Levels
| ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Pressure (bar) | 150 | 200 | 250 |
| Temperature (°C) | 40 | 50 | 60 |
| Dynamic time (min) | 30 | 50 | 70 |
| Modifier (methanol: ethanol, v/v) | 1:3 | 1:1 | 3:1 |
| Trial | Pressure (A) | Temperature (B) | Dynamic Time (C) | Modifier (D) | TFC (mg RE/g Dry Material) a | TPC (mg GAE/g Dry Material) a |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 2.56 ± 0.03 cd | 0.53 ± 0.02 c |
| 2 | 1 | 2 | 2 | 2 | 2.62 ± 0.17 cd | 0.73 ± 0.04 c |
| 3 | 1 | 3 | 3 | 3 | 2.20 ± 0.86 cd | 0.39 ± 0.03 c |
| 4 | 2 | 1 | 2 | 3 | 3.40 ± 0.33 abc | 1.55 ± 0.41 b |
| 5 | 2 | 2 | 3 | 1 | 3.60 ± 0.19 abc | 0.53 ± 0.17 c |
| 6 | 2 | 3 | 1 | 2 | 1.60 ± 0.32 d | 0.35 ± 0.04 c |
| 7 | 3 | 1 | 3 | 2 | 4.67 ± 0.36 a | 2.49 ± 0.10 a |
| 8 | 3 | 2 | 1 | 3 | 2.97 ± 0.20 bcd | 0.57 ± 0.03 c |
| 9 | 3 | 3 | 2 | 1 | 4.24 ± 0.01 ab | 0.84 ± 0.11 c |
| TFC (mg RE/g Dry Material)
| TPC (mg GAE/g Dry Material)
| |||||||
|---|---|---|---|---|---|---|---|---|
| Pressure (A) | Temperature (B) | Dynamic Time (C) | Modifier (D) | Pressure (A) | Temperature (B) | Dynamic Time (C) | Modifier (D) | |
| K1 | 7.37 a | 10.63 | 7.12 | 10.39 | 1.65 | 4.57 | 1.45 | 1.90 |
| K2 | 8.60 | 9.18 | 10.26 | 8.88 | 2.44 | 1.84 | 3.13 | 3.57 |
| K3 | 11.87 | 8.03 | 10.46 | 8.57 | 3.90 | 1.58 | 3.41 | 2.51 |
| k1 | 2.46 b | 3.54 | 2.37 | 3.46 | 0.55 | 1.52 | 0.48 | 0.63 |
| k2 | 2.87 | 3.06 | 3.42 | 2.96 | 0.81 | 0.61 | 1.04 | 1.19 |
| k3 | 3.96 | 2.68 | 3.49 | 2.86 | 1.30 | 0.53 | 1.14 | 0.84 |
| R | 4.50 c | 2.59 | 3.34 | 1.81 | 2.25 | 2.98 | 1.96 | 1.67 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| TFC | |||||
| Corrected Model a | 21.341 | 8 | 2.667 | 17.180 | 0.000 |
| Pressure | 9.638 | 2 | 4.819 | 31.040 | 0.000 |
| Temperature | 2.745 | 2 | 1.373 | 8.840 | 0.002 |
| Dynamic time | 6.500 | 2 | 3.250 | 20.930 | 0.000 |
| Modifier | 2.458 | 2 | 1.229 | 7.910 | 0.003 |
| TPC | |||||
| Corrected Model b | 9.911 | 8 | 1.239 | 19.700 | 0.000 |
| Pressure | 3.635 | 2 | 1.817 | 28.900 | 0.000 |
| Temperature | 3.945 | 2 | 1.972 | 31.370 | 0.000 |
| Dynamic time | 1.716 | 2 | 0.858 | 13.64 | 0.000 |
| Modifier | 0.616 | 2 | 0.308 | 4.890 | 0.020 |
| TFC | TPC | DPPH | FIC | |
|---|---|---|---|---|
| TFC | 1.000 | 0.710 ** | 0.674 ** | −0.740 ** |
| TPC | 1.000 | 0.850 * | −0.568 * | |
| DPPH | 1.000 | −0.677 ** |
| Dihydromyricetin | Vitexin-2″-O-rhamnoside | Vitexin | Rutin | Quercetin-3-galactoside | Quercitrin | Myricetin | Luteolin | Quercetin | Apigenin | Kaempferol | Total Content | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 b | 210.01 ± 8.33 de | - | 1.12 ± 0.01 c | - | - | - | 0.43 ± 0.11 c | - | - | 0.16 ± 0.02 b | 0.59 ± 0.02 c | 212.31 ± 8.48 de |
| 2 | 386.23 ± 31.04 d | - | 0.23 ± 0.07 e | 3.03 ± 0.31 ab | - | - | 0.68 ± 0.06 c | 0.12 ± 0.00 d | 0.05 ± 0.00 ab | 0.28 ± 0.02 b | 0.92 ± 0.01 b | 391.53 ± 31.49 d |
| 3 | 43.88±16.55 e | - | 0.27 ± 0.03 de | 2.46 ± 0.13 bc | - | - | - | 0.12 ± 0.00 d | 0.06 ± 0.00 ab | 0.28 ± 0.01 b | 1.11 ± 0.01 a | 48.16 ± 16.43 e |
| 4 | 1476.48 ± 38.08 b | 0.34 ± 0.14 abc | 0.45 ± 0.07 ed | 3.83 ± 0.11 a | 0.18 ± 0.06 b | 0.74 ± 0.06 a | 2.83 ± 0.34 b | 0.45 ± 0.16 b | 0.05 ± 0.00 ab | 1.46 ± 0.14 a | 0.86 ± 0.03 b | 1488.66 ± 39.48 b |
| 5 | 436.20 ± 3.68 d | 0.32 ± 0.18 bc | 0.56 ± 0.18 d | 3.70 ± 0.86 a | - | - | - | 0.17 ± 0.02 cd | 0.05 ± 0.01 ab | 1.63 ± 0.12 a | - | 442.62 ± 2.29 d |
| 6 | 34.41 ± 5.49 e | - | - | 1.70 ± 0.05 c | - | - | - | 0.15 ± 0.04 cd | 0.04 ± 0.01 b | 1.54 ± 0.06 a | - | 37.84 ± 5.42 e |
| 7 | 2534.42 ± 195.93 a | 0.51 ± 0.00 ab | 2.31 ± 0.00 b | - | 0.39 ± 0.03 a | 0.39 ± 0.03 b | 8.68 ± 0.35 a | 1.15 ± 0.06 a | 0.04 ± 0.00 b | 1.07 ± 0.39 a | 0.21 ± 0.08 d | 2550.48 ± 196.73 a |
| 8 | 412.01 ± 27.92 d | 0.47 ± 0.13 ab | 2.07 ± 0.00 b | - | - | - | 0.93 ± 0.10 c | 0.22 ± 0.07 bcd | 0.05 ± 0.01 ab | 0.27 ± 0.02 b | 0.90 ± 0.02 b | 416.94 ± 28.23 d |
| 9 | 808.13 ± 40.73 c | 0.69 ± 0.03 a | 3.16 ± 0.14 a | - | - | - | 1.16 ± 0.24 c | 0.41 ± 0.05 bc | 0.07 ± 0.00 a | 0.35 ± 0.03 b | 0.95 ± 0.08 ab | 814.92 ± 40.22 c |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.; Ying, L.; Sun, D.; Zhang, S.; Zhu, Y.; Xu, P. Supercritical Carbon Dioxide Extraction of Bioactive Compounds from Ampelopsis grossedentata Stems: Process Optimization and Antioxidant Activity. Int. J. Mol. Sci. 2011, 12, 6856-6870. https://doi.org/10.3390/ijms12106856
Wang Y, Ying L, Sun D, Zhang S, Zhu Y, Xu P. Supercritical Carbon Dioxide Extraction of Bioactive Compounds from Ampelopsis grossedentata Stems: Process Optimization and Antioxidant Activity. International Journal of Molecular Sciences. 2011; 12(10):6856-6870. https://doi.org/10.3390/ijms12106856
Chicago/Turabian StyleWang, Yuefei, Le Ying, Da Sun, Shikang Zhang, Yuejin Zhu, and Ping Xu. 2011. "Supercritical Carbon Dioxide Extraction of Bioactive Compounds from Ampelopsis grossedentata Stems: Process Optimization and Antioxidant Activity" International Journal of Molecular Sciences 12, no. 10: 6856-6870. https://doi.org/10.3390/ijms12106856




