Quantum Dots-Based Immunofluorescent Imaging of Stromal Fibroblasts Caveolin-1 and Light Chain 3B Expression and Identification of Their Clinical Significance in Human Gastric Cancer
Abstract
:1. Introduction
2. Results and Discussion
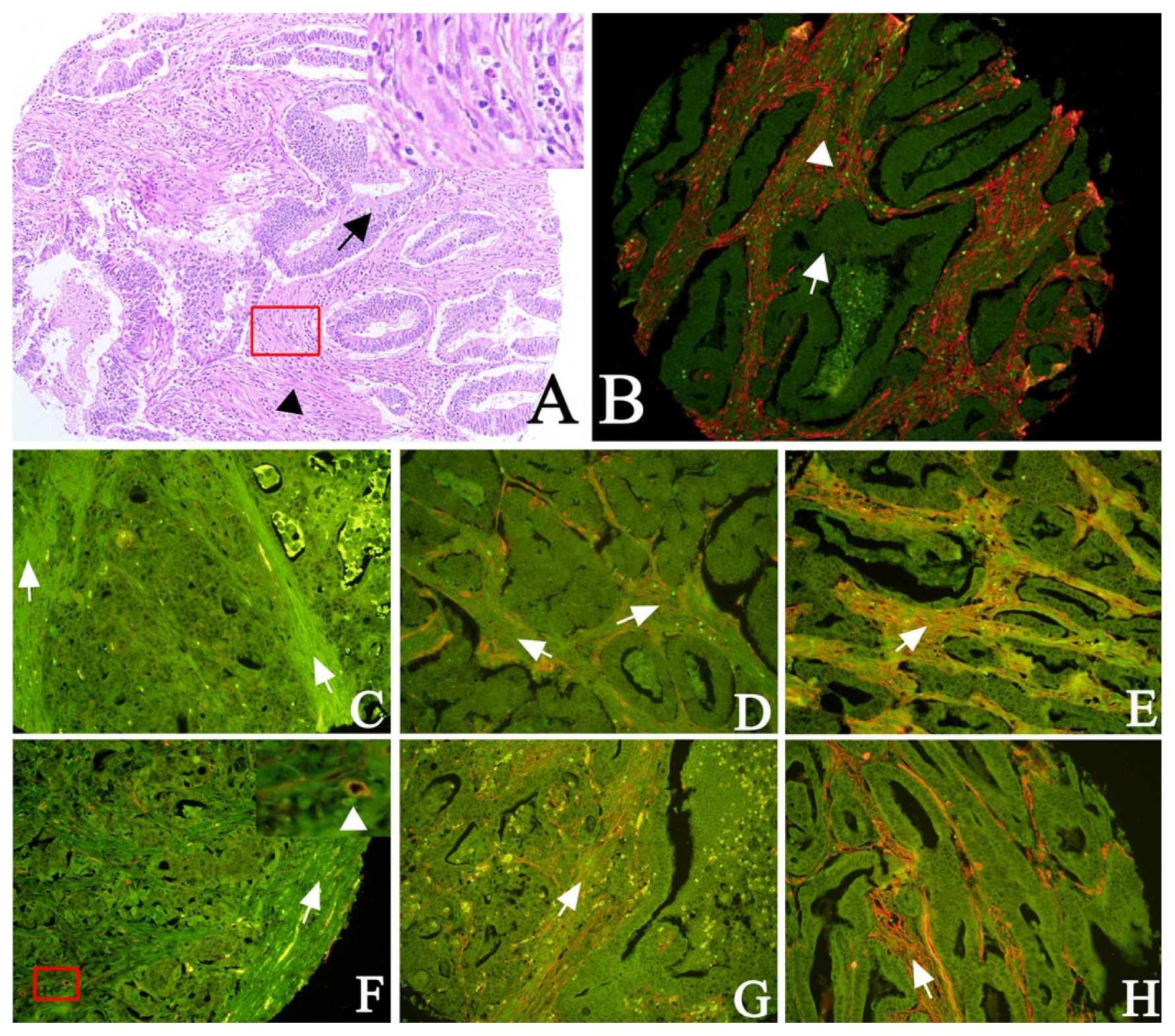
2.1. Expression of Cav-1 and LC3B in GC
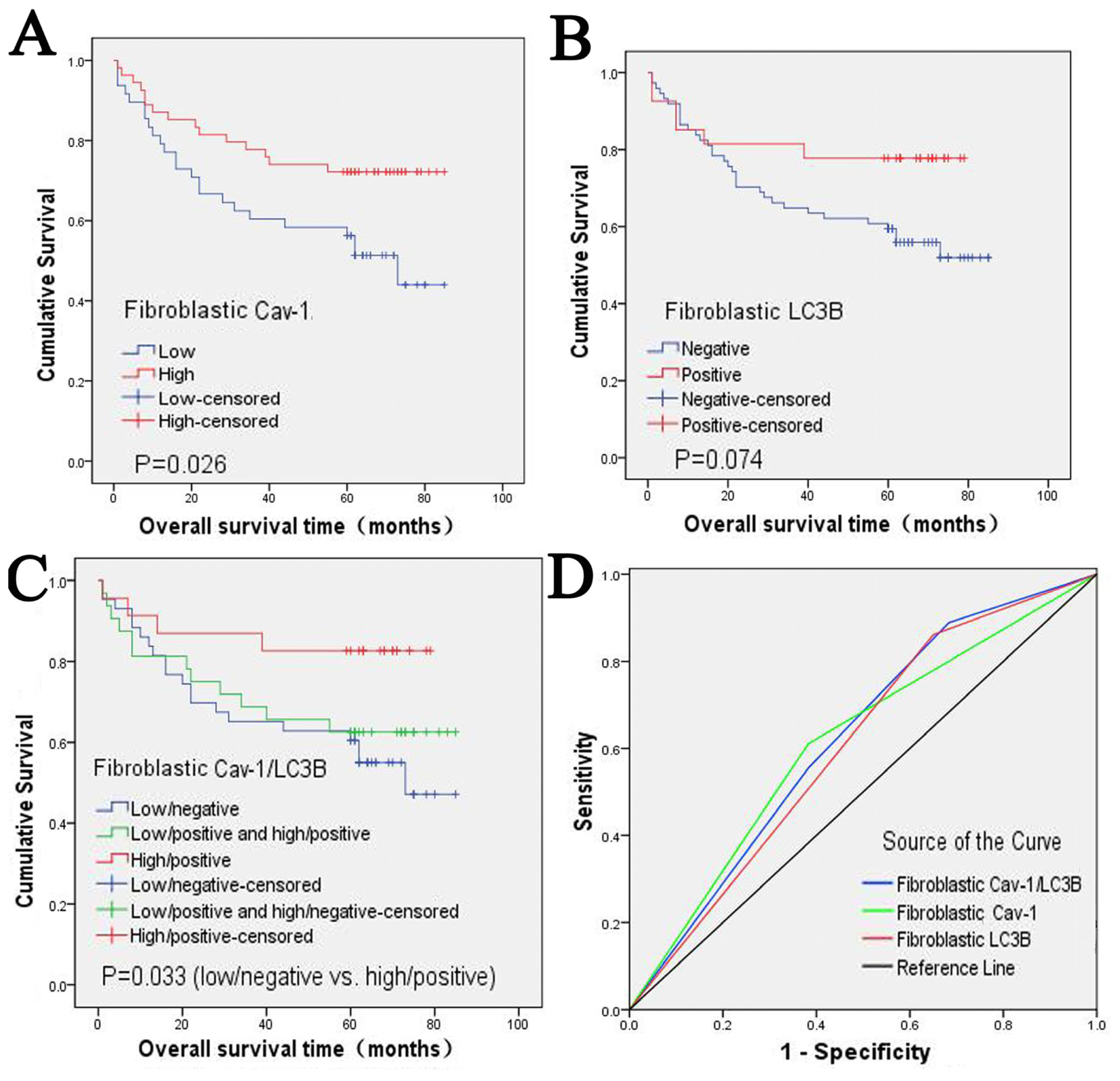
2.2. Clinical Significance and Prognostic Value of Fibroblastic Cav-1 and LC3B
2.3. The Correlation between Fibroblastic Cav-1 and LC3B
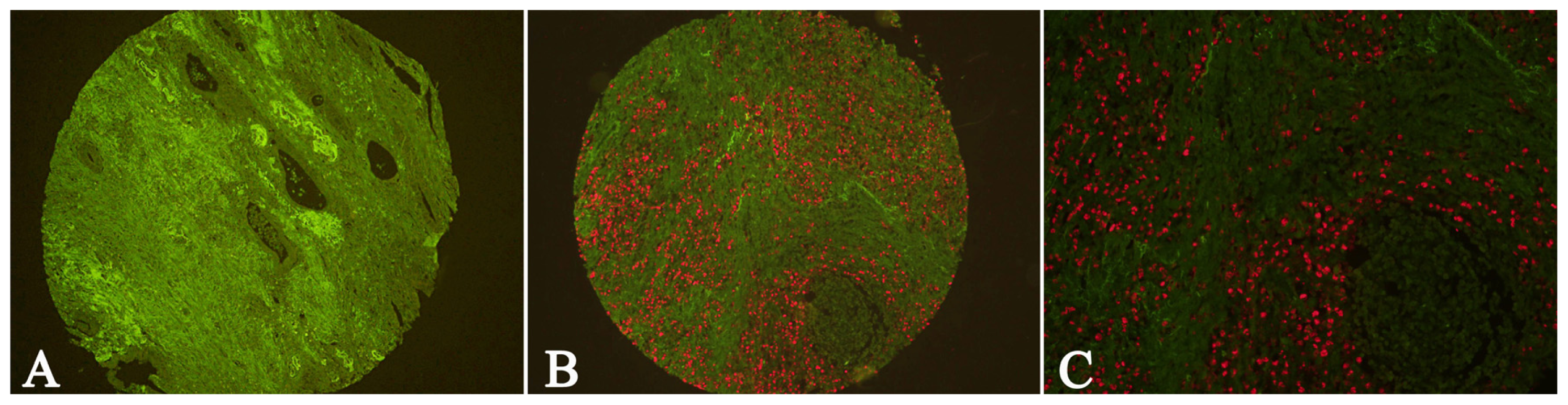
2.4. EBV Infection Affected Expression of Fibroblastic Cav-1 and LC3B
2.5. Discussion
3. Experimental Section
3.1. Patients and Tissue Samples
3.2. Tissue Microarray Construction
3.3. QDs-Based Immunofluorescence Histochemistry
3.4. QDs-Based Double Immunofluorescence Labeling
3.5. QDs-Based Fluorescence in situ Hybridization
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Sotgia, F.; Martinez-Outschoorn, U.E.; Howell, A.; Pestell, R.G.; Pavlides, S.; Lisanti, M.P. Caveolin-1 and cancer metabolism in the tumor microenvironment: Markers, models, and mechanisms. Annu. Rev. Pathol 2012, 7, 423–467. [Google Scholar]
- Nakasone, E.S.; Askautrud, H.A.; Kees, T.; Park, J.H.; Plaks, V.; Ewald, A.J.; Fein, M.; Rasch, M.G.; Tan, Y.X.; Qiu, J.; et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 2012, 21, 488–503. [Google Scholar]
- Simpkins, S.A.; Hanby, A.M.; Holliday, D.L.; Speirs, V. Clinical and functional significance of loss of caveolin-1 expression in breast cancer-associated fibroblasts. J. Pathol 2012, 227, 490–498. [Google Scholar]
- Witkiewicz, A.K.; Dasgupta, A.; Sotgia, F.; Mercier, I.; Pestell, R.G.; Sabel, M.; Kleer, C.G.; Brody, J.R.; Lisanti, M.P. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am. J. Pathol 2009, 174, 2023–2034. [Google Scholar]
- Paulsson, J.; Sjoblom, T.; Micke, P.; Ponten, F.; Landberg, G.; Heldin, C.H.; Bergh, J.; Brennan, D.J.; Jirstrom, K.; Ostman, A. Prognostic significance of stromal platelet-derived growth factor beta-receptor expression in human breast cancer. Am. J. Pathol 2009, 175, 334–341. [Google Scholar]
- Witkiewicz, A.K.; Dasgupta, A.; Sammons, S.; Er, O.; Potoczek, M.B.; Guiles, F.; Sotgia, F.; Brody, J.R.; Mitchell, E.P.; Lisanti, M.P. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol. Ther 2010, 10, 135–143. [Google Scholar]
- Martinez-Outschoorn, U.E.; Pavlides, S.; Whitaker-Menezes, D.; Daumer, K.M.; Milliman, J.N.; Chiavarina, B.; Migneco, G.; Witkiewicz, A.K.; Martinez-Cantarin, M.P.; Flomenberg, N.; et al. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: Implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle 2010, 9, 2423–2433. [Google Scholar]
- Schafer, M.; Werner, S. Cancer as an overhealing wound: An old hypothesis revisited. Nat. Rev. Mol. Cell Biol 2008, 9, 628–638. [Google Scholar]
- Di Vizio, D.; Morello, M.; Sotgia, F.; Pestell, R.G.; Freeman, M.R.; Lisanti, M.P. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle 2009, 8, 2420–2424. [Google Scholar]
- Li, M.; Li, C.; Li, D.; Xie, Y.; Shi, J.; Li, G.; Guan, Y.; Zhang, P.; Peng, F.; Xiao, Z.; et al. Periostin, a stroma-associated protein, correlates with tumor invasiveness and progression in nasopharyngeal carcinoma. Clin. Exp. Metastasis 2012. [Google Scholar] [CrossRef]
- Adegboyega, P.A.; Rodriguez, S.; McLarty, J. Stromal expression of actin is a marker of aggressiveness in basal cell carcinoma. Hum. Pathol 2010, 41, 1128–1137. [Google Scholar]
- Gallego, E.; Vicioso, L.; Alvarez, M.; Hierro, I.; Perez-Villa, L.; Blanes, A.; Matilla, A. Stromal expression of vascular endothelial growth factor C is relevant to predict sentinel lymph node status in melanomas. Virchows Arch 2011, 458, 621–630. [Google Scholar]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin 2011, 61, 69–90. [Google Scholar]
- Yang, L. Incidence and mortality of gastric cancer in China. World J. Gastroenterol 2006, 12, 17–20. [Google Scholar]
- Goetz, J.G.; Lajoie, P.; Wiseman, S.M.; Nabi, I.R. Caveolin-1 in tumor progression: The good, the bad and the ugly. Cancer Metastasis Rev 2008, 27, 715–735. [Google Scholar]
- Zhao, X.; He, Y.; Chen, H. Autophagic tumor stroma: Mechanisms and roles in tumor growth and progression. Int. J. Cancer 2012. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Whitaker-Menezes, D.; Pavlides, S.; Chiavarina, B.; Bonuccelli, G.; Casey, T.; Tsirigos, A.; Migneco, G.; Witkiewicz, A.; Balliet, R.; et al. The autophagic tumor stroma model of cancer or “battery-operated tumor growth”: A simple solution to the autophagy paradox. Cell Cycle 2010, 9, 4297–4306. [Google Scholar]
- Martinez-Outschoorn, U.E.; Trimmer, C.; Lin, Z.; Whitaker-Menezes, D.; Chiavarina, B.; Zhou, J.; Wang, C.; Pavlides, S.; Martinez-Cantarin, M.P.; Capozza, F.; et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle 2010, 9, 3515–3533. [Google Scholar]
- Martinez-Outschoorn, U.E.; Pavlides, S.; Howell, A.; Pestell, R.G.; Tanowitz, H.B.; Sotgia, F.; Lisanti, M.P. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int. J. Biochem. Cell Biol 2011, 43, 1045–1051. [Google Scholar]
- Chiavarina, B.; Whitaker-Menezes, D.; Martinez-Outschoorn, U.E.; Witkiewicz, A.K.; Birbe, R.C.; Howell, A.; Pestell, R.G.; Smith, J.; Daniel, R.; Sotgia, F.; et al. Pyruvate kinase expression (PKM1 and PKM2) in cancer-associated fibroblasts drives stromal nutrient production and tumor growth. Cancer Biol. Ther 2011, 12, 1101–1103. [Google Scholar]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar]
- Chen, H.; Xue, J.; Zhang, Y.; Zhu, X.; Gao, J.; Yu, B. Comparison of quantum dots immunofluorescence histochemistry and conventional immunohistochemistry for the detection of caveolin-1 and PCNA in the lung cancer tissue microarray. J. Mol. Histol 2009, 40, 261–268. [Google Scholar]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar]
- Peng, C.W.; Liu, X.L.; Chen, C.; Liu, X.; Yang, X.Q.; Pang, D.W.; Zhu, X.B.; Li, Y. Patterns of cancer invasion revealed by QDs-based quantitative multiplexed imaging of tumor microenvironment. Biomaterials 2011, 32, 2907–2917. [Google Scholar]
- Chen, C.; Peng, J.; Sun, S.R.; Peng, C.W.; Li, Y.; Pang, D.W. Tapping the potential of quantum dots for personalized oncology: Current status and future perspectives. Nanomedicine (Lond) 2012, 7, 411–428. [Google Scholar]
- Parak, W.J.; Pellegrino, T.; Plank, C. Labelling of cells with quantum dots. Nanotechnology 2005, 16, R9–R25. [Google Scholar]
- Akiba, S.; Koriyama, C.; Herrera-Goepfert, R.; Eizuru, Y. Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Sci 2008, 99, 195–201. [Google Scholar]
- Song, H.J.; Kim, K.M. Pathology of epstein-barr virus-associated gastric carcinoma and its relationship to prognosis. Gut Liver 2011, 5, 143–148. [Google Scholar]
- Santibanez, J.F.; Blanco, F.J.; Garrido-Martin, E.M.; Sanz-Rodriguez, F.; del Pozo, M.A.; Bernabeu, C. Caveolin-1 interacts and cooperates with the transforming growth factor-beta type I receptor ALK1 in endothelial caveolae. Cardiovasc. Res 2008, 77, 791–799. [Google Scholar]
- Razani, B.; Zhang, X.L.; Bitzer, M.; von Gersdorff, G.; Bottinger, E.P.; Lisanti, M.P. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J. Biol. Chem 2001, 276, 6727–6738. [Google Scholar]
- Elenbaas, B.; Weinberg, R.A. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp. Cell Res 2001, 264, 169–184. [Google Scholar]
- Witkiewicz, A.K.; Casimiro, M.C.; Dasgupta, A.; Mercier, I.; Wang, C.; Bonuccelli, G.; Jasmin, J.F.; Frank, P.G.; Pestell, R.G.; Kleer, C.G.; et al. Towards a new “stromal-based” classification system for human breast cancer prognosis and therapy. Cell Cycle 2009, 8, 1654–1658. [Google Scholar]
- Qian, N.; Ueno, T.; Kawaguchi-Sakita, N.; Kawashima, M.; Yoshida, N.; Mikami, Y.; Wakasa, T.; Shintaku, M.; Tsuyuki, S.; Inamoto, T.; et al. Prognostic significance of tumor/stromal caveolin-1 expression in breast cancer patients. Cancer Sci 2011, 102, 1590–1596. [Google Scholar]
- Koo, J.S.; Park, S.; Kim, S.I.; Lee, S.; Park, B.W. The impact of caveolin protein expression in tumor stroma on prognosis of breast cancer. Tumour Biol 2011, 32, 787–799. [Google Scholar]
- El-Gendi, S.M.; Mostafa, M.F.; El-Gendi, A.M. Stromal caveolin-1 expression in breast carcinoma. Correlation with early tumor recurrence and clinical outcome. Pathol. Oncol. Res 2012, 18, 459–469. [Google Scholar]
- Choi, K.S. Autophagy and cancer. Exp. Mol. Med 2012, 44, 109–120. [Google Scholar]
- Klionsky, D.J.; Abdalla, F.C.; Abeliovich, H.; Abraham, R.T.; Acevedo-Arozena, A.; Adeli, K.; Agholme, L.; Agnello, M.; Agostinis, P.; Aguirre-Ghiso, J.A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophage 2012, 8, 445–544. [Google Scholar]
- Sotgia, F.; Martinez-Outschoorn, U.E.; Lisanti, M.P. Mitochondrial oxidative stress drives tumor progression and metastasis: Should we use antioxidants as a key component of cancer treatment and prevention? BMC Med. 2011, 9. [Google Scholar] [CrossRef]
- Chen, C.; Peng, J.; Xia, H.; Wu, Q.; Zeng, L.; Xu, H.; Tang, H.; Zhang, Z.; Zhu, X.; Pang, D.; et al. Quantum-dot-based immunofluorescent imaging of HER2 and ER provides new insights into breast cancer heterogeneity. Nanotechnology 2010, 21. [Google Scholar] [CrossRef]
- Liu, X.L.; Peng, C.W.; Chen, C.; Yang, X.Q.; Hu, M.B.; Xia, H.S.; Liu, S.P.; Pang, D.W.; Li, Y. Quantum dots-based double-color imaging of HER2 positive breast cancer invasion. Biochem. Biophys. Res. Commun 2011, 409, 577–582. [Google Scholar]
- Peng, C.W.; Tian, Q.; Yang, G.F.; Fang, M.; Zhang, Z.L.; Peng, J.; Li, Y.; Pang, D.W. Quantum-dots based simultaneous detection of multiple biomarkers of tumor stromal features to predict clinical outcomes in gastric cancer. Biomaterials 2012, 33, 5742–5752. [Google Scholar]
- Chen, C.; Peng, J.; Xia, H.S.; Yang, G.F.; Wu, Q.S.; Chen, L.D.; Zeng, L.B.; Zhang, Z.L.; Pang, D.W.; Li, Y. Quantum dots-based immunofluorescence technology for the quantitative determination of HER2 expression in breast cancer. Biomaterials 2009, 30, 2912–2918. [Google Scholar]
- Li, M.; Chen, H.; Diao, L.; Zhang, Y.; Xia, C.; Yang, F. Caveolin-1 and VEGF-C promote lymph node metastasis in the absence of intratumoral lymphangiogenesis in non-small cell lung cancer. Tumori 2010, 96, 734–743. [Google Scholar]
- Sun, J.; Gao, J.; Hu, J.B.; Fan, L.F.; Zhu, X.B.; Subahan, R.; Chen, H.L. Expression of Cav-1 in tumour cells, rather than in stromal tissue, may promote cervical squamous cell carcinoma proliferation, and correlates with high-risk HPV infection. Oncol. Rep 2012, 27, 1733–1740. [Google Scholar]
- Vauhkonen, M.; Vauhkonen, H.; Sipponen, P. Pathology and molecular biology of gastric cancer. Best Pract. Res. Clin. Gastroenterol 2006, 20, 651–674. [Google Scholar]
- Peng, J.; Chen, H.-L.; Zhu, X.-B.; Yang, G.-F.; Zhang, Z.-L.; Tian, Z.-Q.; Pang, D.-W. Detection of epstein-barr virus infection in gastric carcinomas using quantum dot-based fluorescence in-situ hybridization. J. Nanosci. Nanotechnol 2011, 11, 9725–9730. [Google Scholar]


| Features | n | Fibroblastic Cav-1 | p | n | Fibroblastic LC3B | p | ||
|---|---|---|---|---|---|---|---|---|
| Low (%) | High (%) | Negative (%) | Positive (%) | |||||
| Age | 0.724 | 0.287 | ||||||
| <58 | 55 | 28 (50.9) | 27 (40.1) | 50 | 39 (78.0) | 11 (22.0) | ||
| ≥58 | 63 | 30 (47.6) | 33 (52.4) | 51 | 35 (68.6) | 16 (31.4) | ||
| Gender | 0.186 | 0.384 | ||||||
| Male | 85 | 45 (52.9) | 40 (47.1) | 72 | 51 (70.8) | 21 (29.2) | ||
| Female | 33 | 13 (39.4) | 20 (60.6) | 29 | 23 (79.3) | 6 (20.7) | ||
| Depth of invasion # | 0.903 | 0.032 | ||||||
| T1 + T2 | 36 | 18 (50.0) | 18 (50.0) | 32 | 19 (59.4) | 13 (40.6) | ||
| T3 + T4 | 82 | 40 (48.8) | 42 (51.2) | 69 | 55 (79.7) | 14 (20.3) | ||
| Lymph node status # | 0.474 | 0.992 | ||||||
| N0 | 39 | 21 (53.8) | 18 (46.2) | 30 | 22 (73.3) | 8 (26.7) | ||
| N1 + N2 | 79 | 37 (46.8) | 42 (53.2) | 71 | 52 (73.2) | 19 (26.8) | ||
| TNM stage # | 0.725 | 0.914 | ||||||
| 0+Ia+Ib+II | 53 | 27 (50.9) | 26 (49.1) | 44 | 32 (72.7) | 12 (27.3) | ||
| IIIa+ IIIb+ IV | 65 | 31 (47.7) | 34 (52.3) | 57 | 42 (73.7) | 15 (26.3) | ||
| Grade of AC | 0.792 | 0.065 | ||||||
| Well and moderately | 43 | 20 (46.5) | 23 (53.5) | 34 | 20 (58.8) | 14 (41.2) | ||
| Poorly | 59 | 29 (49.2) | 30 (50.8) | 53 | 41 (77.4) | 12 (22.6) | ||
| Lauren classification | 0.316 | 0.142 | ||||||
| Intestinal-type | 65 | 28 (43.1) | 37 (56.9) | 51 | 31 (60.8) | 20 (39.2) | ||
| Diffuse-type | 45 | 26 (57.8) | 19 (42.2) | 42 | 36 (85.7) | 6 (14.3) | ||
| Mixed-type | 8 | 4 (50.0) | 4 (50.0) | 8 | 7 (87.5) | 1 (12.5) | ||
| HER-2 | 0.967 | 0.628 | ||||||
| Positive | 23 | 11 (47.8) | 12 (52.2) | 23 | 16 (69.6) | 7 (30.4) | ||
| Negative | 89 | 43 (48.3) | 46 (51.7) | 75 | 56 (74.7) | 19 (25.3) | ||
| Tumor cell type | 0.565 | 0.189 | ||||||
| AC | 102 | 49 (48.0) | 53 (52.0) | 87 | 61 (70.1) | 26 (29.9) | ||
| MAC | 11 | 7 (63.6) | 4 (36.4) | 10 | 9 (90.0) | 1 (10.0) | ||
| UC | 5 | 2 (40.0) | 3 (60.0) | 4 | 4 (100.0) | 0 (0) | ||
| Fibroblastic Cav-1 | 0.000 | |||||||
| Low | 45 | 42 (93.3) | 3 (6.7) | |||||
| High | 51 | 28 (54.9) | 23 (45.1) | |||||
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| p value | HR (95%CI) | p value | HR (95%CI) | |
| Sex | ||||
| Men vs. women | 0.732 | 1.098 (0.638, 1.890) | ||
| Age | ||||
| <56 vs. ≥56 | 0.199 | 0.728 (0.445, 1.193) | ||
| TNM stage | ||||
| I–II vs. III–IV | 0.003 | 0.543 (0.256, 0.803) | 0.001 | 4.344 (1.895, 9.958) |
| T classification | ||||
| T1-T2 vs. T3-T4 | 0.001 | 0.317 (0.137, 0.732) | 0.483 | |
| Lymph node | ||||
| No vs. Yes | 0.034 | 0.528 (0.275, 0.987) | 0.663 | |
| Lauren classification | ||||
| Intestinal-type vs. Diffuse-type | 0.882 | 0.965 (0.603, 1.543) | ||
| Grade of AC | ||||
| Well and moderately vs. poorly | 0.638 | 0.873 (0.493, 1.546) | ||
| Fibroblastic Cav-1 | ||||
| High vs. Low | 0.021 | 0.556 (0.332, 0.929) | 0.029 | 0.474 (0.242, 0.928) |
| Fibroblastic LC3B | ||||
| Positive vs. Negative | 0.041 | 0.498 (0.235, 1.055) | 0.180 | |
| EBV infection | ||||
| Yes vs. No | 0.347 | 0.725 (0.355, 1.481) | ||
| Variables | Fibroblastic Cav-1 | Fibroblastic LC3B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (%) | High (%) | r | p | Negative (%) | Positive (%) | r | p | ||
| EBV Infection | Negative | 48 (49.5) | 49 (50.0) | 0.092 | 0.877 | 60(74.1) | 21 (25.9) | 0.037 | 0.712 |
| Positive | 10 (47.6) | 11 (52.4) | 14(70.0) | 6 (30.0) | |||||
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, Y.; Zhao, X.; Gao, J.; Fan, L.; Yang, G.; Cho, W.C.-s.; Chen, H. Quantum Dots-Based Immunofluorescent Imaging of Stromal Fibroblasts Caveolin-1 and Light Chain 3B Expression and Identification of Their Clinical Significance in Human Gastric Cancer. Int. J. Mol. Sci. 2012, 13, 13764-13780. https://doi.org/10.3390/ijms131113764
He Y, Zhao X, Gao J, Fan L, Yang G, Cho WC-s, Chen H. Quantum Dots-Based Immunofluorescent Imaging of Stromal Fibroblasts Caveolin-1 and Light Chain 3B Expression and Identification of Their Clinical Significance in Human Gastric Cancer. International Journal of Molecular Sciences. 2012; 13(11):13764-13780. https://doi.org/10.3390/ijms131113764
Chicago/Turabian StyleHe, Yuyu, Xianda Zhao, Jun Gao, Lifang Fan, Guifang Yang, William Chi-shing Cho, and Honglei Chen. 2012. "Quantum Dots-Based Immunofluorescent Imaging of Stromal Fibroblasts Caveolin-1 and Light Chain 3B Expression and Identification of Their Clinical Significance in Human Gastric Cancer" International Journal of Molecular Sciences 13, no. 11: 13764-13780. https://doi.org/10.3390/ijms131113764







