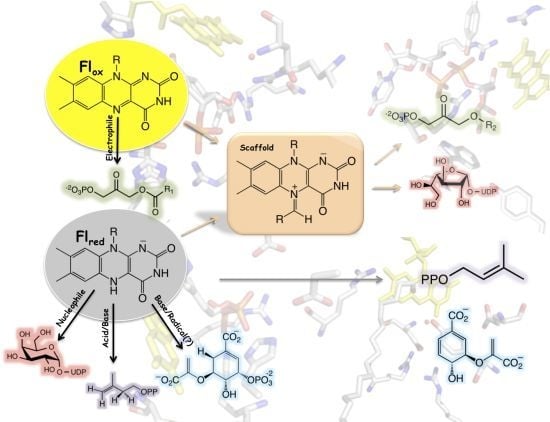
Noncanonical Reactions of Flavoenzymes
Abstract
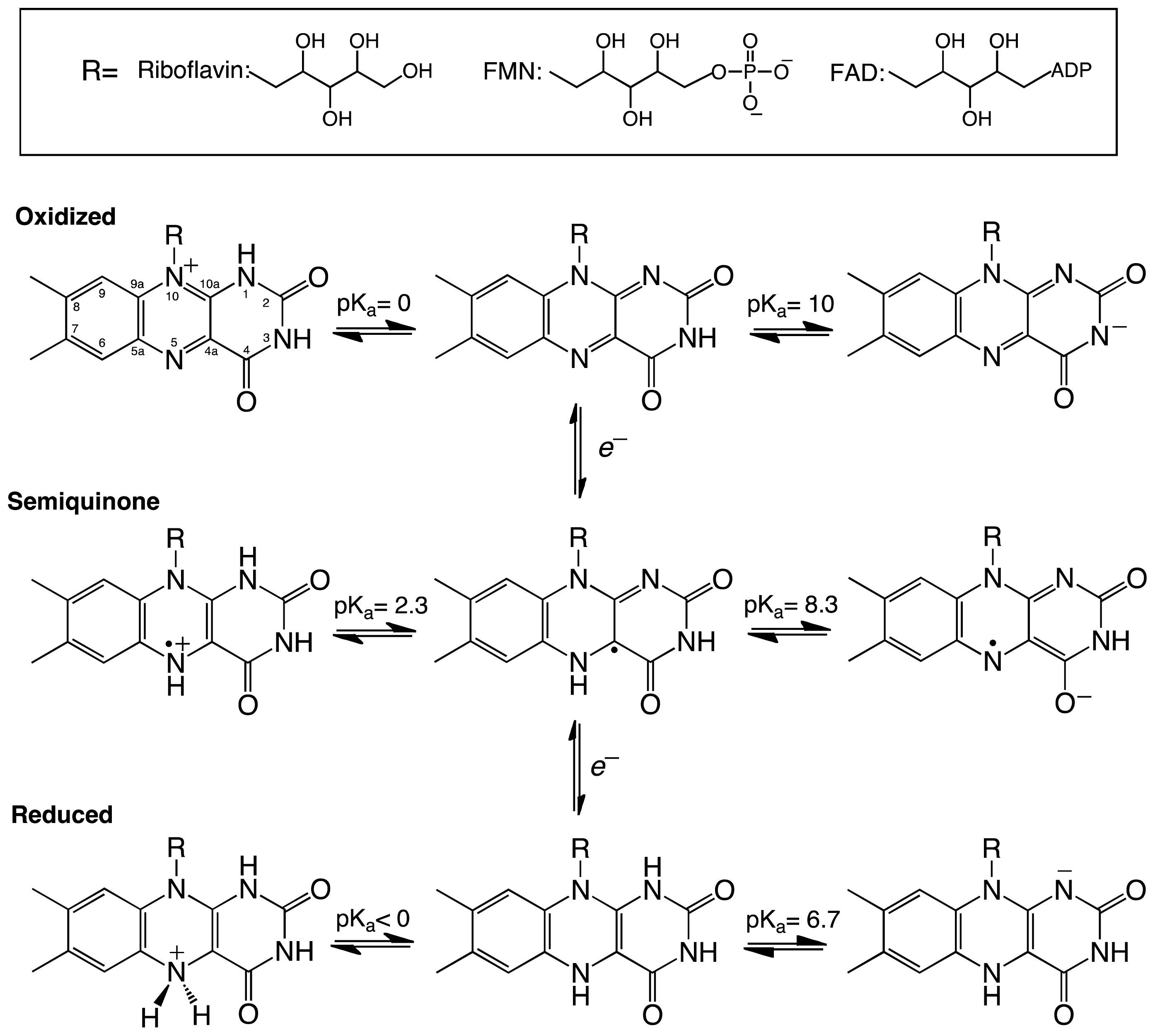
:1. Introduction
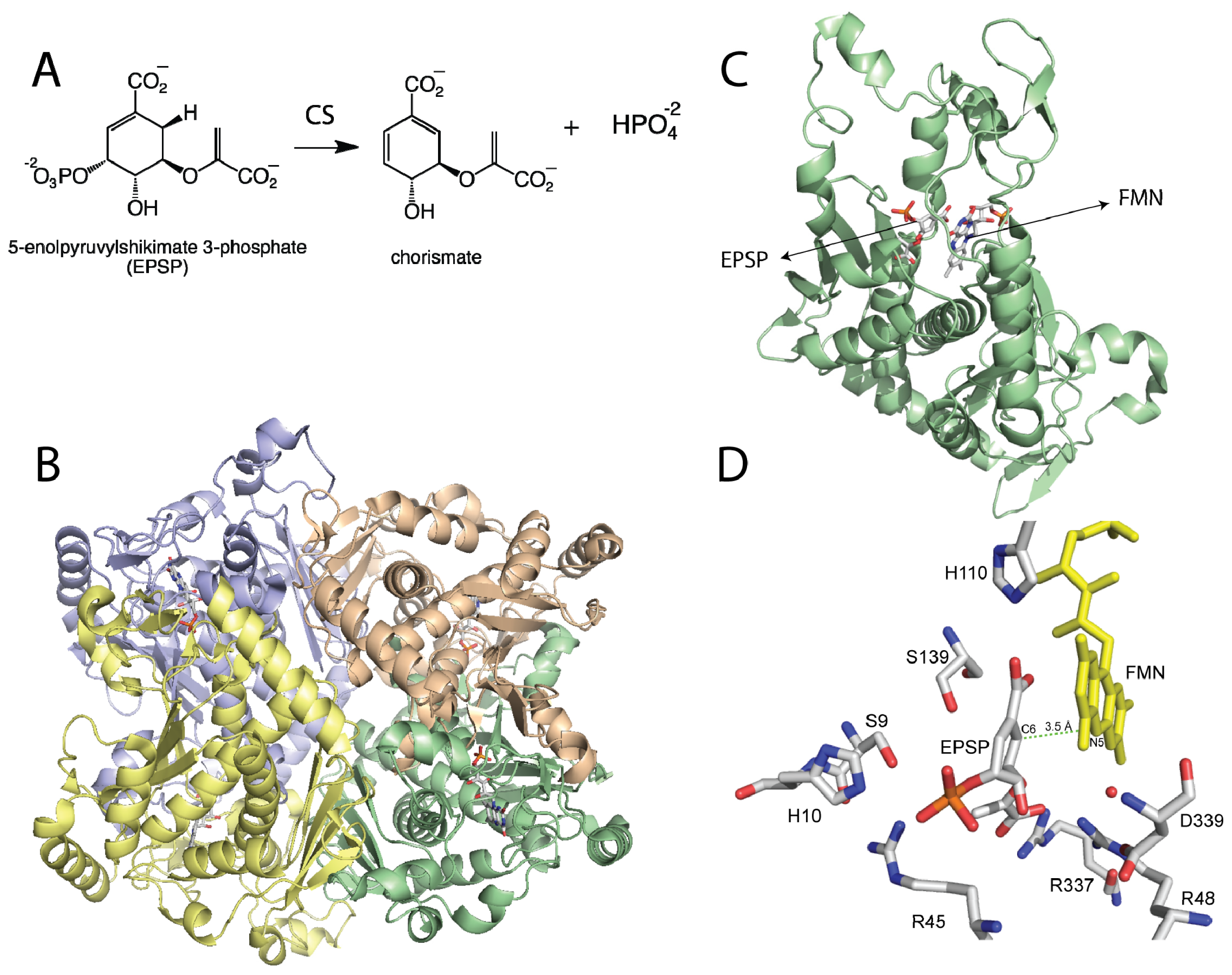
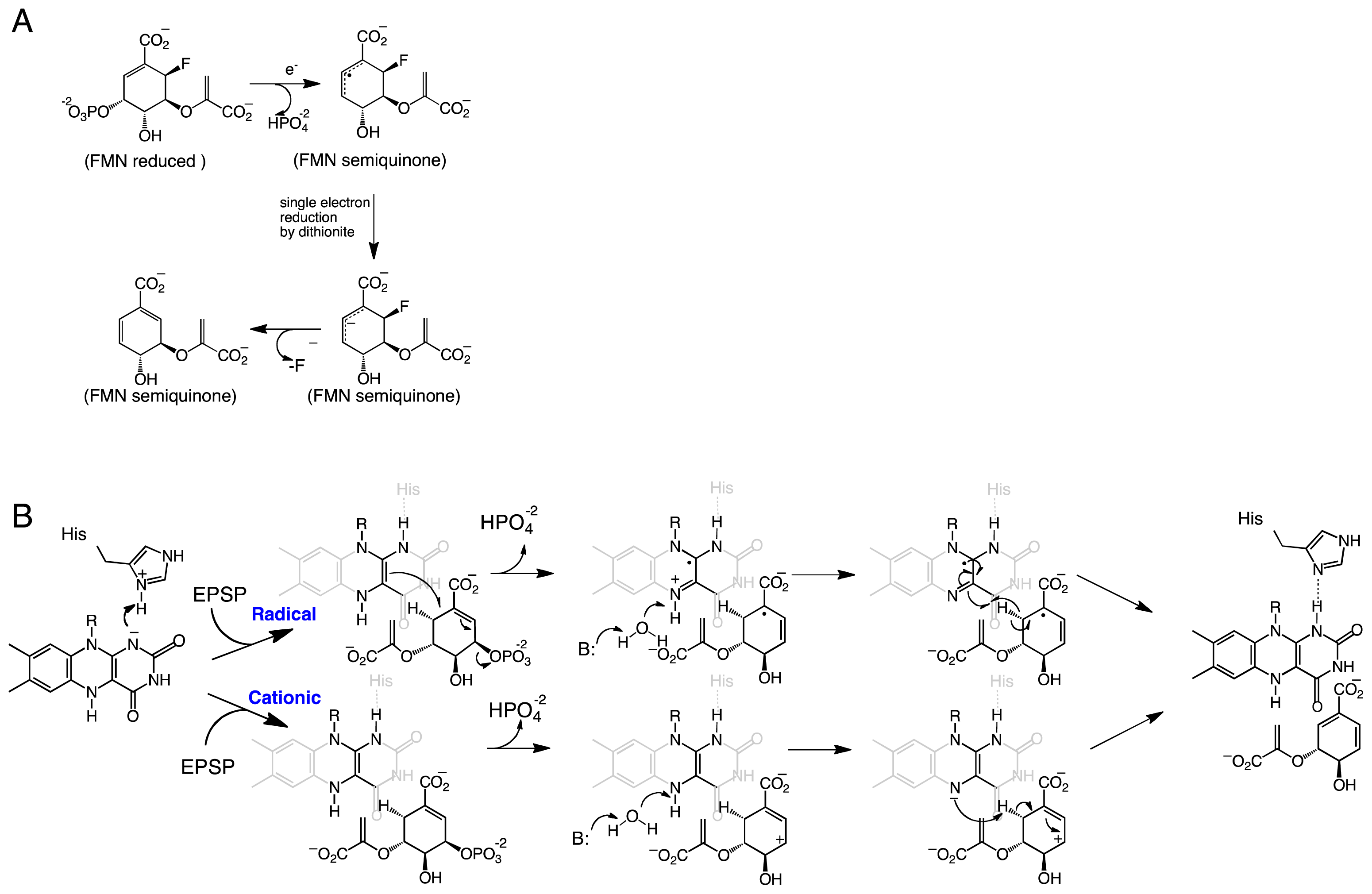
2. Chorismate Synthase
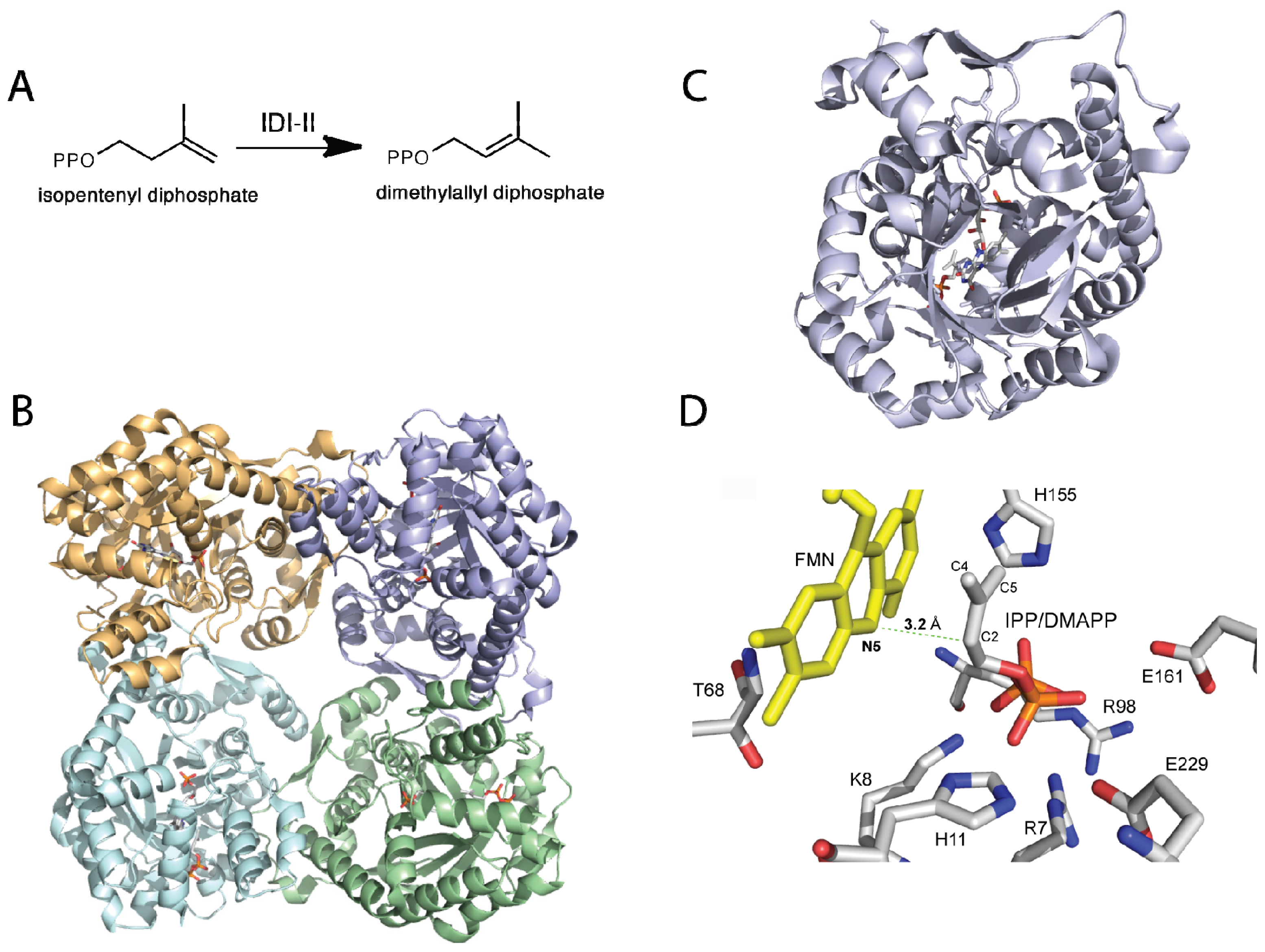
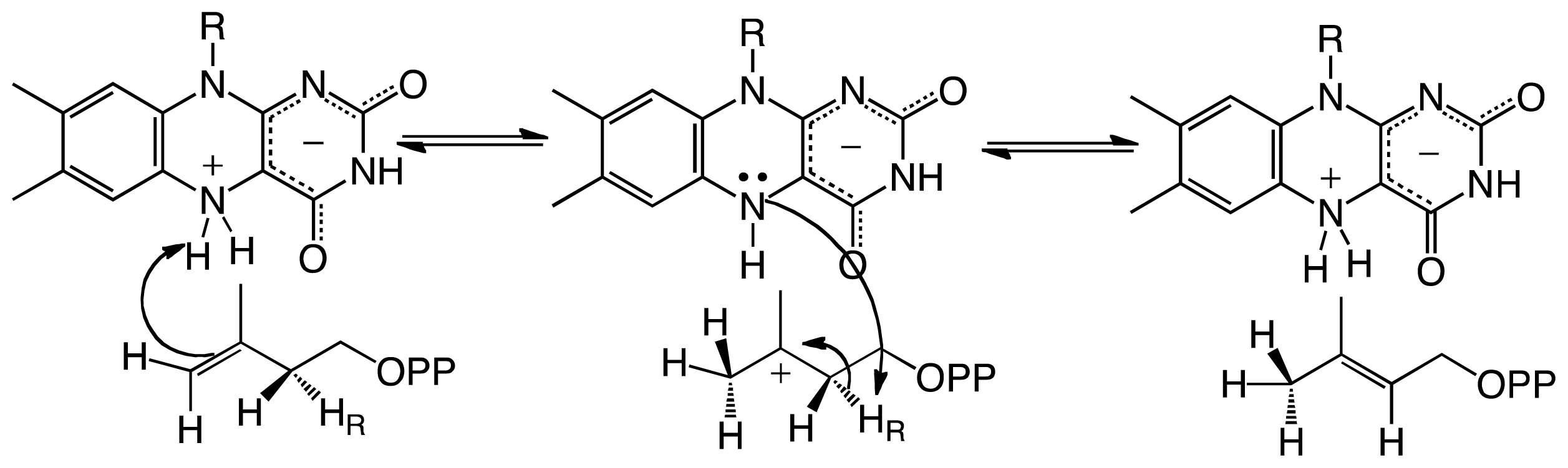
3. Type II Isopentenyl Diphosphate/Dimethylallyl Diphosphate Isomerase
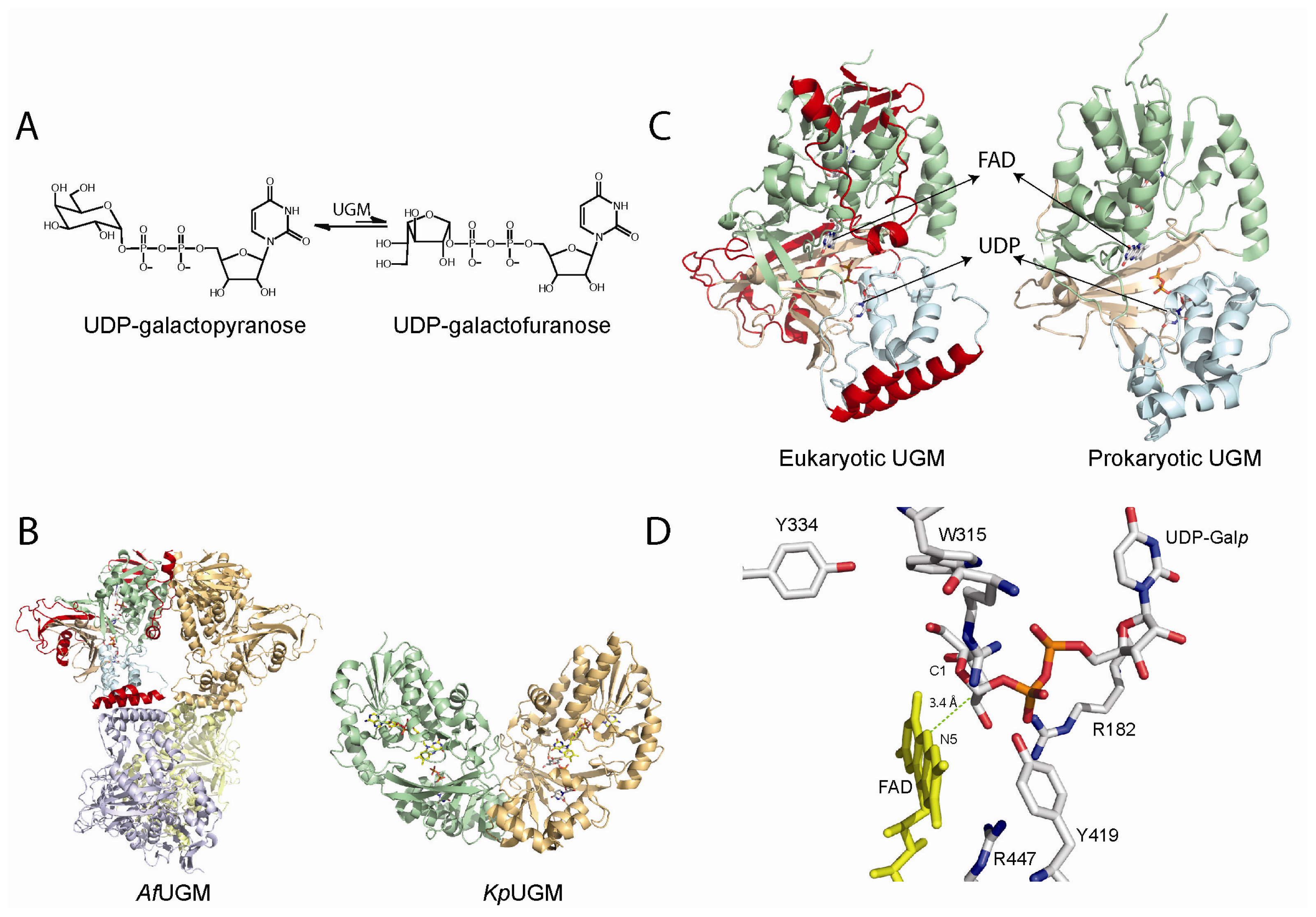
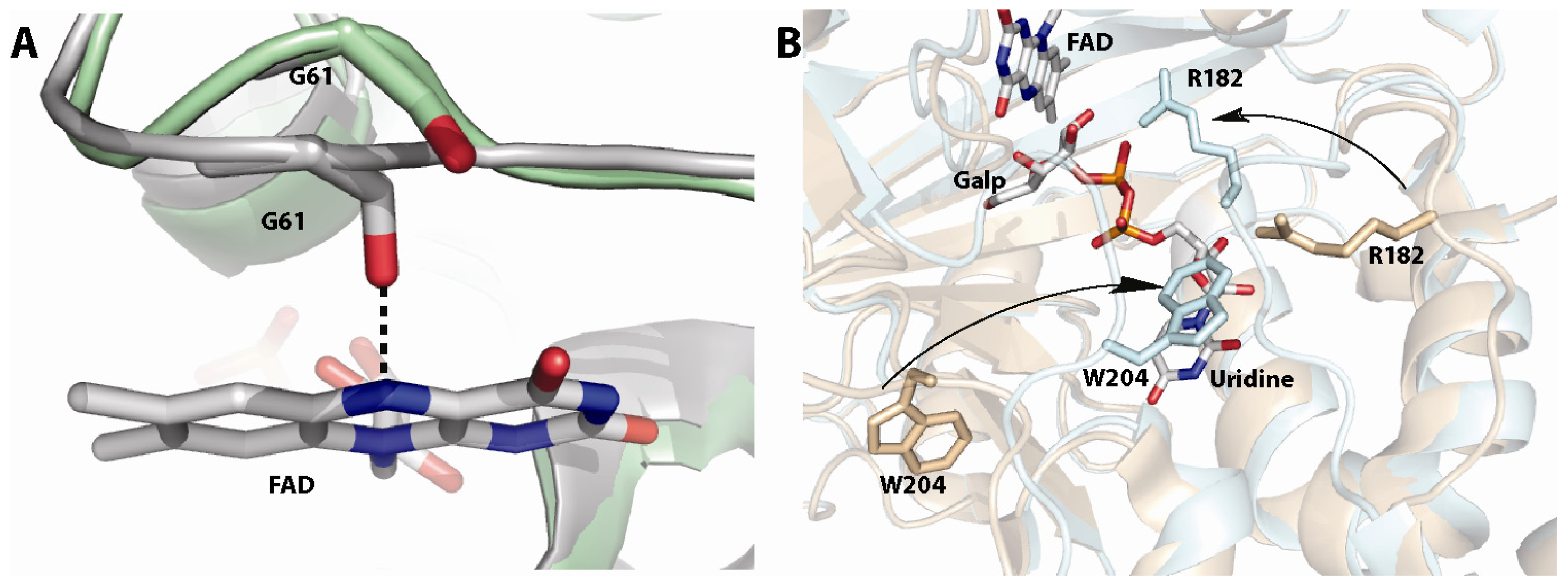
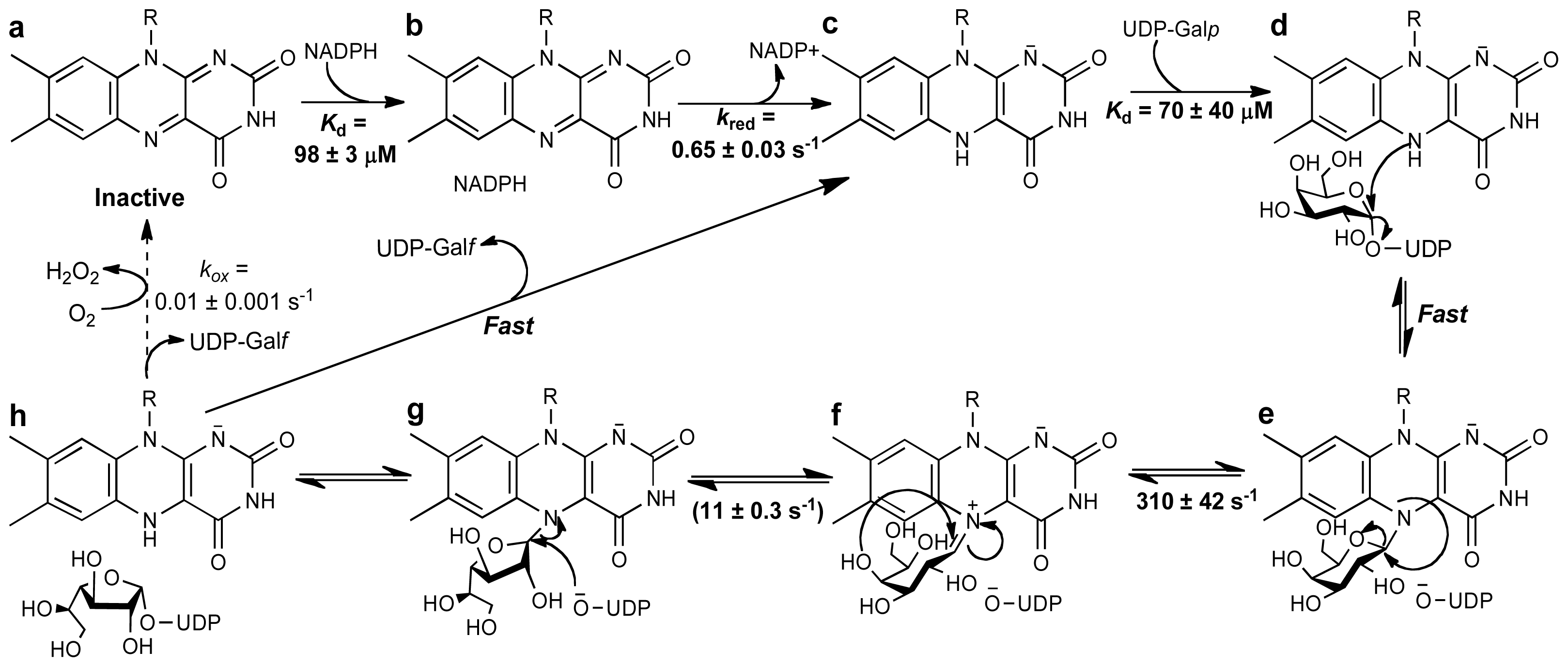
4. UDP-Galactopyranose Mutase
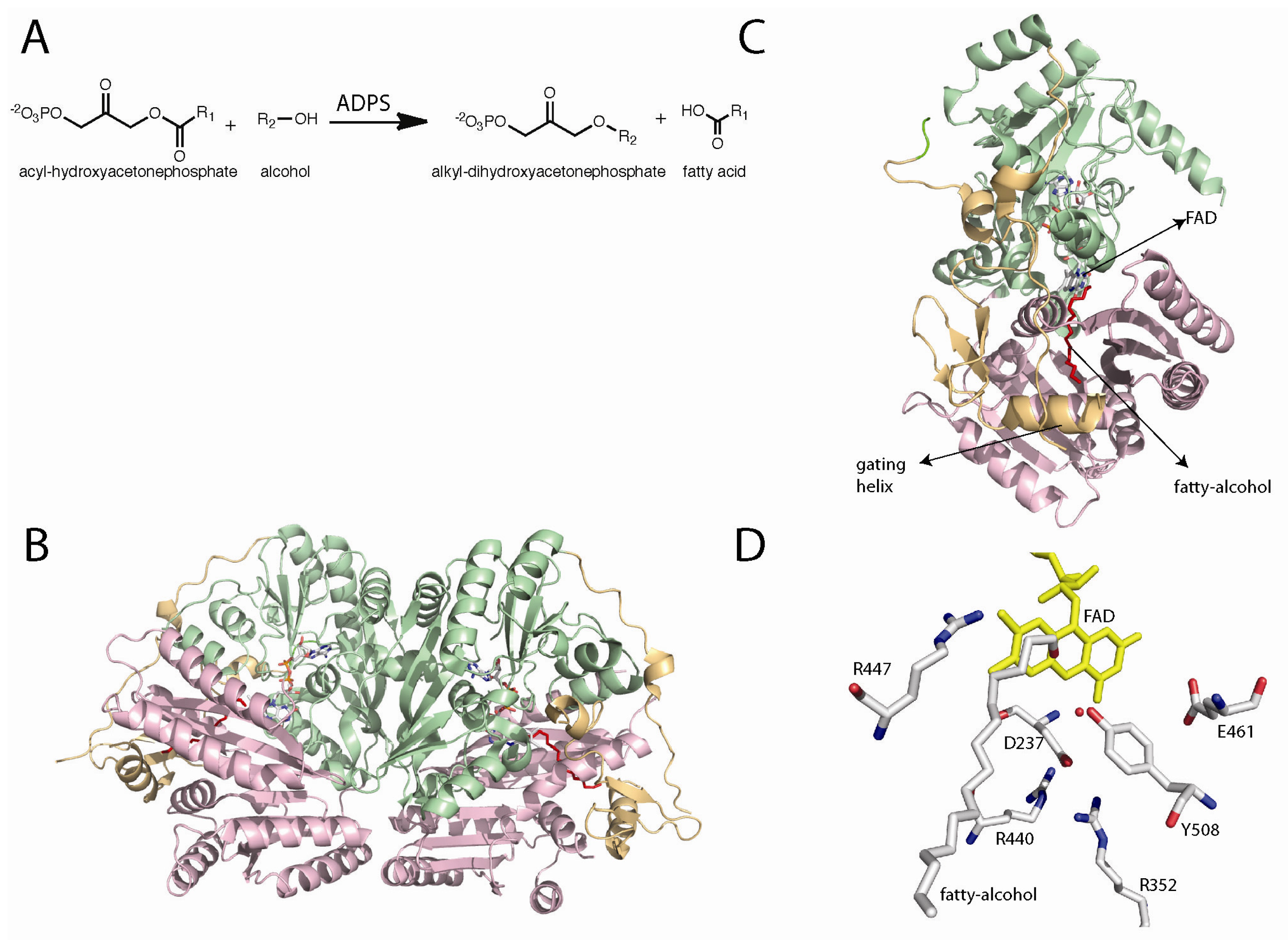
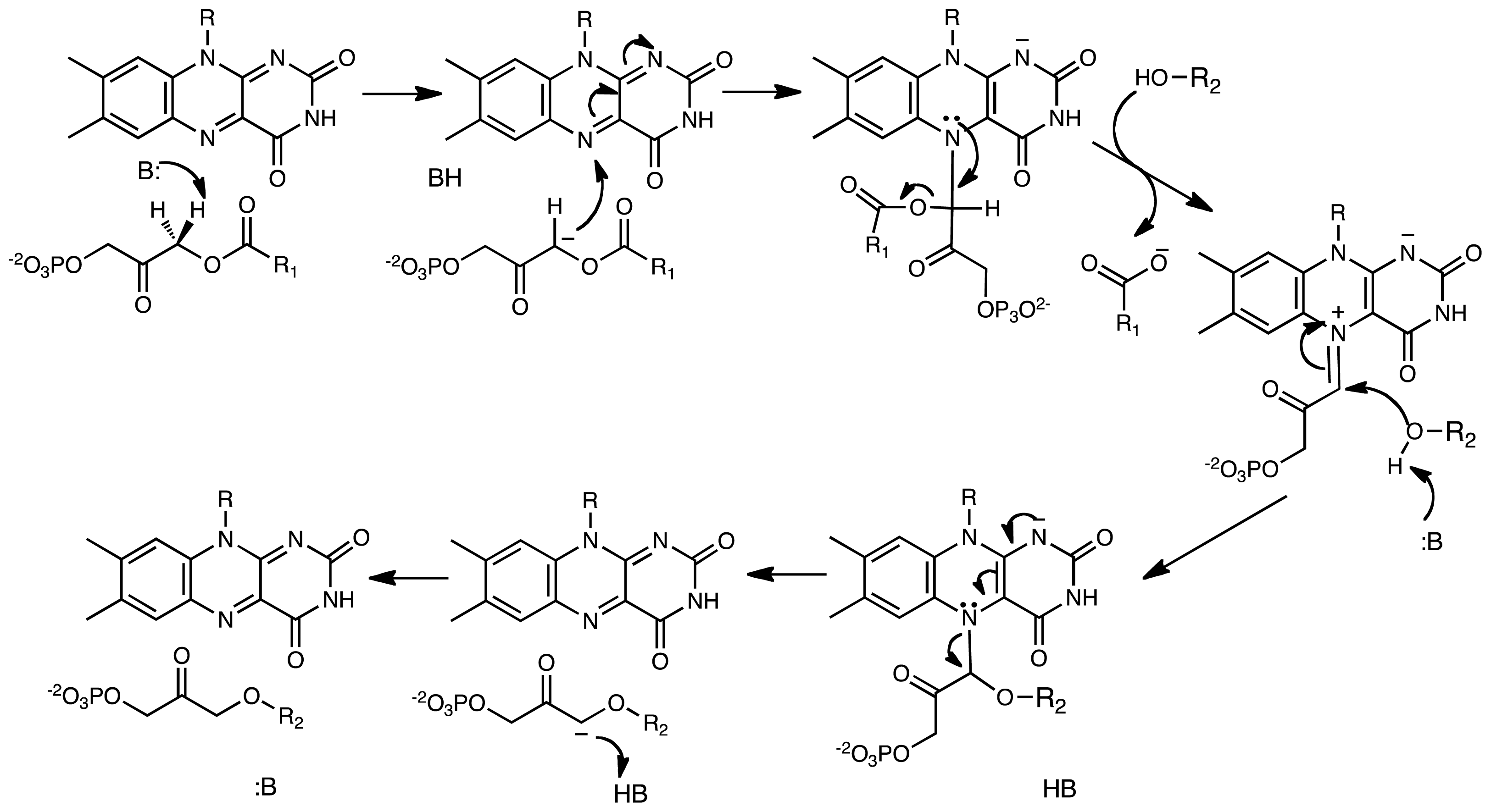
5. Alkyl-dihydroxyacetonephosphate Synthase
6. Concluding Remarks
Acknowledgments
References
- Anfinsen, C.B. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. [Google Scholar]
- Sanchez, G.R.; Chaiken, I.M.; Anfinsen, C.B. Structure-function relationships at the active site of nuclease-T′. J. Biol. Chem 1973, 248, 3653–3659. [Google Scholar]
- Glasner, M.E.; Gerlt, J.A.; Babbitt, P.C. Evolution of enzyme superfamilies. Curr. Opin. Chem. Biol 2006, 10, 492–497. [Google Scholar]
- Frey, P.A.; Hegeman, A.D. Enzymatic Reaction Mechanisms; Oxford University Press: New York, NY, USA, 2007; pp. 126–188. [Google Scholar]
- Perutz, M.F. Haemoglobin: Structure, function and synthesis. Br. Med. Bull 1976, 32, 193–194. [Google Scholar]
- Werck-Reichhart, D.; Feyereisen, R. Cytochromes P450: A success story. Genome Biol. 2000, 1, 3003:1–3003:9. [Google Scholar]
- Fraaije, M.W.; Mattevi, A. Flavoenzymes: Diverse catalysts with recurrent features. Trends Biochem. Sci 2000, 25, 126–132. [Google Scholar]
- Van Berkel, W.J.; Kamerbeek, N.M.; Fraaije, M.W. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J. Biotechnol 2006, 124, 670–689. [Google Scholar]
- Mansoorabadi, S.O.; Thibodeaux, C.J.; Liu, H.W. The diverse roles of flavin coenzymes—nature’s most versatile thespians. J. Org. Chem 2007, 72, 6329–6342. [Google Scholar]
- Massey, V. The chemical and biological versatility of riboflavin. Biochem. Soc. Trans 2000, 28, 283–296. [Google Scholar]
- Muller, F. Chemistry and Biochemistry of Flavoenzymes; CRC Press: Boca Raton, FL, USA, 1991; p. 26. [Google Scholar]
- Massey, V. Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem 1994, 269, 22459–22462. [Google Scholar]
- Fitzpatrick, P.F. Carbanion versus hydride transfer mechanisms in flavoprotein-catalyzed dehydrogenations. Bioorg. Chem 2004, 32, 125–139. [Google Scholar]
- Sobrado, P.; Fitzpatrick, P.F. Analysis of the role of the active site residue Arg98 in the flavoprotein tryptophan 2-monooxygenase, a member of the l-amino oxidase family. Biochemistry 2003, 42, 13826–13832. [Google Scholar]
- Chocklett, S.W.; Sobrado, P. Aspergillus fumigatus SidA is a highly specific ornithine hydroxylase with bound flavin cofactor. Biochemistry 2010, 49, 6777–6783. [Google Scholar]
- Bornemann, S. Flavoenzymes that catalyse reactions with no net redox change. Nat. Prod. Rep 2002, 19, 761–772. [Google Scholar]
- Macheroux, P.; Schmid, J.; Amrhein, N.; Schaller, A. A unique reaction in a common pathway: Mechanism and function of chorismate synthase in the shikimate pathway. Planta 1999, 207, 325–334. [Google Scholar]
- Bornemann, S.; Lowe, D.J.; Thorneley, R.N. Escherichia coli chorismate synthase. Biochem. Soc. Trans 1996, 24, 84–88. [Google Scholar]
- Hill, R.K.; Newkome, G.R. Stereochemistry of chorismic acid biosynthesis. J. Am. Chem. Soc 1969, 91, 5893–5894. [Google Scholar]
- Floss, H.G.; Onderka, D.K.; Carroll, M. Stereochemistry of the 3-deoxy-d-arabino-heptulosonate 7-phosphate synthetase reaction and the chorismate synthetase reaction. J. Biol. Chem 1972, 247, 736–744. [Google Scholar]
- Onderka, D.K.; Floss, H.G. Steric course of the chorismate synthetase reaction and the 3-deoxy-d-arabino-heptulosonate 7-phosphate (DAHP) synthetase reaction. J. Am. Chem. Soc 1969, 91, 5894–5896. [Google Scholar]
- Hasan, N.; Nester, E.W. Purification and properties of chorismate synthase from Bacillus subtilis. J. Biol. Chem 1978, 253, 4993–4998. [Google Scholar]
- Bornemann, S.; Ramjee, M.K.; Balasubramanian, S.; Abell, C.; Coggins, J.R.; Lowe, D.J.; Thorneley, R.N. Escherichia coli chorismate synthase catalyzes the conversion of (6S)-6-fluoro-5-enolpyruvylshikimate-3-phosphate to 6-fluorochorismate. Implications for the enzyme mechanism and the antimicrobial action of (6S)-6-fluoroshikimate. J. Biol. Chem 1995, 270, 22811–22815. [Google Scholar]
- Hill, R.K.; Bock, M.G. Stereochemistry of 1,4-conjugate elimination reactions. J. Am. Chem. Soc 1978, 100, 637–639. [Google Scholar]
- Bornemann, S.; Lowe, D.J.; Thorneley, R.N. The transient kinetics of Escherichia coli chorismate synthase: Substrate consumption, product formation, phosphate dissociation, and characterization of a flavin intermediate. Biochemistry 1996, 35, 9907–9916. [Google Scholar]
- Kitzing, K.; Auweter, S.; Amrhein, N.; Macheroux, P. Mechanism of chorismate synthase. Role of the two invariant histidine residues in the active site. J. Biol. Chem 2004, 279, 9451–9461. [Google Scholar]
- Rauch, G.; Ehammer, H.; Bornemann, S.; Macheroux, P. Mutagenic analysis of an invariant aspartate residue in chorismate synthase supports its role as an active site base. Biochemistry 2007, 46, 3768–3774. [Google Scholar]
- Maclean, J.; Ali, S. The structure of chorismate synthase reveals a novel flavin binding site fundamental to a unique chemical reaction. Structure 2003, 11, 1499–1511. [Google Scholar]
- Macheroux, P.; Bornemann, S.; Ghisla, S.; Thorneley, R.N. Studies with flavin analogs provide evidence that a protonated reduced FMN is the substrate-induced transient intermediate in the reaction of Escherichia coli chorismate synthase. J. Biol. Chem 1996, 271, 25850–25858. [Google Scholar]
- Hawkes, T.R.; Lewis, T.; Coggins, J.R.; Mousdale, D.M.; Lowe, D.J.; Thorneley, R.N. Chorismate synthase. Pre-steady-state kinetics of phosphate release from 5-enolpyruvylshikimate 3-phosphate. Biochem. J 1990, 265, 899–902. [Google Scholar]
- Osborne, A.; Thorneley, R.N.; Abell, C.; Bornemann, S. Studies with substrate and cofactor analogues provide evidence for a radical mechanism in the chorismate synthase reaction. J. Biol. Chem 2000, 275, 35825–35830. [Google Scholar]
- Fitzpatrick, T.B.; Killer, P.; Thomas, R.M.; Jelesarov, I.; Amrhein, N.; Macheroux, P. Chorismate synthase from the hyperthermophile Thermotoga maritima combines thermostability and increased rigidity with catalytic and spectral properties similar to mesophilic counterparts. J. Biol. Chem 2001, 276, 18052–18059. [Google Scholar]
- Macheroux, P.; Petersen, J.; Bornemann, S.; Lowe, D.J.; Thorneley, R.N. Binding of the oxidized, reduced, and radical flavin species to chorismate synthase. An investigation by spectrophotometry, fluorimetry, and electron paramagnetic resonance and electron nuclear double resonance spectroscopy. Biochemistry 1996, 35, 1643–1652. [Google Scholar]
- Land, E.J.; Swallow, A.J. One-electron reactions in biochemical systems as studied by pulse radiolysis. II. Riboflavin. Biochemistry 1969, 8, 2117–2125. [Google Scholar]
- Rauch, G.; Ehammer, H.; Bornemann, S.; Macheroux, P. Replacement of two invariant serine residues in chorismate synthase provides evidence that a proton relay system is essential for intermediate formation and catalytic activity. FEBS J 2008, 275, 1464–1473. [Google Scholar]
- Kuzuyama, T.; Seto, H. Diversity of the biosynthesis of the isoprene units. Nat. Prod. Rep 2003, 20, 171–183. [Google Scholar]
- Lee, S.; Poulter, C.D. Escherichia coli type I isopentenyl diphosphate isomerase: Structural and catalytic roles for divalent metals. J. Am. Chem. Soc 2006, 128, 11545–11550. [Google Scholar]
- Carrigan, C.N.; Poulter, C.D. Zinc is an essential cofactor for type I isopentenyl diphosphate:dimethylallyl diphosphate isomerase. J. Am. Chem. Soc 2003, 125, 9008–9009. [Google Scholar]
- Wouters, J.; Oudjama, Y.; Barkley, S.J.; Tricot, C.; Stalon, V.; Droogmans, L.; Poulter, C.D. Catalytic mechanism of Escherichia coli isopentenyl diphosphate isomerase involves Cys-67, Glu-116, and Tyr-104 as suggested by crystal structures of complexes with transition state analogues and irreversible inhibitors. J. Biol. Chem 2003, 278, 11903–11908. [Google Scholar]
- Wouters, J.; Oudjama, Y.; Stalon, V.; Droogmans, L.; Poulter, C.D. Crystal structure of the C67A mutant of isopentenyl diphosphate isomerase complexed with a mechanism-based irreversible inhibitor. Proteins 2004, 54, 216–221. [Google Scholar]
- Kittleman, W.; Thibodeaux, C.J.; Liu, Y.N.; Zhang, H.; Liu, H.W. Characterization and mechanistic studies of type II isopentenyl diphosphate:dimethylallyl diphosphate isomerase from Staphylococcus aureus. Biochemistry 2007, 46, 8401–8413. [Google Scholar]
- Johnston, J.B.; Walker, J.R.; Rothman, S.C.; Poulter, C.D. Type-2 isopentenyl diphosphate isomerase. Mechanistic studies with cyclopropyl and epoxy analogues. J. Am. Chem. Soc 2007, 129, 7740–7741. [Google Scholar]
- Rothman, S.C.; Johnston, J.B.; Lee, S.; Walker, J.R.; Poulter, C.D. Type II isopentenyl diphosphate isomerase: Irreversible inactivation by covalent modification of flavin. J. Am. Chem. Soc. 2008, 130, 4906–4913. [Google Scholar]
- Thibodeaux, C.J.; Mansoorabadi, S.O.; Kittleman, W.; Chang, W.C.; Liu, H.W. Evidence for the involvement of acid/base chemistry in the reaction catalyzed by the type II isopentenyl diphosphate/dimethylallyl diphosphate isomerase from Staphylococcus aureus. Biochemistry 2008, 47, 2547–2558. [Google Scholar]
- Macheroux, P.; Ghisla, S.; Sanner, C.; Ruterjans, H.; Muller, F. Reduced flavin: NMR investigation of N5-H exchange mechanism, estimation of ionisation constants and assessment of properties as biological catalyst. BMC Biochemistry 2005, 6, 26. [Google Scholar]
- Unno, H.; Yamashita, S.; Ikeda, Y.; Sekiguchi, S.Y.; Yoshida, N.; Yoshimura, T.; Kusunoki, M.; Nakayama, T.; Nishino, T.; Hemmi, H. New role of flavin as a general acid-base catalyst with no redox function in type 2 isopentenyl-diphosphate isomerase. J. Biol. Chem 2009, 284, 9160–9167. [Google Scholar]
- De Ruyck, J.; Pouyez, J.; Rothman, S.C.; Poulter, D.; Wouters, J. Crystal structure of type 2 isopentenyl diphosphate isomerase from Thermus thermophilus in complex with inorganic pyrophosphate. Biochemistry 2008, 47, 9051–9053. [Google Scholar]
- Thibodeaux, C.J.; Chang, W.C.; Liu, H.W. Linear free energy relationships demonstrate a catalytic role for the flavin mononucleotide coenzyme of the type II isopentenyl diphosphate:dimethylallyl diphosphate isomerase. J. Am. Chem. Soc 2010, 132, 9994–9996. [Google Scholar]
- Calveras, J.; Thibodeaux, C.J.; Mansoorabadi, S.O.; Liu, H.W. Stereochemical studies of the type II isopentenyl diphosphate-dimethylallyl diphosphate isomerase implicate the FMN coenzyme in substrate protonation. Chembiochem 2012, 13, 42–46. [Google Scholar]
- Beverley, S.M.; Owens, K.L.; Showalter, M.; Griffith, C.L.; Doering, T.L.; Jones, V.C.; McNeil, M.R. Eukaryotic UDP-galactopyranose mutase (GLF gene) in microbial and metazoal pathogens. Eukaryotic Cell 2005, 4, 1147–1154. [Google Scholar]
- Pedersen, L.L.; Turco, S.J. Galactofuranose metabolism: A potential target for antimicrobial chemotherapy. Cell Mol. Life Sci 2003, 60, 259–266. [Google Scholar]
- Pan, F.; Jackson, M.; Ma, Y.; McNeil, M. Cell wall core galactofuran synthesis is essential for growth of mycobacteria. J. Bacteriol 2001, 183, 3991–3998. [Google Scholar]
- Schmalhorst, P.S.; Krappmann, S.; Vervecken, W.; Rohde, M.; Muller, M.; Braus, G.H.; Contreras, R.; Braun, A.; Bakker, H.; Routier, F.H. Contribution of galactofuranose to the virulence of the opportunistic pathogen Aspergillus fumigatus. Eukaryotic Cell 2008, 7, 1268–1277. [Google Scholar]
- Kleczka, B.; Lamerz, A.C.; van Zandbergen, G.; Wenzel, A.; Gerardy-Schahn, R.; Wiese, M.; Routier, F.H. Targeted gene deletion of Leishmania major UDP-galactopyranose mutase leads to attenuated virulence. J. Biol. Chem 2007, 282, 10498–10505. [Google Scholar]
- Oppenheimer, M.; Valenciano, A.L.; Sobrado, P. Biosynthesis of galactofuranose in kinetoplastids: Novel therapeutic targets for treating leishmaniasis and chagas’ disease. Enzyme Res 2011, 2011, 415976. [Google Scholar]
- Ferguson, M.A. The surface glycoconjugates of trypanosomatid parasites. Philos. Trans. Roy. Soc. London 1997, 352, 1295–1302. [Google Scholar]
- Sobrado, P. Functional Expression and Purification of UDP-Galactopyranose Mutase from Trypanosoma cruzi. In Flavins and Flavoproteins; Frago, S., Gomez-Moreno, C., Medina, M., Eds.; Prensas Universitarias de Zaragoza: Zarazoza, Spain, 2008; pp. 509–513. [Google Scholar]
- Dhatwalia, R.; Singh, H.; Oppenheimer, M.; Karr, D.B.; Nix, J.C.; Sobrado, P.; Tanner, J.J. Crystal structures and small-angle X-ray scattering analysis of UDP-galactopyranose mutase from the pathogenic fungus Aspergillus fumigatus. J. Biol. Chem 2012, 287, 9041–9051. [Google Scholar]
- Dhatwalia, R.; Singh, H.; Oppenheimer, M.; Sobrado, P.; Tanner, J.J. Crystal Structures of Trypanosoma cruzi UDP-Galactopyranose Mutase Implicate Flexibility of the Histidine Loop in Enzyme Activation. Biochemistry 2012, 51, 4968–4979. [Google Scholar]
- Beis, K.; Srikannathasan, V.; Liu, H.; Fullerton, S.W.; Bamford, V.A.; Sanders, D.A.; Whitfield, C.; McNeil, M.R.; Naismith, J.H. Crystal structures of Mycobacteria tuberculosis and Klebsiella pneumoniae UDP-galactopyranose mutase in the oxidised state and Klebsiella pneumoniae UDP-galactopyranose mutase in the (active) reduced state. J. Mol. Biol 2005, 348, 971–982. [Google Scholar]
- Sanders, D.A.; Staines, A.G.; McMahon, S.A.; McNeil, M.R.; Whitfield, C.; Naismith, J.H. UDP-galactopyranose mutase has a novel structure and mechanism. Nat. Struct. Biol 2001, 8, 858–863. [Google Scholar]
- Gruber, T.D.; Westler, W.M.; Kiessling, L.L.; Forest, K.T. X-ray crystallography reveals a reduced substrate complex of UDP-galactopyranose mutase poised for covalent catalysis by flavin. Biochemistry 2009, 48, 9171–9173. [Google Scholar]
- Chad, J.M.; Sarathy, K.P.; Gruber, T.D.; Addala, E.; Kiessling, L.L.; Sanders, D.A. Site-directed mutagenesis of UDP-galactopyranose mutase reveals a critical role for the active-site, conserved arginine residues. Biochemistry 2007, 46, 6723–6732. [Google Scholar]
- Oppenheimer, M.; Valenciano, A.L.; Kizjakina, K.; Qi, J.; Sobrado, P. Chemical mechanism of UDP-galactopyranose mutase from Trypanosoma cruzi: A potential drug target against Chagas’ disease. PLoS One 2012, 7, e32918. [Google Scholar]
- Oppenheimer, M.; Valenciano, A.L.; Sobrado, P. Isolation and characterization of functional Leishmania major virulence factor UDP-galactopyranose mutase. Biochem. Biophys. Res. Commun 2011, 407, 552–556. [Google Scholar]
- Oppenheimer, M.; Poulin, M.B.; Lowary, T.L.; Helm, R.F.; Sobrado, P. Characterization of recombinant UDP-galactopyranose mutase from Aspergillus fumigatus. Arch. Biochem. Biophys 2010, 502, 31–38. [Google Scholar]
- Zhang, Q.; Liu, H.-W. Studies of UDP-Galactopyranose mutase from Escherichia coli: An unusual role of reduced fad in its catalysis. J. Am. CHem. Soc 2000, 122, 9065–9070. [Google Scholar]
- Barlow, J.N.; Girvin, M.E.; Blanchard, J.S. Positional Isotope Exchange Catalyzed by UDP-Galactopyranose Mutase. J. Am. Chem. Soc. 1999, 121, 6968–6969. [Google Scholar]
- Zhang, Q.; Liu, H. Mechanistic investigation of UDP-galactopyranose mutase from Escherichia coli using 2- and 3-fluorinated UDP-galactofuranose as probes. J. Am. Chem. Soc 2001, 123, 6756–6766. [Google Scholar]
- Fullerton, S.W.; Daff, S.; Sanders, D.A.; Ingledew, W.J.; Whitfield, C.; Chapman, S.K.; Naismith, J.H. Potentiometric analysis of UDP-galactopyranose mutase: Stabilization of the flavosemiquinone by substrate. Biochemistry 2003, 42, 2104–2109. [Google Scholar]
- Huang, Z.; Zhang, Q.; Liu, H.W. Reconstitution of UDP-galactopyranose mutase with 1-deaza-FAD and 5-deaza-FAD: Analysis and mechanistic implications. Bioorg. Chem 2003, 31, 494–502. [Google Scholar]
- Soltero-Higgin, M.; Carlson, E.E.; Gruber, T.D.; Kiessling, L.L. A unique catalytic mechanism for UDP-galactopyranose mutase. Nat. Struct. Mol. Biol 2004, 11, 539–543. [Google Scholar]
- Caravano, A.; Sinay, P.; Vincent, S.P. 1,4-Anhydrogalactopyranose is not an intermediate of the mutase catalyzed UDP-galactopyranose/furanose interconversion. Bioorg. Med. Chem. Lett 2006, 16, 1123–1125. [Google Scholar]
- Barlow, J.N.; Blanchard, J.S. Enzymatic synthesis of UDP-(3-deoxy-3-fluoro)-d-galactose and UDP-(2-deoxy-2-fluoro)-d-galactose and substrate activity with UDP-galactopyranose mutase. Carbohydr. Res 2000, 328, 473–480. [Google Scholar]
- Partha, S.K.; van Straaten, K.E.; Sanders, D.A. Structural basis of substrate binding to UDP-galactopyranose mutase: Crystal structures in the reduced and oxidized state complexed with UDP-galactopyranose and UDP. J. Mol. Biol 2009, 394, 864–877. [Google Scholar]
- Van Straaten, K.E.; Routier, F.H.; Sanders, D.A. Structural Insight into the Unique Substrate Binding Mechanism and Flavin Redox State of UDP-galactopyranose Mutase from Aspergillus fumigatus. J. Biol. Chem 2012, 287, 10780–10790. [Google Scholar]
- Sun, H.G.; Ruszczycky, M.W.; Chang, W.C.; Thibodeaux, C.J.; Liu, H.W. Nucleophilic participation of reduced flavin coenzyme in mechanism of UDP-galactopyranose mutase. J. Biol. Chem 2012, 287, 4602–4608. [Google Scholar]
- Barlow, J.N.; Marcinkeviciene, J.; Blancherd, J.S. The Enzymatic Converstion of UDP-Galactopyranose to UDP-Galactofuranose. In Enzymatic Mechanims; Frey, P.A., Norththrop, D.B., Eds.; IOS Press: Amsterdam, The Netherland, 1999; pp. 98–106. [Google Scholar]
- Hajra, A.K. Acyl dihydroxyacetone phosphate: Precursor of alkyl ethers. Biochem. Biophys. Res. Commun 1970, 39, 1037–1044. [Google Scholar]
- Van den Bosch, H.; Schalkwijk, C.G.; Schrakamp, G.; Wanders, R.J.; Schutgens, R.B.; Schram, A.W.; Tager, J.M. Aberration in de novo ether lipid biosynthesis in peroxisomal disorders. Prog. Clin. Biol. Res 1988, 282, 139–150. [Google Scholar]
- De Vet, E.C.; van den Bosch, H. Alkyl-dihydroxyacetonephosphate synthase. Cell Biochem. Biophys 2000, 32, 117–121. [Google Scholar]
- De Vet, E.C.; Hilkes, Y.H.; Fraaije, M.W.; van den Bosch, H. Alkyl-dihydroxyacetonephosphate synthase. Presence and role of flavin adenine dinucleotide. J. Biol. Chem 2000, 275, 6276–6283. [Google Scholar]
- Van den Bosch, H.; Schutgens, R.B.; Wanders, R.J.; Tager, J.M. Biochemistry of peroxisomes. Annu. Rev. Biochem 1992, 61, 157–197. [Google Scholar]
- De Vet, E.C.; Ijlst, L.; Oostheim, W.; Dekker, C.; Moser, H.W.; van Den Bosch, H.; Wanders, R.J. Ether lipid biosynthesis: Alkyl-dihydroxyacetonephosphate synthase protein deficiency leads to reduced dihydroxyacetonephosphate acyltransferase activities. J. Lipid Res 1999, 40, 1998–2003. [Google Scholar]
- White, A.L.; Modaff, P.; Holland-Morris, F.; Pauli, R.M. Natural history of rhizomelic chondrodysplasia punctata. Am. J Med. Genet 2003, 118A, 332–342. [Google Scholar]
- Razeto, A.; Mattiroli, F.; Carpanelli, E.; Aliverti, A.; Pandini, V.; Coda, A.; Mattevi, A. The crucial step in ether phospholipid biosynthesis: Structural basis of a noncanonical reaction associated with a peroxisomal disorder. Structure 2007, 15, 683–692. [Google Scholar]
- De Vet, E.C.; van den Bosch, H. Characterization of recombinant guinea pig alkyl-dihydroxyacetonephosphate synthase expressed in Escherichia coli. Kinetics, chemical modification and mutagenesis. Biochim. Biophys. Acta 1999, 1436, 299–306. [Google Scholar]
- De Vet, E.C.; Prinsen, H.C.; van den Bosch, H. Nucleotide sequence of a cDNA clone encoding a Caenorhabditis elegans homolog of mammalian alkyl-dihydroxyacetonephosphate synthase: Evolutionary switching of peroxisomal targeting signals. Biochem. Biophys. Res. Commun 1998, 242, 277–281. [Google Scholar]
- De Vet, E.C.; van den Bosch, H. Nucleotide sequence of alkyl-dihydroxyacetonephosphate synthase cDNA from Dictyostelium discoideum. Biochem. Biophys. Res. Commun 1998, 252, 629–633. [Google Scholar]
- De Vet, E.C.; van den Broek, B.T.; van den Bosch, H. Nucleotide sequence of human alkyl-dihydroxyacetonephosphate synthase cDNA reveals the presence of a peroxisomal targeting signal 2. Biochim. Biophys. Acta 1997, 1346, 25–29. [Google Scholar]
- Brown, A.J.; Snyder, F. Alkyldihydroxyacetone-P synthase. Solubilization, partial purification, new assay method, and evidence for a ping-pong mechanism. J. Biol. Chem 1982, 257, 8835–8839. [Google Scholar]
- Brown, A.J.; Snyder, F. Solubilization of alkyldihydroxyacetone-P synthase from Ehrlich ascites cell microsomal membranes. Biochem. Biophys. Res. Commun 1979, 90, 278–284. [Google Scholar]
- Friedberg, S.J.; Heifetz, A. The formation of tritiated O-alkyl lipid from acyldihydroxyacetone phosphate in the presence of tritiated water. Biochemistry 1975, 14, 570–574. [Google Scholar]
- Friedberg, S.J.; Heifetz, A.; Greene, R.C. Loss of hydrogen from dihydroxyacetone phosphate during glyceryl ether synthesis. J. Biol. Chem 1971, 246, 5822–5827. [Google Scholar]
- Friedberg, S.J.; Heifetz, A.; Greene, R.C. Studies on the mechanism of O-alkyl lipid synthesis. Biochemistry 1972, 11, 297–301. [Google Scholar]
- Brown, A.J.; Glish, G.L.; McBay, E.H.; Snyder, F. Alkyldihydroxyacetonephosphate synthase mechanism: 18O studies of fatty acid release from acyldihydroxyacetone phosphate. Biochemistry 1985, 24, 8012–8016. [Google Scholar]
- Friedberg, S.J.; Weintraub, S.T.; Singer, M.R.; Greene, R.C. The mechanism of ether bond formation in O-alkyl lipid synthesis in Ehrlich ascites tumor. Unusual cleavage of the fatty acid moiety of acyl dihydroxyacetone phosphate. J. Biol. Chem 1983, 258, 136–142. [Google Scholar]
- Fraaije, M.W.; van Berkel, W.J.; Benen, J.A.; Visser, J.; Mattevi, A. A novel oxidoreductase family sharing a conserved FAD-binding domain. Trends Biochem. Sci 1998, 23, 206–207. [Google Scholar]
- Razeto, A.; Mattiroli, F.; Bossi, R.; Coda, A.; Mattevi, A. Identifying a recombinant alkyldihydroxyacetonephosphate synthase suited for crystallographic studies. Protein Expr. Purif 2007, 55, 343–351. [Google Scholar]
- Gadda, G.; Fitzpatrick, P.F. Biochemical and physical characterization of the active FAD-containing form of nitroalkane oxidase from Fusarium oxysporum. Biochemistry 1998, 37, 6154–6164. [Google Scholar]
- Gadda, G.; Edmondson, R.D.; Russell, D.H.; Fitzpatrick, P.F. Identification of the naturally occurring flavin of nitroalkane oxidase from fusarium oxysporum as a 5-nitrobutyl-FAD and conversion of the enzyme to the active FAD-containing form. J. Biol. Chem 1997, 272, 5563–5570. [Google Scholar]
- Heroux, A.; Bozinovski, D.M.; Valley, M.P.; Fitzpatrick, P.F.; Orville, A.M. Crystal Structures of Intermediates in the Nitroalkane Oxidase Reaction. Biochemistry 2009, 48, 3407–3416. [Google Scholar]
- Nagpal, A.; Valley, M.P.; Fitzpatrick, P.F.; Orville, A.M. Crystal structures of nitroalkane oxidase: Insights into the reaction mechanism from a covalent complex of the flavoenzyme trapped during turnover. Biochemistry 2006, 45, 1138–1150. [Google Scholar]
- Fitzpatrick, P.F.; Orville, A.M.; Nagpal, A.; Valley, M.P. Nitroalkane oxidase, a carbanion-forming flavoprotein homologous to acyl-CoA dehydrogenase. Arch. Biochem. Biophys 2005, 433, 157–165. [Google Scholar]
| Role of flavin cofactor | Flavin redox state * | Enzymes |
|---|---|---|
| Acid/base chemistry | FMNred | Type II isopentenyl diphosphate isomerase |
| Radical/base | FMNred | Chorismate synthase |
| Nucleophile/scaffold | FADred | UDP-galactopyranose mutase |
| Electrophile/scaffold | FADox | Alkyl-dihydryoxyacetonephosphate synthase |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sobrado, P. Noncanonical Reactions of Flavoenzymes. Int. J. Mol. Sci. 2012, 13, 14219-14242. https://doi.org/10.3390/ijms131114219
Sobrado P. Noncanonical Reactions of Flavoenzymes. International Journal of Molecular Sciences. 2012; 13(11):14219-14242. https://doi.org/10.3390/ijms131114219
Chicago/Turabian StyleSobrado, Pablo. 2012. "Noncanonical Reactions of Flavoenzymes" International Journal of Molecular Sciences 13, no. 11: 14219-14242. https://doi.org/10.3390/ijms131114219