Comparison of In Situ Polymerization and Solution-Dispersion Techniques in the Preparation of Polyimide/Montmorillonite (MMT) Nanocomposites
Abstract
:1. Introduction
2. Results and Discussion
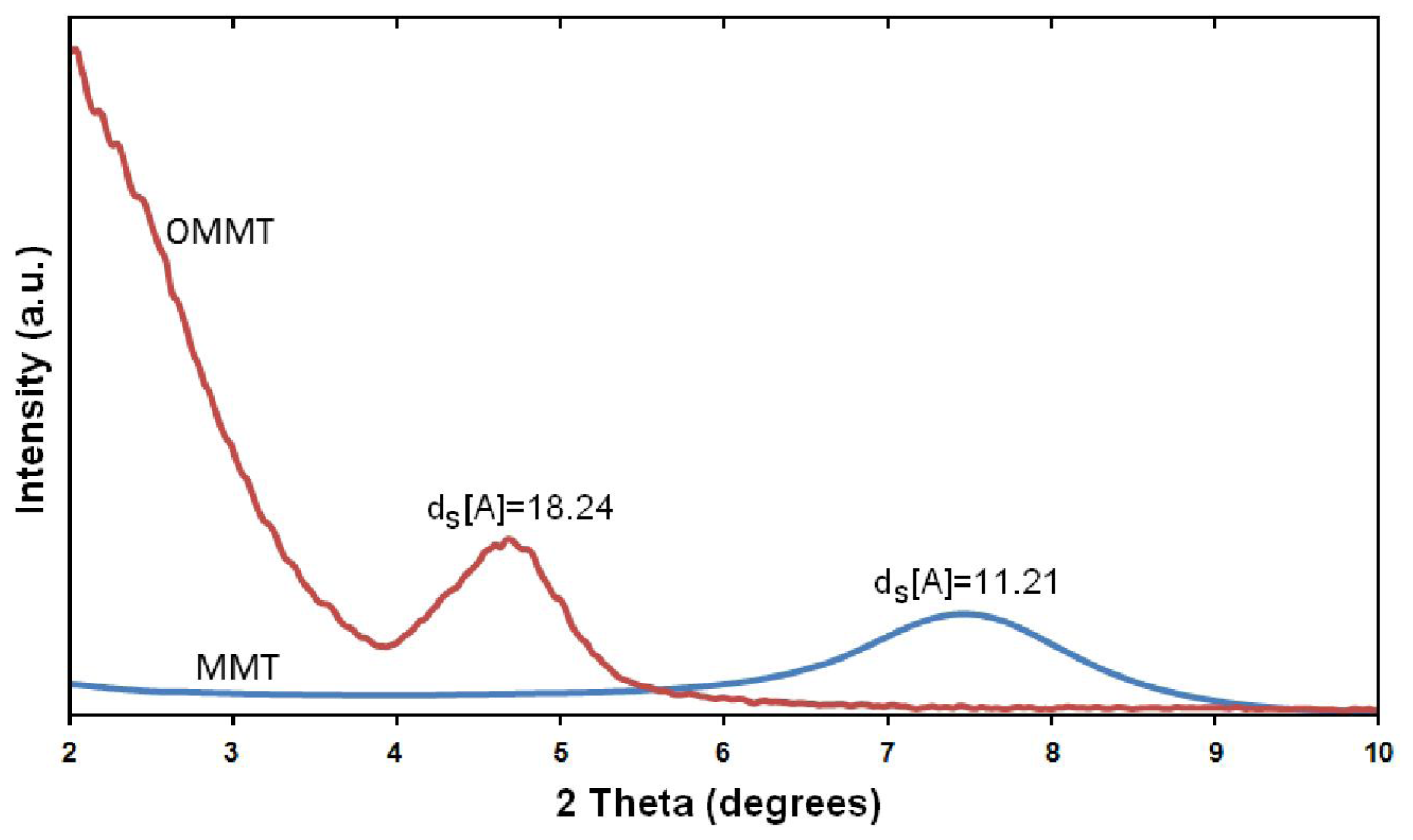
2.1. X-ray Diffraction and Thermogravimetric Analysis (TGA) Study of OMMT Structure
2.2. Chemical Analysis of PAA and PI by Fourier Transform Infrared Spectroscopy
2.3. Chemical Analysis of PI/MMT NC by Fourier Transform Infrared Spectroscopy
2.4. X-ray Diffraction Study of PI/MMT NCs Structure
2.5. Transmission Electron Microscopy (TEM) Images of PI/ MMT NCs Film
2.6. Thermal Properties Study of PI/MMT NCs
3. Experimental
3.1. Materials
3.2. Modification of MMT
3.3. Preparation of the PI/MMT NCs via In Situ Polymerization
3.4. Preparation of PI/MMT NCs via Solution-Dispersion Technique
3.5. Characterization
4. Conclusions
- The dispersion of MMT in the PI matrices by in situ polymerization technique is higher than solution-dispersion technique.
- The PI/MMT NCs have higher thermal stability compared to pristine PI.
- The thermal stability increased with the OMMT loading for both techniques; however, this factor is higher for the in situ polymerization techniques.
Acknowledgements
References
- Pan, L-Y; Zhang, M-S; Wang, K. High-temperature-resistant polyimide/montmorillonite nanocomposite foams by solid blending. Polym Eng Sci 2011, 51, 1397–1403. [Google Scholar]
- Hergenrother, PM; Watson, KA; Smith, JG, Jr; Connell, JW; Yokota, R. Polyimides from 2,3,3′,4′-biphenyltetracarboxylic dianhydride and aromatic diamines. Polymer 2002, 43, 5077–5093. [Google Scholar]
- Mazoniene, E; Bendoraitiene, J; Peciulyte, L; Diliunas, S; Zemaitaitis, A. (Co)polyimides from commonly used monomers, and their nanocomposites. Prog Solid State Chem 2006, 34, 201–211. [Google Scholar]
- Gu, A; Kuo, SW; Chang, FC. A novel preparation of polyimide/clay hybrid films with low coefficient of thermal expansion. J Appl Polym Sci 2001, 79, 289–294. [Google Scholar]
- Tyan, HL; Liu, YC; Wei, KH. Thermally and mechanically enhanced clay/polyimide nanocomposite via reactive organoclay. Chem Mater 1999, 11, 1942–1947. [Google Scholar]
- Tyan, HL; Liu, YC; Wei, KH. Enhancement of imidization of poly(amic acid) through forming poly(amic acid)/organoclay nanocomposites. Polymer 1999, 40, 4877–4886. [Google Scholar]
- Hsaio, SH; Liou, GS; Chang, LM. Synthesis and properties of organosoluble polyimide/clay hybrids. J Appl Polym Sci 2001, 80, 2067–2073. [Google Scholar]
- Chen, H-S; Chen, C-M; Chang, G-Y; Lee, S-Y. Study on nanodispersion of PI/clay nanocomposite by temporal analyses. Mater Chem Phys 2006, 96, 244–252. [Google Scholar]
- Yano, K; Usuki, A; Okada, A; Kurauchi, T; Kamigaito, O. Synthesis and properties of polyimide-clay hybrid. J Polym Sci Part A Polym Chem 1993, 31, 2493–2498. [Google Scholar]
- Lan, T; Kaviratna, PD; Pinnavaia, TJ. On the nature of polyimide-clay hybrid composites. Chem Mater 1994, 6, 573–575. [Google Scholar]
- Yang, Y; Zhu, ZK; Yin, J; Wang, X; Qi, Z. Preparation and properties of hybrids of organo-soluble polyimide and montmorillonite with various chemical surface modification methods. Polymer 1999, 40, 4407–4414. [Google Scholar]
- Zhang, YH; Dang, ZM; Fu, SY; Xin, JH; Deng, JG; Wu, JT; Yang, SY; Li, LF; Yan, Q. Dielectric and dynamic mechanical properties of polyimide-clay nanocomposite films. Chem Phys Lett 2005, 401, 553–557. [Google Scholar]
- Lucilene, BP; Ana Rita, M; Francisco, R; Valenzuela, D. Organoclays: Properties, preparation and applications. Appl Clay Sci 2008, 42, 8–24. [Google Scholar]
- Huang, JC; Zhu, ZK; Yin, J; Qian, XF; Sun, YY. Poly(etherimide)/montmorillonite nanocomposites prepared by melt intercalation: Morphology, solvent resistance properties and thermal properties. Polymer 2001, 42, 873–877. [Google Scholar]
- Chang, JH; Park, DK; Ihn, KJ. Polyimide nanocomposite with a hexadecylamine clay: Synthesis and characterization. J Appl Polym Sci 2002, 84, 294–2301. [Google Scholar]
- Lili, C; Paul, DR. Polymer nanocomposites from organoclays: Structure and properties. Macromol Symp 2011, 301, 9–15. [Google Scholar]
- Okamoto, M; Morita, S; Taguchi, H; Kim, Y; Kotaka, T; Tateyama, H. Synthesis and structure of smectic clay/poly(methyl methacrylate) and clay/polystyrene nanocomposites via in situ intercalative polymerization. Polymer 2000, 41, 3887–3890. [Google Scholar]
- Aranda, P; Ruiz-Hitzky, E. Poly(ethylene oxide)-silicate intercalation materials. Chem Mater 1992, 4, 1395–1403. [Google Scholar]
- Vaia, R; Ishii, H; Giannelis, E. Synthesis and properties of two-dimensional nanostructures by direct intercalation of polymer melts in layered silicates. Chem Mater 1993, 5, 1694–1696. [Google Scholar]
- Delozier, DM; Orwell, RA; Cahoon, JF; Johnston, NJ; Smith, JG, Jr. Preparation and characterization of polyimide/organoclay nanocomposites. Polymer 2002, 43, 813–822. [Google Scholar]
- Rathanawan, M; Wittaya, L; Anuvat, S; Schwank, JW. Preparation, structure, properties and thermal behaviour of rigid-rod polyimide/montmorillonite nanocomposites. Compos Sci Technol 2001, 61, 1253–1264. [Google Scholar]
- Yu, YH; Yeh, JM; Liou, SJ; Chang, YP. Organo-soluble polyimide (TBAPP–OPDA)/clay nanocomposite materials with advanced anticorrosive properties prepared from solution dispersion technique. Acta Mater 2004, 52, 475–486. [Google Scholar]
- Leszczyńskaa, A; Njugunab, J; Pielichowskia, K; Banerjeec, JR. Polymer/montmorillonite nanocomposites with improved thermal properties: Part I. Factors influencing thermal stability and mechanisms of thermal stability improvement. Thermochim Acta 2007, 453, 75–96. [Google Scholar] [Green Version]








© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ahmad, M.B.; Gharayebi, Y.; Salit, M.S.; Hussein, M.Z.; Shameli, K. Comparison of In Situ Polymerization and Solution-Dispersion Techniques in the Preparation of Polyimide/Montmorillonite (MMT) Nanocomposites. Int. J. Mol. Sci. 2011, 12, 6040-6050. https://doi.org/10.3390/ijms12096040
Ahmad MB, Gharayebi Y, Salit MS, Hussein MZ, Shameli K. Comparison of In Situ Polymerization and Solution-Dispersion Techniques in the Preparation of Polyimide/Montmorillonite (MMT) Nanocomposites. International Journal of Molecular Sciences. 2011; 12(9):6040-6050. https://doi.org/10.3390/ijms12096040
Chicago/Turabian StyleAhmad, Mansor Bin, Yadollah Gharayebi, Mohd. Sapuan Salit, Mohd. Zobir Hussein, and Kamyar Shameli. 2011. "Comparison of In Situ Polymerization and Solution-Dispersion Techniques in the Preparation of Polyimide/Montmorillonite (MMT) Nanocomposites" International Journal of Molecular Sciences 12, no. 9: 6040-6050. https://doi.org/10.3390/ijms12096040
APA StyleAhmad, M. B., Gharayebi, Y., Salit, M. S., Hussein, M. Z., & Shameli, K. (2011). Comparison of In Situ Polymerization and Solution-Dispersion Techniques in the Preparation of Polyimide/Montmorillonite (MMT) Nanocomposites. International Journal of Molecular Sciences, 12(9), 6040-6050. https://doi.org/10.3390/ijms12096040



