Recognition-Mediated Assembly of Quantum Dot Polymer Conjugates with Controlled Morphology
Abstract
:1. Introduction
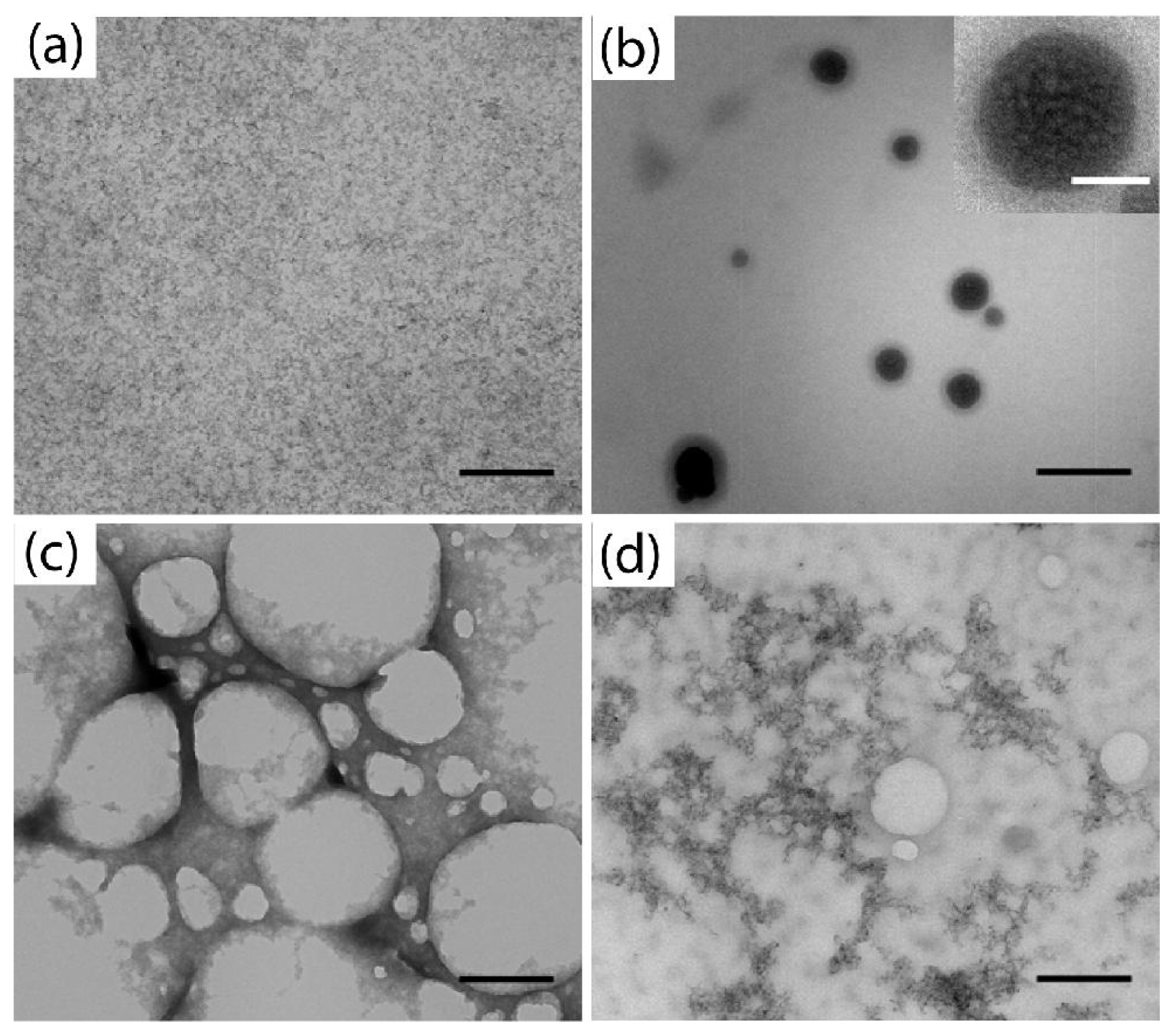
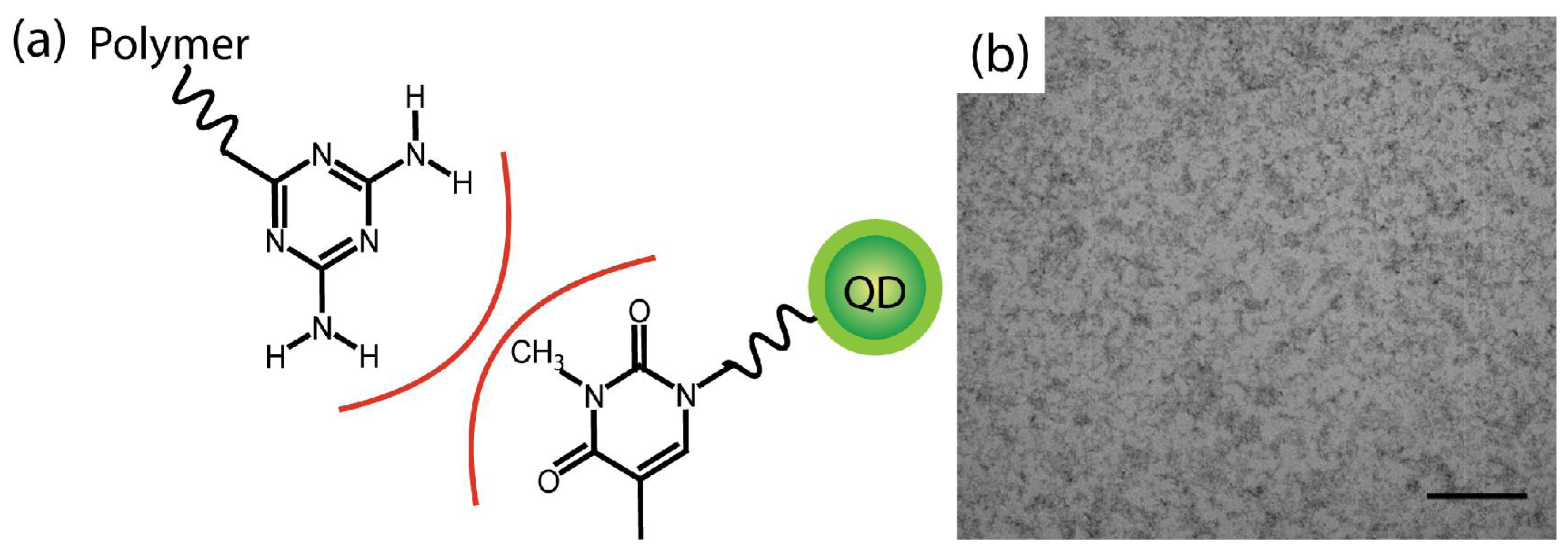
2. Results and Discussion
3. Experimental Section
Synthesis of TOPO functionalized CdSe/ZnS quantum dots (QDs)
Ligand exchange reaction
Synthesis of diblock co-polymer [32]
Preparation of QD-polymer aggregates
Characterization
4. Conclusions
Acknowledgments
References
- Brus, LE. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J Chem Phys 1984, 80, 4403–4409. [Google Scholar]
- Alivisatos, AP. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar]
- Tang, ZY; Kotov, NA; Giersig, M. Spontaneous organization of single CdTe nanoparticles into luminescent nanowires. Science 2002, 297, 237–240. [Google Scholar]
- Coe, SW; Woo, K; Bawendi, M; Bulovic, V. Electroluminescence from single monolayers of nanocrystals in molecular organic devices. Nature 2002, 420, 800–803. [Google Scholar]
- Tessler, N; Medvedev, V; Kazes, M; Kan, S; Banin, U. Efficient near-infrared polymer nanocrystal light-emitting diodes. Science 2002, 295, 1506–1508. [Google Scholar]
- McDonald, SA; Konstantatos, G; Zhang, S; Cyr, PW; Klem, EJD; Levina, L; Sargent, EH. Solution-processed PbS quantum dot infrared photodetectors and photovoltaics. Nat Mater 2005, 4, 138–142. [Google Scholar]
- Choudhury, KR; Kim, WJ; Sahoo, Y; Lee, KS; Prasad, PN. Solution-processed pentacene quantum-dot polymeric nanocomposite for infrared photodetection. Appl Phys Lett 2006, 89, 051109–051113. [Google Scholar]
- Qi, D; Fischbein, M; Drndic, M; Selmic, S. Efficient polymer-nanocrystal quantum-dot photodetectors. Appl Phys Lett 2005, 86, 093103–093113. [Google Scholar]
- Talapin, DV; Murray, CB. PbSe Nanocrystal solids for n- and p-channel thin film field-effect transistors. Science 2005, 310, 86–89. [Google Scholar]
- Gur, I; Fromer, NA; Geier, ML; Alivisatos, AP. Air-stable all-inorganic nanocrystal solar cells processed from solution. Science 2005, 310, 462–465. [Google Scholar]
- Huynh, WU; Dittmer, JJ; Alivisatos, AP. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar]
- Winiarz, JG; Zhang, L; Lal, M; Friend, CS; Prasad, PN. Observation of the photorefractive effect in a hybrid organic-inorganic nanocomposite. J Am Chem Soc 1999, 121, 5287–5295. [Google Scholar]
- Collier, CP; Vossmeyer, T; Heath, JR. Nanocrystal superlattices. Annu Rev Phys Chem 1998, 49, 371–404. [Google Scholar]
- Murray, CB; Kagan, CR; Bawendi, MG. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu Rev Mater Sci 2000, 30, 545–610. [Google Scholar]
- Balazs, AC; Emrick, T; Russell, TP. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar]
- Lin, Y; Boker, A; He, JB; Sill, K; Xiang, HQ; Abetz, C; Li, XF; Wang, J; Emrick, T; Long, S; Wang, Q; Balas, A; Russell, TP. Self-directed self-assembly of nanoparticle/copolymer mixtures. Nature 2005, 434, 55–59. [Google Scholar]
- Medintz, IL; Clapp, AR; Mattoussi, H; Goldman, ER; Fisher, B; Mauro, JM. Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat Mater 2003, 2, 630–638. [Google Scholar]
- Medintz, IL; Uyeda, HT; Goldman, ER; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 2005, 4, 435–446. [Google Scholar]
- Zou, W; Du, Z; Li, H; Zhang, C. Fabrication of surface-modified CdSe quantum dots by self-assembly of a functionalizable comb polymer. Polym Int 2011, 60, 751–757. [Google Scholar]
- Wang, M; Zhang, M; Li, J; Kumar, S; Walker, GC; Scholes, GD; Winnik, MA. Self-assembly of colloidal quantum dots on the scaffold of triblock copolymer micelles. ACS Appl Mater Interfaces 2010, 2, 3160–3169. [Google Scholar]
- Zorn, M; Bae, WK; Kwak, J; Lee, H; Lee, C; Zentel, R; Char, K. Quantum dot-block copolymer hybrids with improved properties and their application to quantum dot light-emitting devices. ACS Nano 2009, 3, 1063–1068. [Google Scholar]
- Sheng, W; Kim, S; Lee, J; Kim, SW; Jensen, K; Bawendi, MG. In-situ encapsulation of quantum dots into polymer microspheres. Langmuir 2006, 22, 3782–3790. [Google Scholar]
- Skaff, H; Sill, K; Emrick, T. Quantum dots tailored with poly(para-phenylene vinylene). J Am Chem Soc 2004, 126, 11322–11325. [Google Scholar]
- Cao, XD; Li, CM; Bao, HF; Bao, QL; Dong, H. Fabrication of strongly fluorescent quantum dot-polymer composite in aqueous solution. Chem Mater 2007, 19, 3773–3779. [Google Scholar]
- Shenhar, R; Norsten, TB; Rotello, VM. Polymer-mediated nanoparticle assembly: Structural control and applications. Adv Mater 2005, 17, 657–669. [Google Scholar]
- Arumugam, P; Xu, H; Srivastava, S; Rotello, VM. ‘Bricks and mortar’ nanoparticle self-assembly using polymers. Polym Int 2007, 56, 461–466. [Google Scholar]
- Boal, AK; Galow, TH; Ilhan, F; Rotello, VM. Binary and ternary polymer-mediated “bricks and mortar” self-assembly of gold and silica nanoparticles. Adv Funct Mater 2001, 11, 461–467. [Google Scholar]
- Boal, AK; Gray, M; Ilhan, F; Clavier, GM; Kapitzky, L; Rotello, VM. ‘Bricks-and-mortar’ self-assembly of nanoparticles. Tetrahedron 2002, 58, 756–770. [Google Scholar]
- Boal, AK; Frankamp, BL; Uzun, O; Tuominen, MT; Rotello, VM. Modulation of spacing and magnetic properties of iron oxide nanoparticles through polymer-mediated “bricks and mortar” self-assembly. Chem Mater 2004, 16, 3252–3256. [Google Scholar]
- Boal, AK; Ilhan, F; DeRouchey, JE; Thurn-Albrecht, T; Russell, TP; Rotello, VM. Self-assembly of nanoparticles into structured spherical and network aggregates. Nature 2000, 404, 746–748. [Google Scholar]
- Frankamp, BL; Uzun, O; Ilhan, F; Boal, AK; Rotello, VM. Recognition-mediated assembly of nanoparticles into micellar structures with diblock copolymers. J Am Chem Soc 2002, 124, 892–893. [Google Scholar]
- Uzun, O; Frankamp, BL; Sanyal, A; Rotello, VM. Recognition-mediated assembly of nanoparticle-diblock copolymer micelles with controlled size. Chem Mater 2006, 18, 5404–5409. [Google Scholar]
- Subramani, C; Dickert, S; Yeh, Y-C; Tuominen, MT; Rotello, VM. Supramolecular functionalization of electron-beam generated nanostructures. Langmuir 2011, 27, 1543–1545. [Google Scholar]
- Deans, R; Ilhan, F; Rotello, VM. Recognition mediated unfolding of a self-assembled polymeric globule. Macromolecules 1999, 32, 4956–4960. [Google Scholar]
- Crooker, SA; Hollingsworth, JA; Tretiak, S; Klimov, VI. Spectrally resolved dynamics of energy transfer in quantum-dot assemblies: Towards engineered energy flows in artificial materials. Phys Rev Lett 2002, 89, 186802:1–186802:4. [Google Scholar]
- Yang, BQ; Schneeloch, JE; Pan, Z; Furis, M; Achermann, M. Radiative lifetimes and orbital symmetry of electronic energy levels of CdS nanocrystals: Size dependence. Phys Rev B 2010, 81, 073401:1–073401:4. [Google Scholar]
- Peng, ZA; Peng, XG. Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J Am Chem Soc 2001, 123, 183–184. [Google Scholar]
- Dabbousi, BO; Rodriguez-Viejo, J; Mikulec, FV; Heine, JR; Mattoussi, H; Ober, R; Jensen, KF; Bawendi, MG. (CdSe)ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J Phys Chem B 1997, 101, 9463–9475. [Google Scholar]
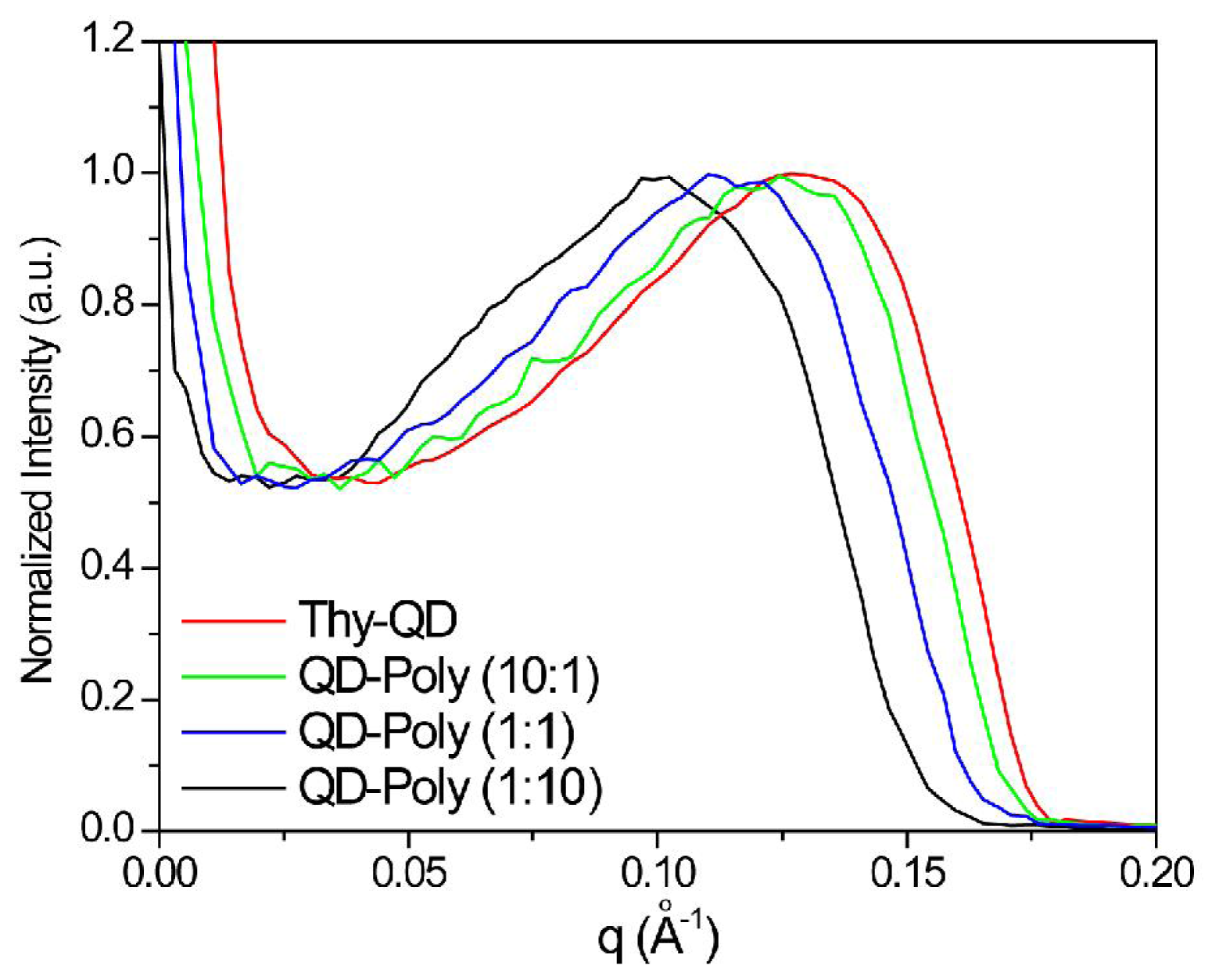



| Thy-QD | Thy-Poly (10:1) | Thy-Poly (1:1) | Thy-Poly (1:10) | |
|---|---|---|---|---|
| q Å−1 | 0.131 | 0.126 | 0.115 | 0.099 |
| d Å−1 | 4.792 | 4.955 | 5.425 | 6.333 |
| A1 | τ1 (ns) | A2 | τ2 (ns) | |
|---|---|---|---|---|
| Thy-QD | 0.45 | 0.61 | 0.55 | 7.0 |
| Thy-Poly (10:1) | 0.43 | 0.60 | 0.57 | 6.40 |
| Thy-Poly (1:10) | 0.45 | 0.60 | 0.55 | 6.56 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nandwana, V.; Subramani, C.; Eymur, S.; Yeh, Y.-C.; Tonga, G.Y.; Tonga, M.; Jeong, Y.; Yang, B.; Barnes, M.D.; Cooke, G.; et al. Recognition-Mediated Assembly of Quantum Dot Polymer Conjugates with Controlled Morphology. Int. J. Mol. Sci. 2011, 12, 6357-6366. https://doi.org/10.3390/ijms12096357
Nandwana V, Subramani C, Eymur S, Yeh Y-C, Tonga GY, Tonga M, Jeong Y, Yang B, Barnes MD, Cooke G, et al. Recognition-Mediated Assembly of Quantum Dot Polymer Conjugates with Controlled Morphology. International Journal of Molecular Sciences. 2011; 12(9):6357-6366. https://doi.org/10.3390/ijms12096357
Chicago/Turabian StyleNandwana, Vikas, Chandramouleeswaran Subramani, Serkan Eymur, Yi-Cheun Yeh, Gulen Yesilbag Tonga, Murat Tonga, Youngdo Jeong, Boqian Yang, Michael D. Barnes, Graeme Cooke, and et al. 2011. "Recognition-Mediated Assembly of Quantum Dot Polymer Conjugates with Controlled Morphology" International Journal of Molecular Sciences 12, no. 9: 6357-6366. https://doi.org/10.3390/ijms12096357




