Different Reactive Oxygen Species Lead to Distinct Changes of Cellular Metal Ions in the Eukaryotic Model Organism Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Results and Discussion
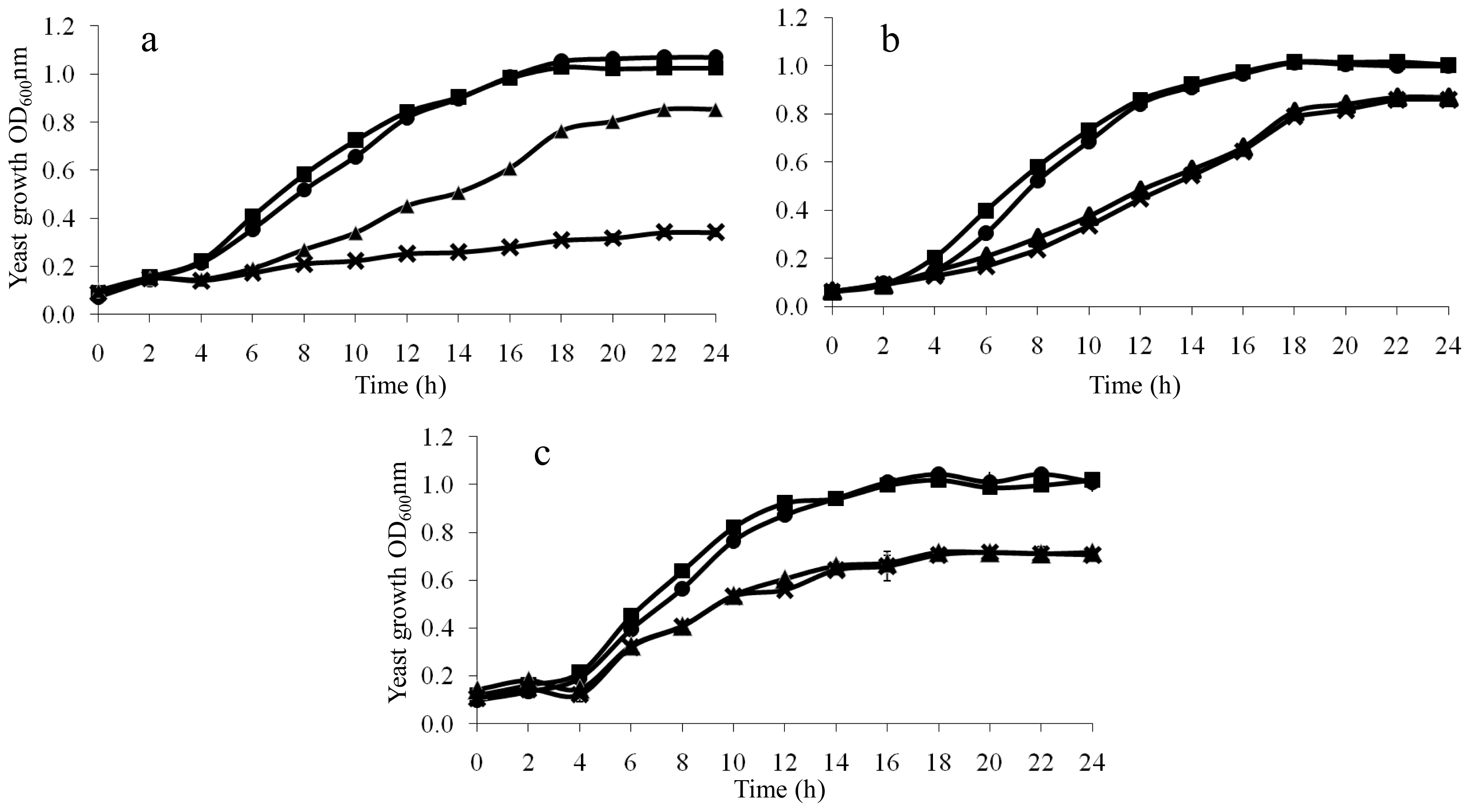
2.1. Determination of Arresting Concentrations of the Oxidants
2.2. Ionomic Profiling Under Seven Individual Oxidants
2.3. Further Validation of the Linkage Between Diamide-Induced Disulfide Stress and Al3+ Accumulation
2.4. Growth Rescue of LAH-Treated Yeast Cells by Additional K+
3. Experimental Section
3.1. Yeast Strain and Culture Conditions
3.2. Oxidants
3.3. Determination of Arresting Concentrations of the Oxidants
3.4. Oxidant Treatment and ICP-AES Analysis
3.5. Characterization of Aluminium Accumulation over a Range of Diamide Concentrations and over a Time Course
3.6. Relationships of Diamide with Al3+, H2O2 with Al3+, and Diamide with Cd2+
3.7. Al3+ Uptake of Yeast Cells in the Presence of Other Elements
4. Conclusions
Acknowledgments
References
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Tainer, J.A.; Roberts, V.A.; Getzoff, E.D. Metal-binding sites in proteins. Curr. Opin. Biotech 1991, 2, 582–591. [Google Scholar]
- Higgins, V.J.; Rogers, P.J.; Dawes, I.W. Application of genome-wide expression analysis to identify molecular markers useful in monitoring industrial fermentations. Appl. Environ. Microbiol 2003, 69, 7535–7540. [Google Scholar]
- Becaria, A.; Lahiri, D.K.; Bondy, S.C.; Chen, D.; Hamadeh, A.; Li, H.; Taylor, R.; Campbell, A. Aluminum and copper in drinking water enhance inflammatory or oxidative events specifically in the brain. J. Neuroimmunol 2006, 176, 16–23. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J 1984, 219, 1–14. [Google Scholar]
- Gasch, A.P.; Spellman, P.T.; Kao, C.M.; Carmel-Harel, O.; Eisen, M.B.; Storz, G.; Botstein, D.; Brown, P.O. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 2000, 11, 4241–4257. [Google Scholar]
- Thorpe, G.W.; Fong, C.S.; Alic, N.; Higgins, V.J.; Dawes, I.W. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: Oxidative-stress-response genes. Proc. Natl. Acad. Sci. USA 2004, 101, 6564–6569. [Google Scholar]
- Eide, D.; Clark, S.; Nair, T.M.; Gehl, M.; Gribskov, M.; Guerinot, M.; Harper, J. Characterization of the yeast ionome: A genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome Biol 2005, 6, R77–R89. [Google Scholar]
- Danku, J.M.C.; Gumaelius, L.; Baxter, I.; Salt, D.E. A high-throughput method for Saccharomyces cerevisiae (yeast) ionomics. J. Anal. Atom. Spectrom 2008, 24, 103–107. [Google Scholar]
- Salt, D.E.; Baxter, I.; Lahner, B. Ionomics and the study of the plant ionome. Annu. Rev. Plant Biol 2008, 59, 709–733. [Google Scholar]
- Flattery-O’Brien, J.; Dawes, I. Hydrogen peroxide causes RAD9-dependent cell cycle arrest in G2 in Saccharomyces cerevisiae whereas menadione causes G1 arrest independent of RAD9 function. J. Biol. Chem 1998, 273, 8564–8571. [Google Scholar]
- Alic, N.; Higgins, V.J.; Dawes, I.W. Lipid peroxidation and the cell cycle in Saccharomyces cerevisiae. Yeast 2001, 18, S190–S190. [Google Scholar]
- Fong, C.S.; Temple, M.D.; Alic, N.; Chiu, J.; Durchdewald, M.; Thorpe, G.; Higgins, V.J.; Dawes, I.W. Oxidant-induced cell-cycle delay in Saccharomyces cerevisiae: The involvement of the SWI6 transcription factor. FEMS Yeast Res 2008, 8, 386–399. [Google Scholar]
- Ng, C.H.; Tan, S.X.; Perrone, G.G.; Thorpe, G.W.; Higgins, V.J.; Dawes, I.W. Adaptation to hydrogen peroxide in Saccharomyces cerevisiae: The role of NADPH-generating systems and the SKN7 transcription factor. Free Radic. Biol. Med 2008, 44, 1131–1145. [Google Scholar]
- Leichert, L.I.O.; Scharf, C.; Hecker, M. Global characterization of disulfide stress in Bacillus subtilis. J. Bacteriol 2003, 185, 1967–1975. [Google Scholar]
- Sweetman, L.L.; Zhang, N.Y.; Peterson, H.; Gopalakrishna, R.; Sevanian, A. Effect of linoleic acid hydroperoxide on endothelial cell calcium homeostasis and phospholipid hydrolysis. Arch. Biochem. Biophys 1995, 323, 97–107. [Google Scholar]
- Rodríguez-Navarro, A.; Rubio, F. High-affinity potassium and sodium transport systems in plants. J. Exp. Bot 2006, 57, 1149–1160. [Google Scholar]
- Bressler, J.P.; Olivi, L.; Cheong, J.H.; Kim, Y.; Maerten, A.; Bannon, D. Metal transporters in intestine and brain: Their involvement in metal-associated neurotoxicities. Hum. Exp. Toxicol 2007, 26, 221–229. [Google Scholar]
- Hirsch, E.C.; Faucheux, B.A. Iron metabolism and Parkinson’s disease. Mov. Disord 1998, 13, 39–45. [Google Scholar]
- Delhaize, E.; Ryan, P.R. Aluminium toxicity and tolerance in plants. Plant Physiol 1995, 107, 315–321. [Google Scholar]
- Becaria, A.; Campbell, A.; Bondy, S.C. Aluminum as a toxicant. Toxicol. Ind. Health 2002, 18, 309–320. [Google Scholar]
- Yumoto, S.; Nagai, H.; Matsuzaki, H.; Matsumura, H.; Tada, W.; Nagatsuma, E.; Kobayashi, K. Aluminium incorporation into the brain of rat fetuses and sucklings. Brain Res. Bull 2001, 55, 229–234. [Google Scholar]
- Exley, C. Aluminium and iron, but neither copper nor zinc, are key to the precipitation of β-sheets of Aβ42 in senile plaque cores in Alzheimer’s disease. J. Alzheimer’s Dis 2006, 10, 173–177. [Google Scholar]
- Walton, J.R. Aluminum in hippocampal neurons from humans with Alzheimer’s disease. Neurotoxicology 2006, 27, 385–394. [Google Scholar]
- Brachmann, C.B.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.C.; Hieter, P.; Boeke, J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998, 14, 115–132. [Google Scholar]
- Wu, M.J.; O’Doherty, P.J.; Fernandez, H.R.; Lyons, V.; Rogers, P.J.; Dawes, I.W.; Higgins, V.J. An antioxidant screening assay based on oxidant-induced growth arrest in Saccharomyces cerevisiae. FEMS Yeast Res 2011, 11, 379–387. [Google Scholar]




© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, M.J.; O’Doherty, P.J.; Murphy, P.A.; Lyons, V.; Christophersen, M.; Rogers, P.J.; Bailey, T.D.; Higgins, V.J. Different Reactive Oxygen Species Lead to Distinct Changes of Cellular Metal Ions in the Eukaryotic Model Organism Saccharomyces cerevisiae. Int. J. Mol. Sci. 2011, 12, 8119-8132. https://doi.org/10.3390/ijms12118119
Wu MJ, O’Doherty PJ, Murphy PA, Lyons V, Christophersen M, Rogers PJ, Bailey TD, Higgins VJ. Different Reactive Oxygen Species Lead to Distinct Changes of Cellular Metal Ions in the Eukaryotic Model Organism Saccharomyces cerevisiae. International Journal of Molecular Sciences. 2011; 12(11):8119-8132. https://doi.org/10.3390/ijms12118119
Chicago/Turabian StyleWu, Ming J., Patrick J. O’Doherty, Patricia A. Murphy, Victoria Lyons, Melinda Christophersen, Peter J. Rogers, Trevor D. Bailey, and Vincent J. Higgins. 2011. "Different Reactive Oxygen Species Lead to Distinct Changes of Cellular Metal Ions in the Eukaryotic Model Organism Saccharomyces cerevisiae" International Journal of Molecular Sciences 12, no. 11: 8119-8132. https://doi.org/10.3390/ijms12118119



