Infrared Assisted Production of 3,4-Dihydro-2(1H)-pyridones in Solvent-Free Conditions
Abstract
:1. Introduction
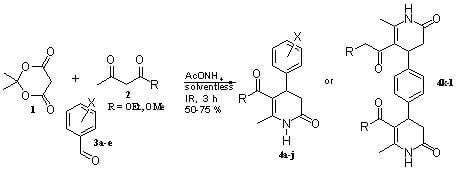
2. Results and Discussion
3. Experimental Section
4-(4’-Fluorophenyl)-5-methoxycarbonyl-6-methyl-3,4-dihydro-2(1H)-pyridone (4c)
5-Ethoxycarbonyl 4-(4’-fluorophenyl)-6-methyl-3,4-dihydro-2(1H)-pyridone (4d)
6-Methyl-4-(2’-methylphenyl)-5-methoxycarbonyl-3,4-dihydro-2(1H)-pyridone (4g)
5-Ethoxycarbonyl-6-methyl-4-(2’-methylphenyl)-3,4-dihydro-2(1H)-pyridone (4h)
Phenylene-1’,4’-di-(4-(6-methyl-5-methoxycarbonyl-3,4-dihydro-2(1H)-pyridone)) (4k)
Phenylene-1’,4’-di-(4-(5-ethoxycarbonyl-6-methyl-3,4-dihydro-2(1H)-pyridone)) (4l)
4. Conclusions
Acknowledgments
References
- Tundo, P; Anastas, P; Black, DC; Breen, J; Collins, T; Memoli, S; Miyamoto, J; Polyakoff, M; Tumas, W. Synthetic pathways and processes in green chemistry. Introductory overview. Pure Appl. Chem 2000, 72, 1207–1228. [Google Scholar]
- Anastas, PT; Williamson, TC. Green Chemistry, Frontiers in Benign Chemical Syntheses and Processes; Oxford University Press: Great Britain, UK, 1998; p. 2. [Google Scholar]
- Wender, PA; Handy, ST; Wright, DL. Towards the ideal synthesis. Chem Ind 1997, 765–769. [Google Scholar]
- Zhu, J; Bienaymé, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Pihlaja, K; Seilo, M. The acidity and general base catalyzed hydrolysis of Meldrum’s acid and its methyl derivates. Acta Chem. Scand 1969, 23, 3003–3010. [Google Scholar]
- Ochoa, E; Suárez, M; Verdecia, Y; Pita, B; Matín, N; Quinteiro, M; Seoane, C; Soto, JL; Duque, J; Pomés, R. Structural study of 3,4-dihydropyridones and furo[3,4-b]-2(1H)-pyridones as potentional calcium channel modulators. Tetrahedron 1998, 54, 12409–12420. [Google Scholar]
- Smith, D; Sammes, PG. Comprehensive Organic Chemistry; Pergamon: Oxford, UK, 1979; Volume 4, p. 3. [Google Scholar]
- Bayley, T; Goe, G; Scriven, E. Heterocyclic Compounds; Newkome, GRW, Ed.; Wiley: New York, NY, USA, 1984; Volume 14, p. 1. [Google Scholar]
- McKillop, A. Comprehensive Heterocyclic Chemistry; McKillop, A, Boulton, A, Eds.; Pergamon: Oxford, UK, 1984; Volume 2, p. 67. [Google Scholar]
- Bristol, JA; Sircar, I; Moos, WH; Evans, DB; Weishaar, RE. Cardiotonic agents 1.4,5-dyhidro-6-[4-(1H-imidazol-1-yl)phenyl]-3(2H)-pyridazinones: Novel positive inotropic agents for the treatment of congestive heart failure. J. Med. Chem 1984, 27, 1099–1101. [Google Scholar]
- Lemke, JV. New inotropic drugs: amrinone and milrinone. J. Med. Assoc. Ga 1987, 76, 854–857. [Google Scholar]
- Iven, H; Brasch, M; Armah, BI. Electrophysiologic effects of saterinone and milrinone in the isolated guinea pig myocardium. Arneim. Forcg. Drugs Res 1988, 38, 1298–1302. [Google Scholar]
- Schultz, AG. Camptothecin. Chem. Rev 1973, 73, 385–405. [Google Scholar]
- Kelly, TR; Bell, SH; Osashi, N; Armstrong-Chong, RJ. Synthesis of (+)-fredericamycin A. J. Am. Chem. Soc 1988, 110, 6471–6480. [Google Scholar]
- Curran, DP; Liu, H. New 4 + 1 radical annulations. A formal total synthesis of (+)-camptothecin. J. Am. Chem. Soc 1992, 114, 5863–5864. [Google Scholar]
- Cox, RJ; O’Hagan, D. Synthesis of isotopically labelled 3-amino-2-phenylpropionic acid and its role as a precursor in the biosynthesis of tenellin and tropic acid. J. Chem. Soc. Perkin Trans 1991, 1, 2537–2540. [Google Scholar]
- Williams, D; Lowder, P; Gu, YG. Studies toward funiculosin. Intramolecular carbonyl condensations using carboxamidimidazolide intermediates. Tetrahedron Lett 1997, 38, 327–330. [Google Scholar]
- Kozikowski, AP; Campiani, G; Sun, LQ; Wang, S; Saxena, A; Doctor, BP. Identification of a more potent analogue of the naturally occurring alkaloid Huperzine a. Predictive molecular modeling of its interactions with ACLE. J. Am. Chem. Soc 1996, 118, 11357–11362. [Google Scholar]
- Brickner, S. Multidrug-resistant bacterial infections: Driving the search for new antibiotics. Chem Ind 1997, 131–135. [Google Scholar]
- Murray, T; Zimmerman, S. 7-amido-1,8-naphthyridines as hydrogen bonding units for the complexation of guanine derivates. The role of 2-alkoxyl groups in decreasing binding affinity. Tetrahedron Lett 1995, 36, 7627–7630. [Google Scholar]
- Jones, G; Katritzky, A; Rees, CW; Scriven, EF. Comprehensive Heterocyclic Chemistry II; Pergamon Press: Oxford, UK, 1996. [Google Scholar]
- Mohrle, H; Weber, H. Oxidation von 1-methyl-und 1,3-dimethylpyridium salzen: überpyridone—I. Tetrahedron 1970, 26, 2953–2958. [Google Scholar]
- Jones, G. The Knoevenagel condensation. Org. React 1967, 15, 204–582. [Google Scholar]
- Alberola, A; Calvo, LA; González-Ortega, A; Sañudo Ruíz, MC; Yustos, P; García, S; García-Rodríguez, E. Regioselective synthesis of 2(1H)-pyridones from β–aminoenones and malonitrile. Reaction mechanism. J. Org. Chem 1999, 64, 9493–9498. [Google Scholar]
- Brun, E; Gil, S; Mestres, R; Parra, M. A new synthetic method to 2-pyridones. Synthesis 2000, 2000, 273–280. [Google Scholar]
- Paulvannan, K; Chen, T. Solid-phase synthesis of 1,2,3,4-tetrahydro-2 pyridones via aza-annulation of enamines. J. Org. Chem 2000, 65, 6160–6166. [Google Scholar]
- Gómez-Pliego, R; Ramírez-San, JE; Miranda, R; Villalobos-Molina, R; Delgado, F; Osnaya, R; Trujillo-Ferrara, JG. Vasodilator effects of bis-dihydropyridines structurally related to nifedipine. Med. Chem 2006, 2, 527–534. [Google Scholar]
- Velasco-Bejarano, B; Trujillo-Ferrara, J; Miranda, R. Preparation of apoptotic inducer, 2,2-diphrnyl-1,3,2-oxazaborolidin-5-ones, under alkaline conditions. Synlett 2007, 6, 921–924. [Google Scholar]
- Velasco, B; Trujillo-Ferrara, JG; Fabila Castillo, LH; Miranda, R; Sánchez-Torres, LE. In vitro apoptotic activity of 2,2-diphenyl-1,3,2-oxazaborolidin-5-ones in L58178Y cells. Life Sci 2007, 80, 1007–1013. [Google Scholar]
- Noguez, O; García, A; Ibarra, C; Cabrera, A; Aceves, JM; Miranda, R. Green synthesis of bis-Biginelli esters, with vasodilatory effects, their mass spectrometric and physical. Trends Org. Chem 2009, 13, 75–82. [Google Scholar]
- Miranda, R; Noguez, O; Velasco, B; Arroyo, G; Penieres, G; Martínez, JO; Delgado, F. Irradiación infrarroja una alternativa para la activación de reacciones y su contribución a la química verde. Educ. Quím 2009, 20, 421–425. [Google Scholar]
- Suárez, M; Verdecia, Y; Ochoa, E; Salfrán, E; Morán, L; Martín, N; Martínez, R; Quinteiro, M; Seoane, C; Soto, JL; Novoa, H; Blaton, N; Peeters, OM; de Ranter, C. Synthesis and structural study of 3,4-dihydro-2(1H)-pyridones and isoxazolo[5,4-b]pyridin-6(7H)-ones. Eur J Org Chem 2000, 2079–2088. [Google Scholar]
- Martínez, R; Suárez, M; Martín, N; Ochoa, E; Seoane, C; Verdecia, Y. Mass spectral fragmentation patterns of new 5-acetyl-4-aryl-6-methyl-2(1H) pyridones. Rapid Commun. Mass Espectrom 1999, 13, 2180–2182. [Google Scholar]
- Suárez, M; Martínez-Álvarez, R; Martín, N; Verdecia, Y; Ochoa, E; Alba, L; Seoane, C; Kayali, N. Electrospray ionisation and ion-trap fragmentation of substituted 3,4-dihydro-2(1H)-pyridin-2-ones. Rapid Commun. Mass Espectrom 2002, 16, 740–754. [Google Scholar]
- Molero, D; Suárez, M; Martínez-Álvarez, R; Verdecia, Y; Martín, N; Seoane, C; Ochoa, E. 1H and 13C spectral assignment of 2(1H)-pyridin-2-ones derivates. Magn. Reson. Chem 2004, 42, 704–708. [Google Scholar]
- Morales, A; Ochoa, E; Suárez, M; González, L; Verdecia, Y; Martín, N; Quinteiro, M; Seoane, C; Soto, JL. Novel hexahydrofuro [3,4-b]-2(1H)-pyridones from 4-aryl substituted 5-alkoxycarbonyl-6-methyl-3,4-dihydropyridones. J. Heterocyclic Chem 1996, 33, 103–107. [Google Scholar]


| Compound | Melting point[b] (°C) | Yield[c]% |
|---|---|---|
| 4a | 197–198(197–198) [12,28] | 75 |
| 4b | 168–171 | 75 |
| 4c | 206–208(207–209) [12,28] | 75 |
| 4d | 184–186(184–186) [29] | 70 |
| 4e | 200–202(201–201) [29] | 75 |
| 4f | 130–133 | 60 |
| 4g | oil | 70 |
| 4h | oil | 60 |
| 4i | 180–183 | 55 |
| 4j | 185–188 | 50 |
| 4k | oil | 65 |
| 4l | oil | 59 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Noguez, M.O.; Marcelino, V.; Rodríguez, H.; Martín, O.; Martínez, J.O.; Arroyo, G.A.; Pérez, F.J.; Suárez, M.; Miranda, R. Infrared Assisted Production of 3,4-Dihydro-2(1H)-pyridones in Solvent-Free Conditions. Int. J. Mol. Sci. 2011, 12, 2641-2649. https://doi.org/10.3390/ijms12042641
Noguez MO, Marcelino V, Rodríguez H, Martín O, Martínez JO, Arroyo GA, Pérez FJ, Suárez M, Miranda R. Infrared Assisted Production of 3,4-Dihydro-2(1H)-pyridones in Solvent-Free Conditions. International Journal of Molecular Sciences. 2011; 12(4):2641-2649. https://doi.org/10.3390/ijms12042641
Chicago/Turabian StyleNoguez, M. Olivia, Vanessa Marcelino, Hortensia Rodríguez, Osnieski Martín, Joel O. Martínez, Gabriel A. Arroyo, Francisco J. Pérez, Margarita Suárez, and René Miranda. 2011. "Infrared Assisted Production of 3,4-Dihydro-2(1H)-pyridones in Solvent-Free Conditions" International Journal of Molecular Sciences 12, no. 4: 2641-2649. https://doi.org/10.3390/ijms12042641