Expression and Clinical Role of Protein of Regenerating Liver (PRL) Phosphatases in Ovarian Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tumors and Patients
2.2. RT-PCR Analysis
2.3. Western Blotting
2.4. Cell Lines and Culture Conditions
2.5. Regulation of PRL-3 Expression
2.6. Statistical Analysis
3. Results
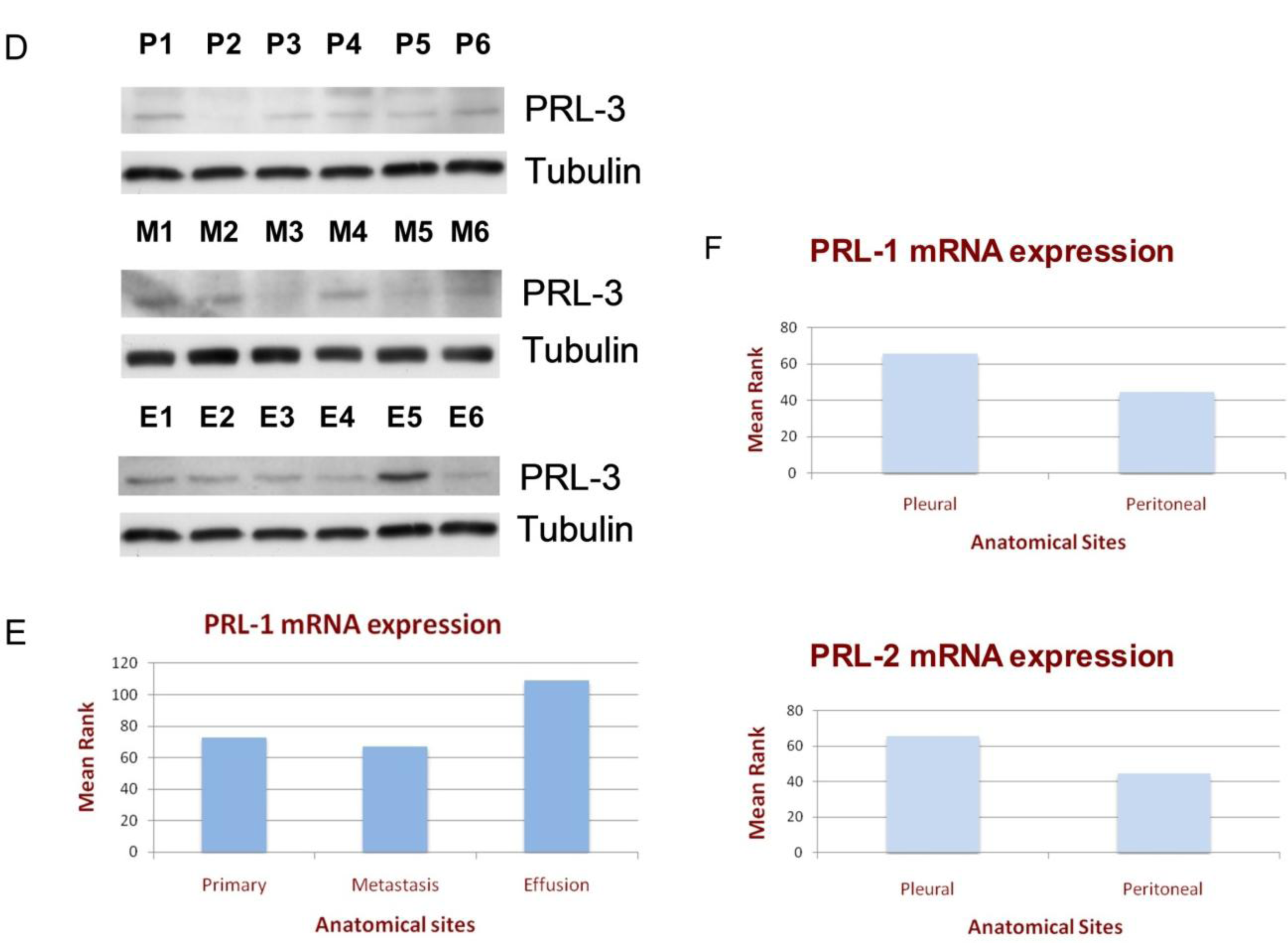
3.1. PRL Enzyme Expression at Various Anatomic Sites
3.2. The Clinical Significance of PRL Enzyme Expression
3.3. PRL-3 Regulation
4. Discussion
5. Conclusion
Acknowledgments
References
- Alonso, A; Sasin, J; Bottini, N; Friedberg, I; Friedberg, I; Osterman, A; Godzik, A; Hunter, T; Dixon, J; Mustelin, T. Protein tyrosine phosphatases in the human genome. Cell 2004, 117, 699–711. [Google Scholar]
- Stoker, AW. Protein tyrosine phosphatases and signaling. J. Endocrinol 2005, 185, 19–33. [Google Scholar]
- van Huijsduijnen, RH; Bombrun, A; Swinnen, D. Selecting protein tyrosine phosphatases as drug targets. Drug Discov. Today 2002, 7, 1013–1019. [Google Scholar]
- Parsons, R. Phosphatases and tumorigenesis. Curr. Opin. Oncol 1998, 10, 88–91. [Google Scholar]
- Mohn, KL; Laz, TM; Hsu, JC; Melby, AE; Bravo, R; Taub, R. The immediate-early growth response in regenerating liver and insulin-stimulated H-35 cells: comparison with serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Mol. Cell Biol 1991, 11, 381–390. [Google Scholar]
- Diamond, RH; Cressman, DE; Laz, TM; Abrams, CS; Taub, R. PRL-1, a unique nuclear protein tyrosine phosphatase, affects cell growth. Mol. Cell Biol 1994, 14, 3752–3762. [Google Scholar]
- Montagna, M; Serova, O; Sylla, BS; Feunteun, J; Lenoir, GM. A 100-kb physical and transcriptional map around the EDH17B2 gene: identification of three novel genes and a pseudogene of a human homologue of the rat PRL-1 tyrosine phosphatase. Hum. Genet 1995, 96, 532–538. [Google Scholar]
- Cates, CA; Michael, RL; Stayrook, KR; Harvey, KA; Burke, YD; Randall, SK; Crowell, PL; Crowell, DN. Prenylation of oncogenic human PTP(CAAX) protein tyrosine phosphatases. Cancer Lett 1996, 110, 49–55. [Google Scholar]
- Zhao, Z; Lee, CC; Monckton, DG; Yazdani, A; Coolbaugh, MI; Li, X; Bailey, J; Shen, Y; Caskey, CT. Characterization and genomic mapping of genes and pseudogenes of a new human protein tyrosine phosphatase. Genomics 1996, 35, 172–181. [Google Scholar]
- Matter, WF; Estridge, T; Zhang, C; Belagaje, R; Stancato, L; Dixon, J; Johnson, B; Bloem, L; Pickard, T; Donaghue, M; et al. Role of PRL-3, a human muscle-specific tyrosine phosphatase, in angiotensin-II signaling. Biochem. Biophys. Res. Commun 2001, 83, 1061–1068. [Google Scholar]
- Zeng, Q; Hong, W; Tan, YH. Mouse PRL-2 and PRL-3, two potentially prenylated protein tyrosine phosphatases homologous to PRL-1. Biochem. Biophys. Res. Commun 1998, 244, 421–427. [Google Scholar]
- Zeng, Q; Dong, JM; Guo, K; Li, J; Tan, HX; Koh, V; Pallen, CJ; Manser, E; Hong, W. PRL-3 and PRL-1 promote cell migration, invasion, and metastasis. Cancer Res 2003, 63, 2716–2722. [Google Scholar]
- Saha, S; Bardelli, A; Buckhaults, P; Velculescu, VE; Rago, C St; Croix, B; Romans, KE; Choti, MA; Lengauer, C; Kinzler, KW; Vogelstein, B. A Phosphatase Associated with Metastasis of Colorectal Cancer. Science 2001, 294, 1343–1346. [Google Scholar]
- Guo, K; Li, J; Tang, JP; Koh, V; Gan, BQ; Zeng, Q. Catalytic domain of PRL-3 plays an essential role in tumor metastasis: formation of PRL-3 tumors inside the blood vessels. Cancer Biol. Ther 2004, 3, 945–951. [Google Scholar]
- Kato, H; Semba, S; Miskad, UA; Seo, Y; Kasuga, M; Yokozaki, H. High expression of PRL-3 promotes cancer cell motility and liver metastasis in human colorectal cancer: a predictive molecular marker of metachronous liver and lung metastases. Clin. Cancer Res 2004, 10, 7318–7328. [Google Scholar]
- Wu, X; Zeng, H; Zhang, X; Zhao, Y; Sha, H; Ge, X; Zhang, M; Gao, X; Xu, Q. Phosphatase of regenerating liver-3 promotes motility and metastasis of mouse melanoma cells. Am. J. Pathol 2004, 164, 2039–2054. [Google Scholar]
- Parker, BS; Argani, P; Cook, BP; Han, L; Chartrand, SD; Zhang, M; Saha, S; Bardelli, A; Jiang, Y; Martin, TB St; et al. Alterations in vascular gene expression in invasive breast carcinoma. Cancer Res 2004, 64, 7857–7866. [Google Scholar]
- Miskad, UA; Semba, S; Kato, H; Yokozaki, H. Expression of PRL-3 phosphatase in human gastric carcinomas: close correlation with invasion and metastasis. Pathobiology 2004, 71, 176–184. [Google Scholar]
- Li, Z; Zhan, W; Wang, Z; Zhu, B; He, Y; Peng, J; Cai, S; Ma, J. Inhibition of PRL-3 gene expression in gastric cancer cell line SGC7901 via microRNA suppressed reduces peritoneal metastasis. Biochem. Biophys. Res. Commun 2006, 348, 229–237. [Google Scholar]
- Jemal, A; Siegel, R; Ward, E; Hao, Y; Xu, J; Murray, T; Thun, MJ. Cancer statistics, 2008. CA Cancer J. Clin 2008, 58, 71–96. [Google Scholar]
- Givant-Horwitz, V; Davidson, B; Goderstad, JM; Nesland, JM; Trope, CG; Reich, R. The PAC-1 dual specificity phosphatase predicts poor outcome in serous ovarian carcinoma. Gynecol. Oncol 2004, 93, 517–523. [Google Scholar]
- Givant-Horwitz, V; Davidson, B; Reich, R. Laminin-induced signaling in tumor cells: the role of the M(r) 67,000 laminin receptor. Cancer Res 2004, 64, 3572–3579. [Google Scholar]
- Guidelines for Amplicon and Primer Design. The primers were designed according to guidelines from Tamar website using Primer 3 (as indicated by Tamar guidelines). Available online: http://www.tamar.co.il/site/item.php?ln=en&item_id=65&main_id=40 (accessed on 10 February 2011).
- Lambert, CA; Colige, AC; Munaut, C; Lapiere, CM; Nusgens, BV. Distinct pathways in the over-expression of matrix metalloproteinases in human fibroblasts by relaxation of mechanical tension. Matrix Biol 2001, 20, 397–408. [Google Scholar]
- Reich, R; Blumenthal, M; Liscovitch, M. Role of phospholipase D in laminin-induced production of gelatinase A (MMP-2) in metastatic cells. Clin. Exp. Metastasis 1995, 13, 134–140. [Google Scholar]
- Williger, BT; Reich, R; Neeman, M; Bercovici, T; Liscovitch, M. Release of gelatinase A (matrix metalloproteinase 2) induced by photolysis of caged phosphatidic acid in HT 1080 metastatic fibrosarcoma cells. J. Biol. Chem 1995, 270, 29656–29659. [Google Scholar]
- Sager, J; Benvenuti, S; Bardelli, A. PRL-3: a phosphatase for metastasis? Cancer Biol. Ther 2004, 3, 952–953. [Google Scholar]
- Polato, F; Codegoni, A; Fruscio, R; Perego, P; Mangioni, C; Saha, S; Bardelli, A; Broggini, M. PRL-3 phosphatase is implicated in ovarian cancer growth. Clin. Cancer Res 2005, 11, 6835–6839. [Google Scholar]
- Ren, T; Jiang, B; Xing, X; Dong, B; Peng, L; Meng, L; Xu, H; Shou, C. Prognostic significance of phosphatase of regenerating liver-3 expression in ovarian cancer. Pathol. Oncol. Res 2009, 15, 555–560. [Google Scholar]
- Elloul, S; Vaksman, O; Stavnes, HT; Trope, CG; Davidson, B; Reich, R. Mesenchymal-to-epithelial transition determinants as characteristics of ovarian carcinoma effusions. Clin. Exp. Metastasis 2010, 27, 161–172. [Google Scholar]
- Wang, H; Quah, SY; Dong, JM; Manser, E; Tang, JP; Zeng, Q. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res 2007, 67, 2922–2926. [Google Scholar]
- Peng, L; Xing, X; Li, W; Qu, L; Meng, L; Lian, S; Jiang, B; Wu, J; Shou, C. PRL-3 promotes the motility, invasion, and metastasis of LoVo colon cancer cells through PRL-3-integrin beta1-ERK1/2 and-MMP2 signaling. Mol. Cancer 2009, 8, 110. [Google Scholar]
- Min, SH; Kim, DM; Heo, YS; Kim, HM; Kim, IC; Yoo, OJ. Downregulation of p53 by phosphatase of regenerating liver 3 is mediated by MDM2 and PIRH2. Life Sci 2010, 86, 66–72. [Google Scholar]



| Parameter | Material (Number of Patients) | |||
|---|---|---|---|---|
| Effusions (90) | Primary (57) | Metastasis (27) | ||
| Age | Mean (range) | 61 (34–79) | 60 (31–84) | 63 (47–80) |
| FIGO Stage | I | 0 | 4 | 2 |
| II | 1 | 5 | 0 | |
| III | 46 | 27 | 16 | |
| IV | 43 | 13 | 9 | |
| NA a | 0 | 8 | 0 | |
| Grade | I | 9 | 9 | 1 |
| II | 21 | 15 | 5 | |
| III b | 51 | 26 | 20 | |
| NA | 9 | 7 | 1 | |
| Residual Disease | ≤1 cm | 32 | 34 | 14 |
| >1 cm | 44 | 14 | 13 | |
| NA | 14 | 9 | 0 | |
| Histology | Serous | 75 | 40 | 19 |
| Mucinous | 1 | 0 | 0 | |
| Clear cell | 4 | 3 | 2 | |
| Endometrioid | 1 | 7 | 3 | |
| Mixed epithelial | 4 | 3 | 0 | |
| Undifferentiated | 2 | 0 | 3 | |
| NA | 3 | 4 | 0 | |
| mRNA | Primer Pairs | Product Size (bp) | Reference |
|---|---|---|---|
| PRL-1 | sense: 5′-GACCTGGATGGGGTAAACCT-3′ | 283 | [23] |
| Antisense: 5′-TGTGACTTCCACAGGAGCTG-3′ | |||
| PRL-2 | Sense: 5′-TTTCCCATCACACTCACACG-3′ | 352 | [23] |
| Antisense:AACACAAGGCACTGCAACAC-3′ | |||
| PRL-3 | Sense: 5′-AGCCCCGTACTTCTTCAGGT-3′ | 198 | [31] |
| Antisense: 5′-GGGACTTCTCAGGTCGTGTC-3′ | |||
| 28s | Sense: 5′-GTTCACCCACTAATAGGGAACGTGA-3′ | 200 | [24] |
| Antisense: 5′-GGATTCTGACTTAGAGGCGTTCAGT-3′ |
| Gene | Heating | Denaturation | Annealing | Extension | No. of Cycles |
|---|---|---|---|---|---|
| PRL-1 | 94 °C, 5 min | 94 °C, 30 s | 60 °C, 30 s | 60 °C, 30 s | 32 |
| PRL-2 | 94 °C, 5 min | 94 °C, 30 s | 60 °C, 30 s | 72 °C, 30 s | 32 |
| PRL-3 | 94 °C, 5 min | 94 °C, 30 s | 58 °C, 1 min | 72 °C, 90 s | 32 |
| 28s | 94 °C, 5 min | 94 °C, 15 s | 63 °C, 20 s | 72 °C, 10 s | 31 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reich, R.; Hadar, S.; Davidson, B. Expression and Clinical Role of Protein of Regenerating Liver (PRL) Phosphatases in Ovarian Carcinoma. Int. J. Mol. Sci. 2011, 12, 1133-1145. https://doi.org/10.3390/ijms12021133
Reich R, Hadar S, Davidson B. Expression and Clinical Role of Protein of Regenerating Liver (PRL) Phosphatases in Ovarian Carcinoma. International Journal of Molecular Sciences. 2011; 12(2):1133-1145. https://doi.org/10.3390/ijms12021133
Chicago/Turabian StyleReich, Reuven, Shany Hadar, and Ben Davidson. 2011. "Expression and Clinical Role of Protein of Regenerating Liver (PRL) Phosphatases in Ovarian Carcinoma" International Journal of Molecular Sciences 12, no. 2: 1133-1145. https://doi.org/10.3390/ijms12021133




