Association of Retinoic Acid Receptors with Extracellular Matrix Accumulation in Rats with Renal Interstitial Fibrosis Disease
Abstract
:1. Introduction
2. Results
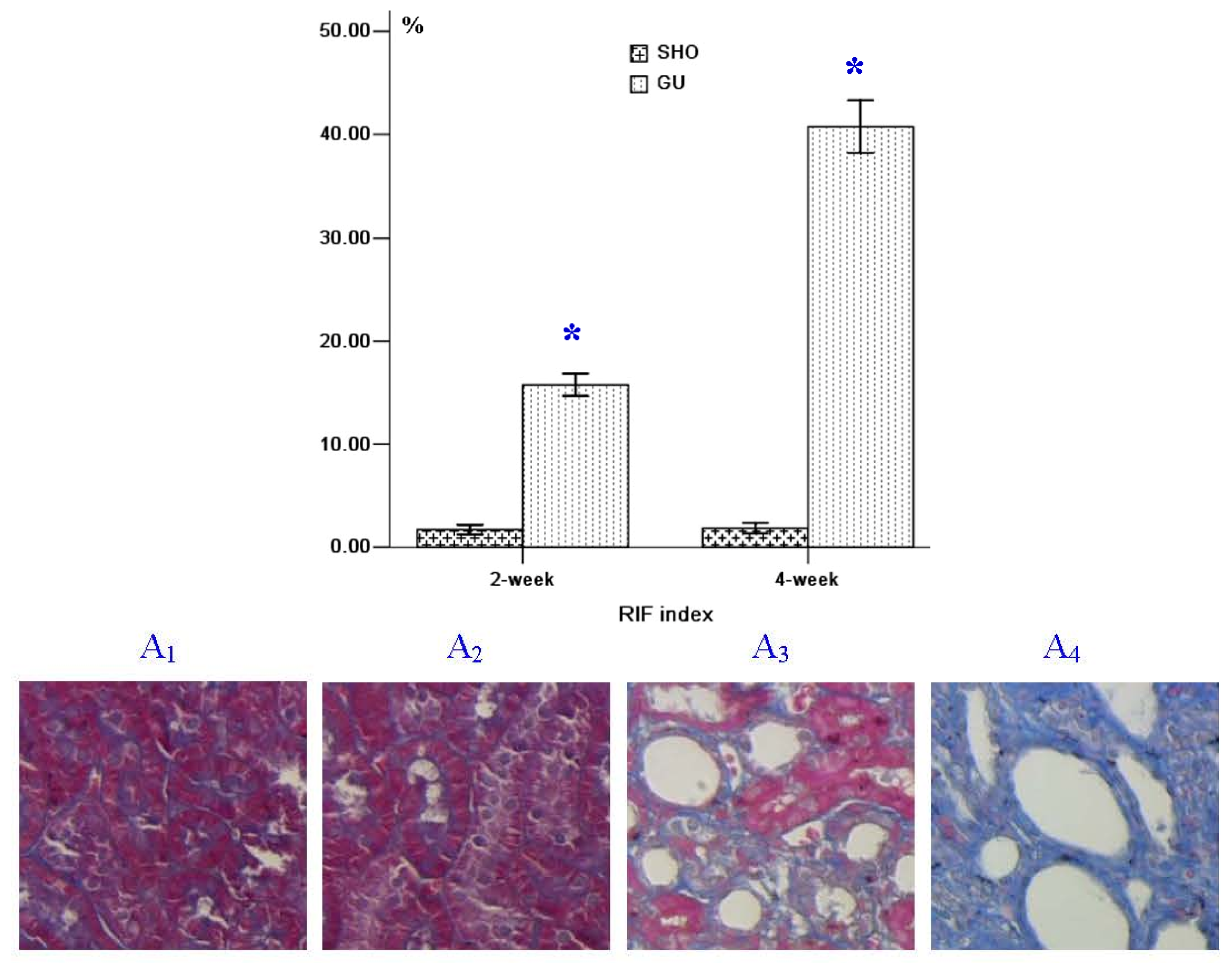
2.1. Renal Morphology
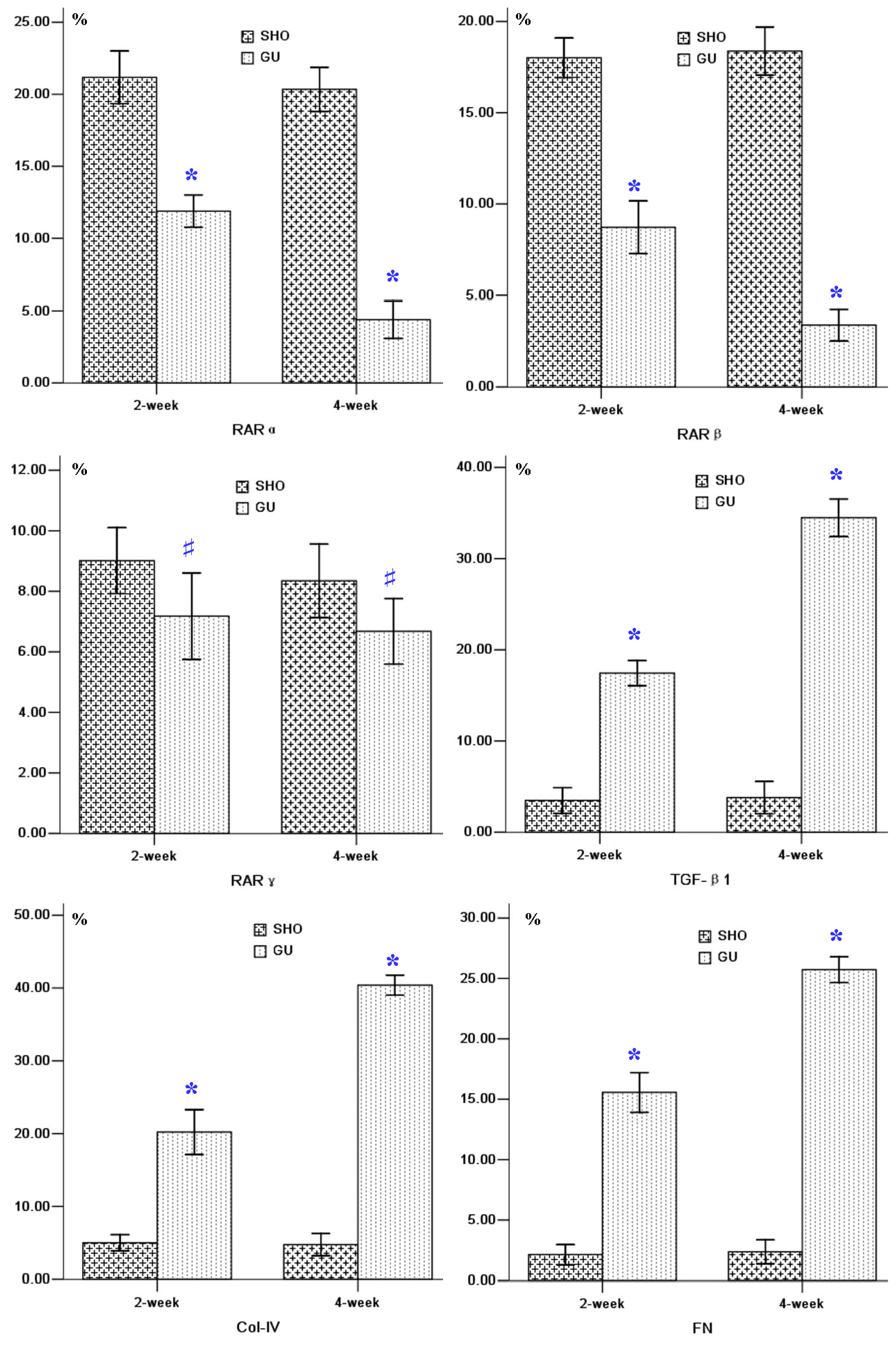
2.2. Protein Expression of RARα, RARβ, RARγ, TGF-β1, Col-IV and FN in Renal Interstitium
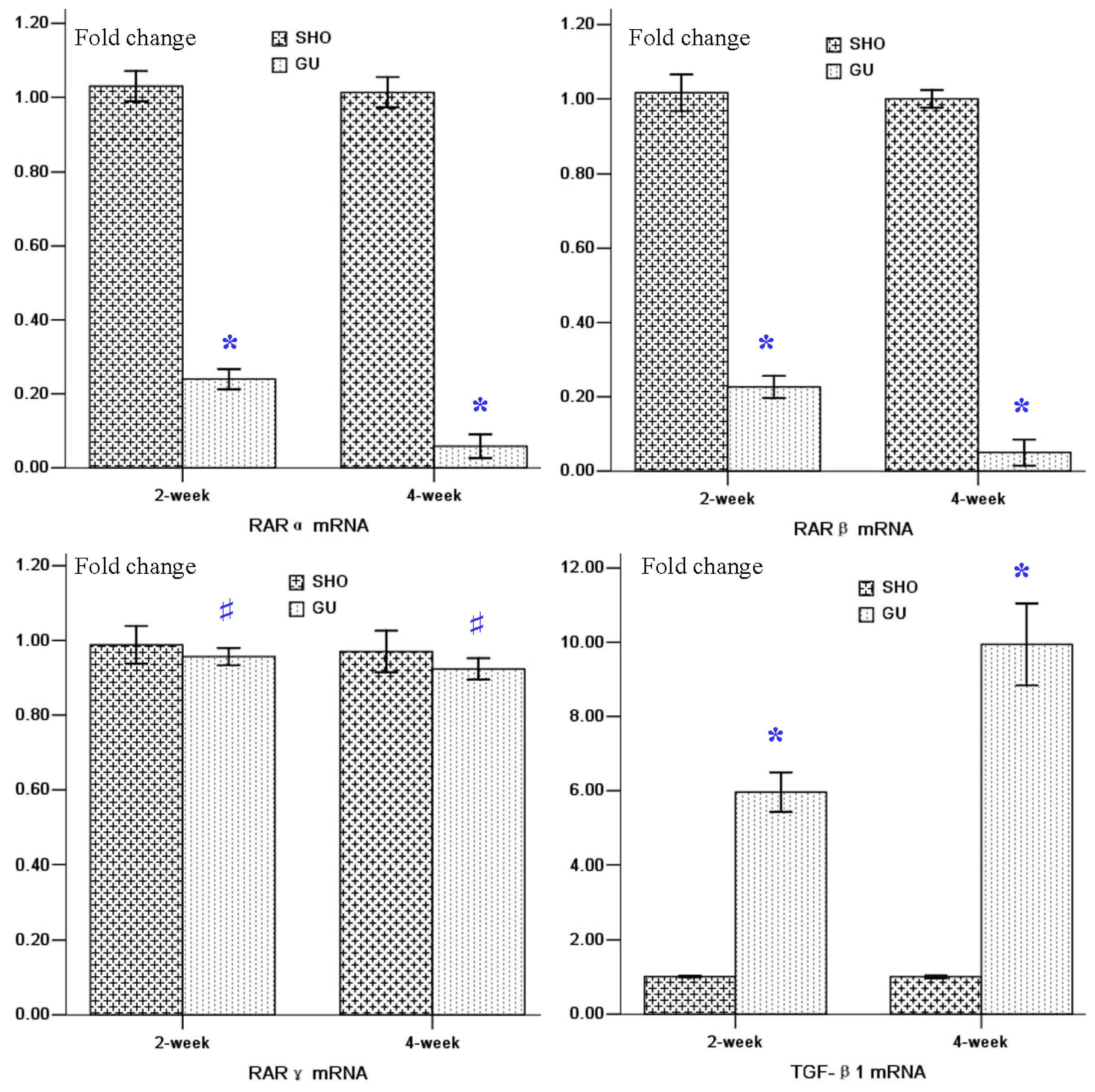
2.3. mRNA Expressions of RARα, RARβ, RARγ and TGF-β1 in Renal Tissue
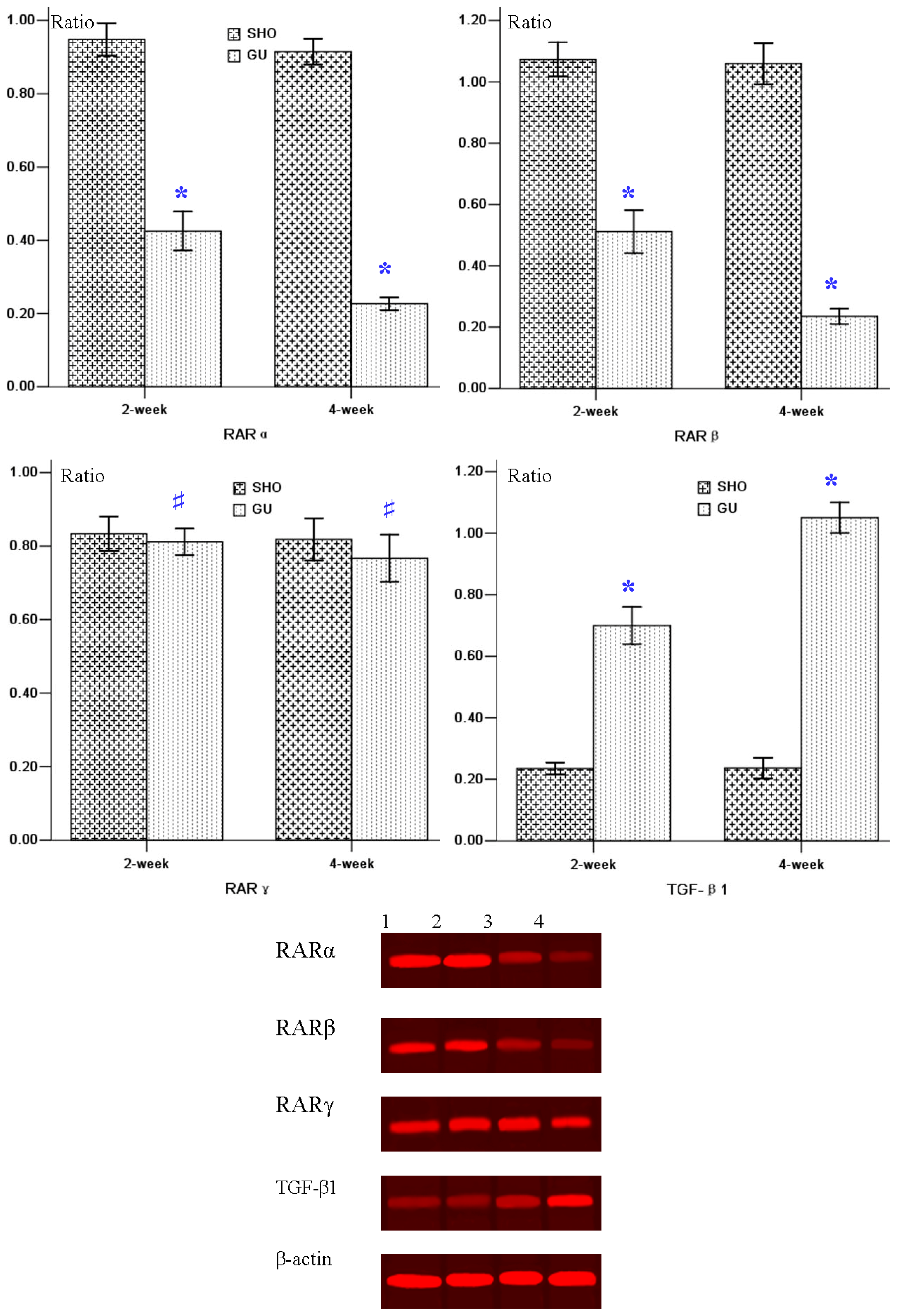
2.4. Western-Blot Analysis for RARα, RARβ, RARγ and TGF-β1 in Renal Tissue
2.5. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Renal Morphology
4.3. Immunohistochemical Analysis of the Protein Expressions of RARα, RARβ, RARγ, TGF-β1, Col-IV and FN
4.4. Real Time Reverse Transcription Polymerase Chain Reaction to Detect mRNA Expressions of RARα, RARβ, RARγ and TGF-β1 in Renal Tissue
4.5. Western-Blot Analysis for RARα, RARβ, RARγ, and TGF-β1 in Renal Tissue
4.6. Statistical Analysis
5. Conclusion
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Duong, V.; Rochette-Egly, C. The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochim. Biophys. Acta 2011, 1812, 1023–1031. [Google Scholar]
- Zhou, T.B.; Qin, Y.H. The potential mechanism for the different expressions of gelatinases induced by all-trans retinoic acid in different cells. J. Recept. Signal Transduct. Res 2012, 32, 129–133. [Google Scholar]
- Sharma, R.P.; Kim, Y.W. Localization of retinoic acid receptors in anterior-human embryo. Exp. Mol. Pathol 1995, 62, 180–189. [Google Scholar]
- Bergheim, I.; Wolfgarten, E.; Bollschweiler, E.; Holscher, A.H.; Bode, C.H.; Parlesak, A. Role of retinoic acid receptors in squamous-cell carcinoma in human esophagus. J. Carcinog 2005, 4, 20. [Google Scholar]
- Macejova, D.; Radikova, Z.; Macho, L.; Liska, J.; Brtko, J. MNU-induced carcinogenesis of rat mammary gland: effect of thyroid hormone on expression of retinoic acid receptors in tumours of mammary gland. Mol. Cell Endocrinol 2005, 244, 47–56. [Google Scholar]
- Zhang, W.; Rashid, A.; Wu, H.; Xu, X.C. Differential expression of retinoic acid receptors and p53 protein in normal, premalignant, and malignant esophageal tissues. J. Cancer Res. Clin. Oncol 2001, 127, 237–242. [Google Scholar]
- Mendelsohn, C.; Lohnes, D.; Decimo, D.; Lufkin, T.; LeMeur, M.; Chambon, P.; Mark, M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 1994, 120, 2749–2771. [Google Scholar]
- Wong, Y.F.; Kopp, J.B.; Roberts, C.; Scambler, P.J.; Abe, Y.; Rankin, A.C.; Dutt, N.; Hendry, B.M.; Xu, Q. Endogenous retinoic acid activity in principal cells and intercalated cells of mouse collecting duct system. PLoS One 2011, 6, e16770. [Google Scholar]
- Terashima, H.; Kato, M.; Yasumo, H.; Tsuchida, H.; Mizuno, M.; Sada, T. A sensitive short-term evaluation of antifibrotic effects using newly established type I collagen reporter transgenic rats. Am. J. Physiol. Renal Physiol 2010, 299, 792–801. [Google Scholar]
- Barnes, J.L.; Glass, W.N. Renal interstitial fibrosis: A critical evaluation of the origin of myofibroblasts. Contrib. Nephrol 2011, 169, 73–93. [Google Scholar]
- Meran, S.; Steadman, R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol 2011, 92, 158–167. [Google Scholar]
- Zhou, T.B.; Qin, Y.H.; Lei, F.Y.; Su, L.N.; Zhao, Y.J.; Huang, W.F. apoE expression in glomerulus and correlation with glomerulosclerosis induced by adriamycin in rats. Ren. Fail 2011, 33, 348–354. [Google Scholar]
- Correa-Costa, M.; Semedo, P.; Monteiro, A.P.; Silva, R.C.; Pereira, R.L.; Goncalves, G.M.; Marques, G.D.; Cenedeze, M.A.; Faleiros, A.C.; Keller, A.C.; et al. Induction of heme oxygenase-1 can halt and even reverse renal tubule-interstitial fibrosis. PLoS One 2010, 5, e14298. [Google Scholar]
- Morisada, N.; Nomura, M.; Nishii, H.; Furuno, Y.; Sakanashi, M.; Sabanai, K.; Toyohira, Y.; Ueno, S.; Watanabe, S.; Tamura, M.; et al. Complete disruption of all nitric oxide synthase genes causes markedly accelerated renal lesion formation following unilateral ureteral obstruction in mice in vivo. J. Pharmacol. Sci 2010, 114, 379–389. [Google Scholar]
- Schaier, M.; Liebler, S.; Schade, K.; Shimizu, F.; Kawachi, H.; Grone, H.J.; Chandraratna, R.; Ritz, E.; Wagner, J. Retinoic acid receptor alpha and retinoid X receptor specific agonists reduce renal injury in established chronic glomerulonephritis of the rat. J. Mol. Med 2004, 82, 116–125. [Google Scholar]
- He, J.C.; Lu, T.C.; Fleet, M.; Sunamoto, M.; Husain, M.; Fang, W.; Neves, S.; Chen, Y.; Shankland, S.; Iyengar, R.; et al. Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J. Am. Soc. Nephrol 2007, 18, 93–102. [Google Scholar]
- Ratnam, K.K.; Feng, X.; Chuang, P.Y.; Verma, V.; Lu, T.C.; Wang, J.; Jin, Y.; Farias, E.F.; Napoli, J.L.; Chen, N.; et al. Role of the retinoic acid receptor-alpha in HIV-associated nephropathy. Kidney Int 2011, 79, 624–634. [Google Scholar]
- Zhong, Y.; Wu, Y.; Liu, R.; Li, Z.; Chen, Y.; Evans, T.; Chuang, P.; Das, B.; He, J.C. Novel retinoic acid receptor alpha agonists for treatment of kidney disease. PLoS One 2011, 6, e27945. [Google Scholar]
- Zhou, T.B.; Qin, Y.H.; Li, Z.Y.; Xu, H.L.; Zhao, Y.J.; Lei, F.Y. All-trans retinoic Acid treatment is associated with prohibitin expression in renal interstitial fibrosis rats. Int. J. Mol. Sci 2012, 13, 2769–2782. [Google Scholar]
- Ma, Y.; Halade, G.V.; Lindsey, M.L. Extracellular matrix and fibroblast communication following myocardial infarction. J. Cardiovasc. Transl. Res. 2012. [Google Scholar] [CrossRef]
- Zhou, T.B.; Qin, Y.H.; Lei, F.Y.; Zhao, Y.J.; Huang, W.F. Association of PAX2 with cell apoptosis in unilateral ureteral obstruction rats. Ren. Fail 2012, 34, 194–202. [Google Scholar]
- Zhang, L.; Zhou, T.B.; Qin, Y.H. Effect of all-trans retinoic acid on unilateral ureteral obstruction model. Nephrology 2012, 17, 521. [Google Scholar]
- Xie, P.; Sun, L.; Nayak, B.; Haruna, Y.; Liu, F.Y.; Kashihara, N.; Kanwar, Y.S. C/EBP-beta modulates transcription of tubulointerstitial nephritis antigen in obstructive uropathy. J. Am. Soc. Nephrol 2009, 20, 807–819. [Google Scholar]
- Zhang, D.; Sun, L.; Xian, W.; Liu, F.; Ling, G.; Xiao, L.; Liu, Y.; Peng, Y.; Haruna, Y.; Kanwar, Y.S. Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-beta/Smad activity. Lab. Invest 2010, 90, 436–447. [Google Scholar]
- Zhou, T.B.; Qin, Y.H.; Zhou, C.; Lei, F.Y.; Zhao, Y.J.; Chen, J.; Su, L.N.; Huang, W.F. Less expression of prohibitin is associated with increased caspase-3 expression and cell apoptosis in renal interstitial fibrosis rats. Nephrology 2012, 17, 189–196. [Google Scholar]
- Zhou, T.B.; Qin, Y.H.; Lei, F.Y.; Su, L.N.; Zhao, Y.J.; Huang, W.F. All-trans retinoic acid regulates the expression of apolipoprotein E in rats with glomerulosclerosis induced by Adriamycin. Exp. Mol. Pathol 2011, 90, 287–294. [Google Scholar]
- Zhou, T.B.; Qin, Y.H.; Ou, C.; Lei, F.Y.; Su, L.N.; Huang, W.F.; Zhao, Y.J. All-trans retinoic acid can regulate the expressions of gelatinases and apolipoprotein E in glomerulosclerosis rats. Vascul. Pharmacol 2011, 55, 169–177. [Google Scholar]
- Sun, D.; Huang, J.; Zhang, Z.; Gao, H.; Li, J.; Shen, M.; Cao, F.; Wang, H. Luteolin limits infarct size and improves cardiac function after myocardium ischemia/reperfusion injury in diabetic rats. PLoS One 2012, 7, e33491. [Google Scholar]
- Funk, A.J.; McCullumsmith, R.E.; Haroutunian, V.; Meador-Woodruff, J.H. Abnormal activity of the MAPK- and cAMP-associated signaling pathways in frontal cortical areas in postmortem brain in schizophrenia. Neuropsychopharmacology 2012, 37, 896–905. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Long, Y.-B.; Qin, Y.-H.; Zhou, T.-B.; Lei, F.-Y. Association of Retinoic Acid Receptors with Extracellular Matrix Accumulation in Rats with Renal Interstitial Fibrosis Disease. Int. J. Mol. Sci. 2012, 13, 14073-14085. https://doi.org/10.3390/ijms131114073
Long Y-B, Qin Y-H, Zhou T-B, Lei F-Y. Association of Retinoic Acid Receptors with Extracellular Matrix Accumulation in Rats with Renal Interstitial Fibrosis Disease. International Journal of Molecular Sciences. 2012; 13(11):14073-14085. https://doi.org/10.3390/ijms131114073
Chicago/Turabian StyleLong, Yao-Bin, Yuan-Han Qin, Tian-Biao Zhou, and Feng-Ying Lei. 2012. "Association of Retinoic Acid Receptors with Extracellular Matrix Accumulation in Rats with Renal Interstitial Fibrosis Disease" International Journal of Molecular Sciences 13, no. 11: 14073-14085. https://doi.org/10.3390/ijms131114073



