Optimization of Pressurized Liquid Extraction of Three Major Acetophenones from Cynanchum bungei Using a Box-Behnken Design
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Variables
2.2. Model Fitting
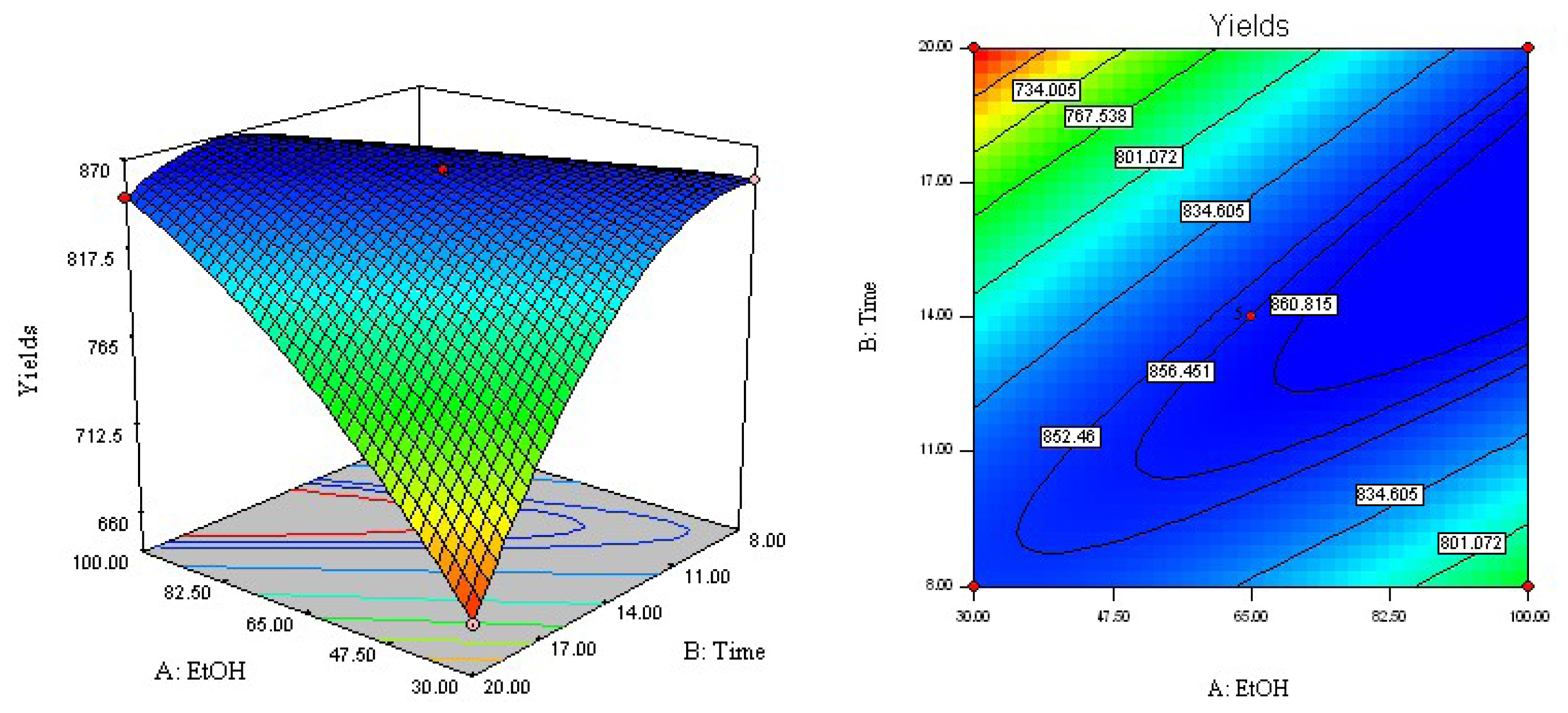
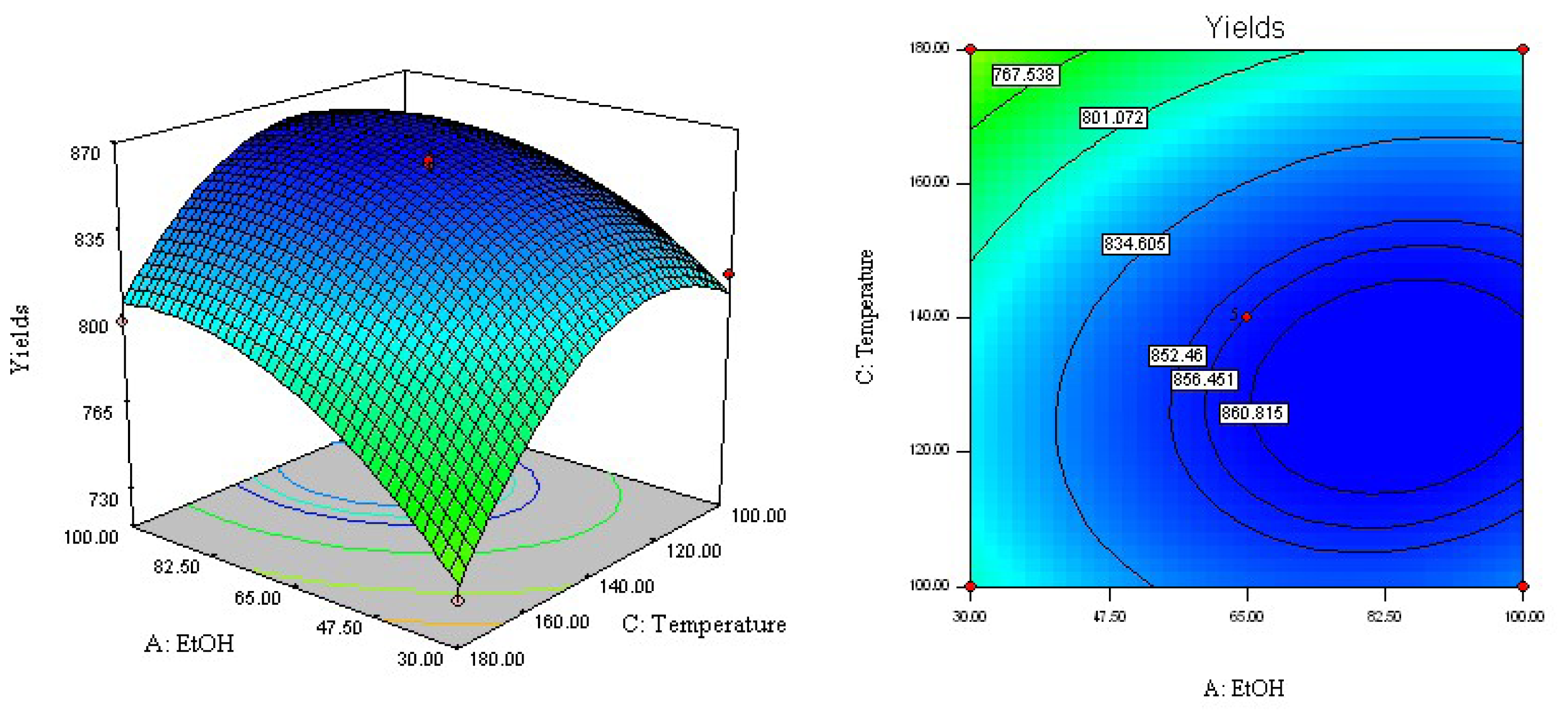
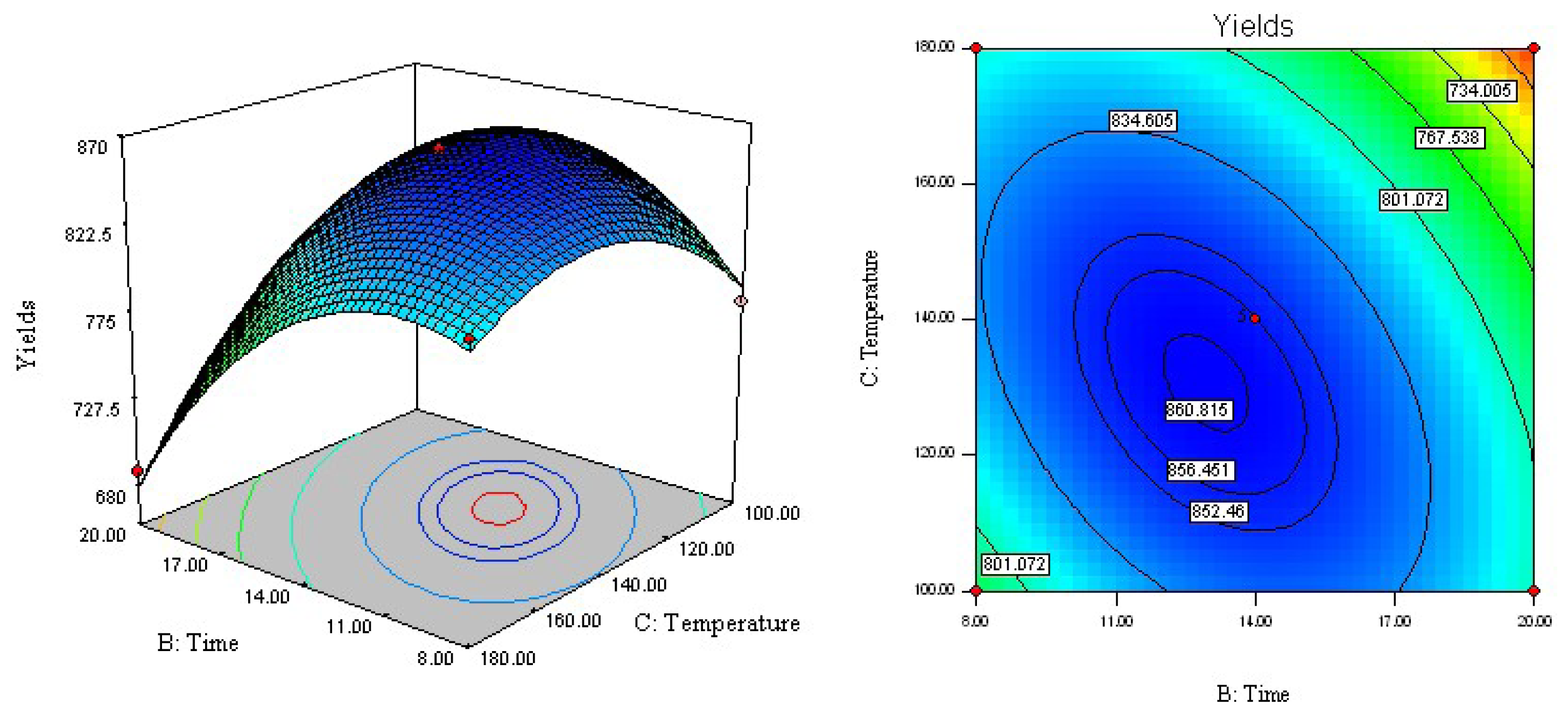
2.3. Analysis of Response Surface
2.4. Optimization of Extraction Parameters and Validation of the Model
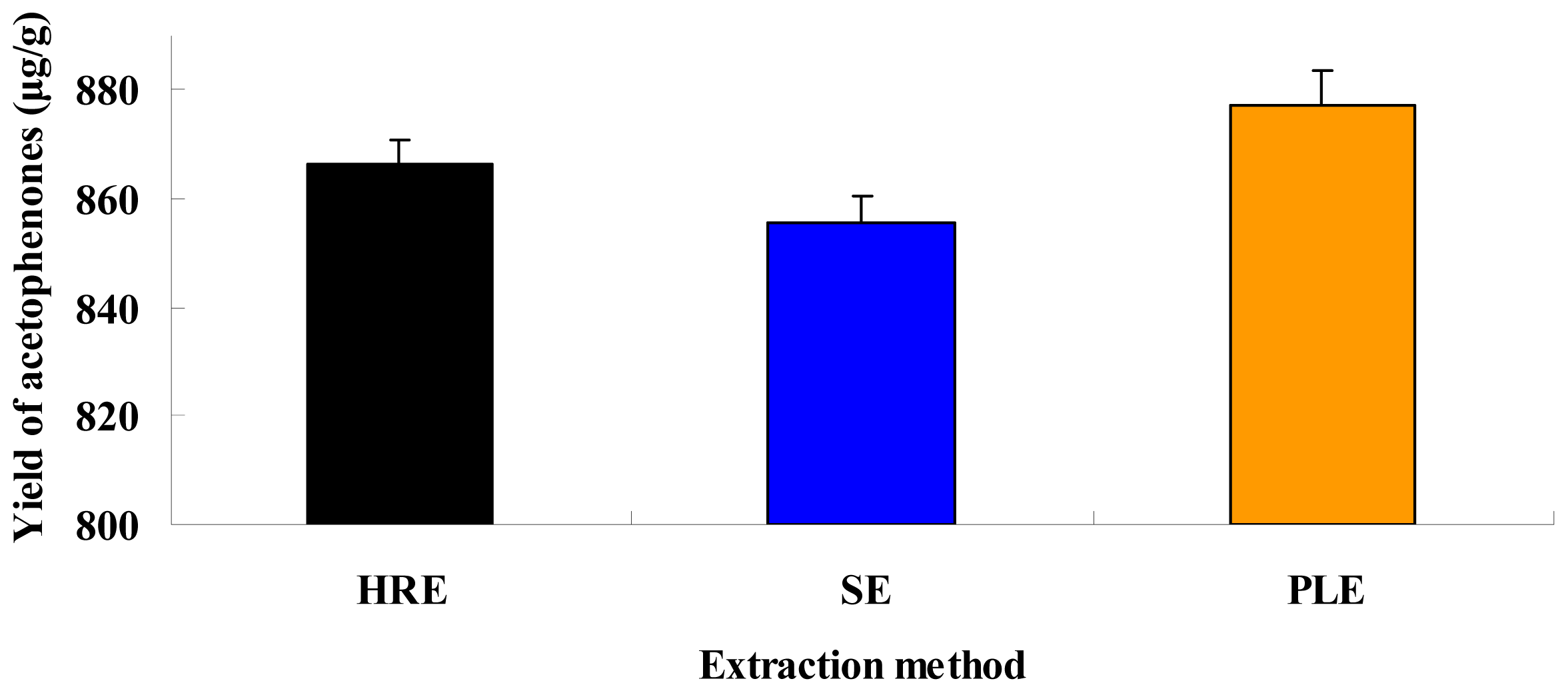
2.5. Comparison of Different Extraction Techniques
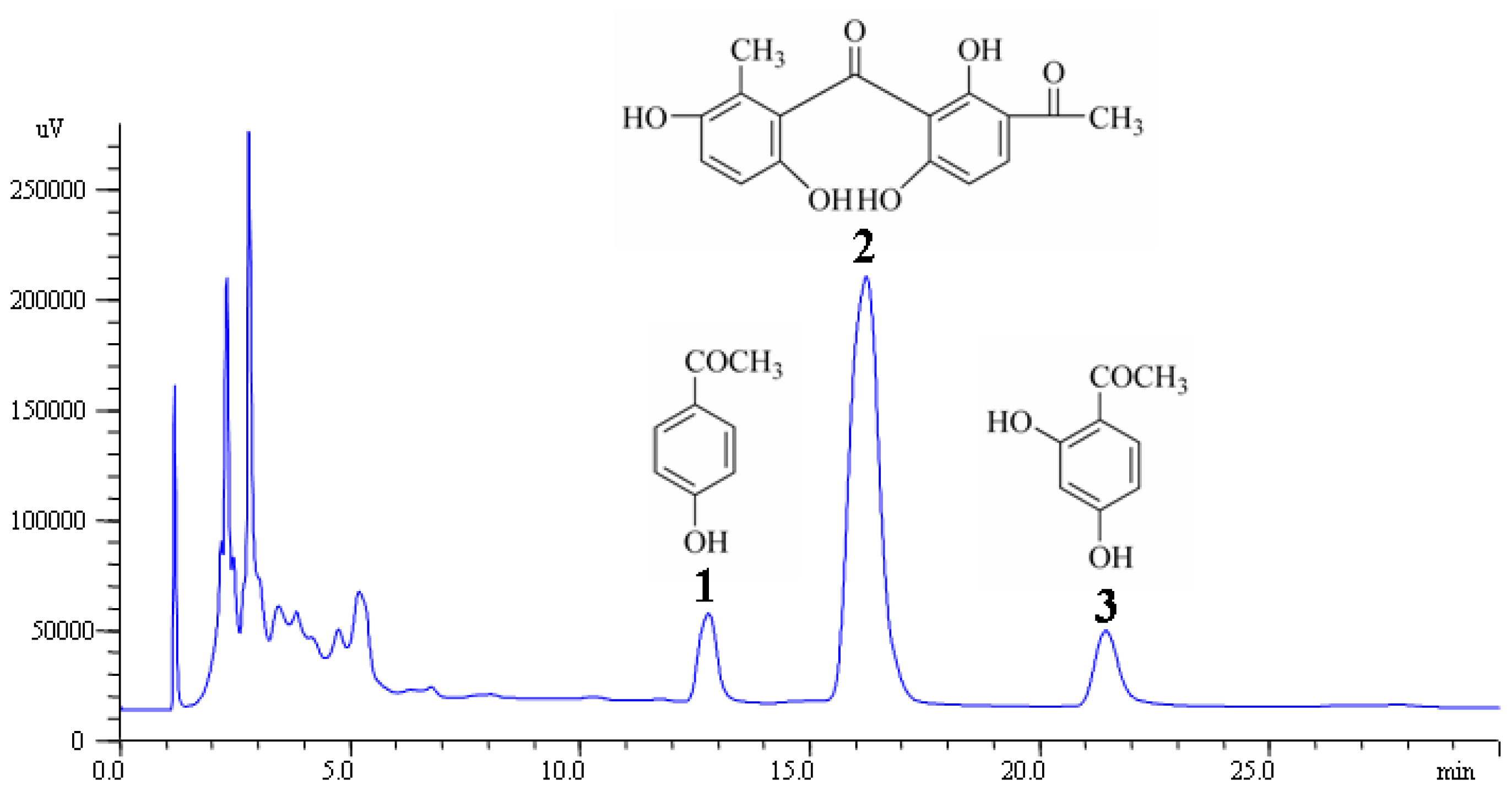
2.6. Validation of the HPLC Method
3. Experimental
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Pressurized Liquid Extraction
3.4. HRE
3.5. SE
3.6. HPLC Analysis of Acetophenones
3.7. Response Surface Modeling
3.8. Data Analysis
4. Conclusions
References
- Pang, Y.J.; Xu, X.T.; Xu, L.C.; Li, Z.F.; Qin, G.P.; Yin, F.J. Experimental study of anti-aging effect of total steroidal glucoside from Cynanchum. bungei Decne (In Chinese). J. Shandong Univ. Trad. Chin. Med 2011, 35, 439–440. [Google Scholar]
- Shan, L.; Liu, R.H.; Shen, Y.H.; Zhang, W.D.; Zhang, C.; Wu, D.Z.; Min, L.; Su, J.; Xu, X.K. Gastroprotective effect of a traditional Chinese herbal drug “Baishouwu” on experimental gastric lesions in rats. J. Ethnopharmacol 2006, 107, 389–394. [Google Scholar]
- Wang, H.; Wang, Q.; Srivastava, R.K.; Gong, S.S.; Lao, L.; Fondell, J.D.; Wang, J.B. Effects of total glycosides from Baishouwu on human breast and prostate cancer cell proliferation. Altern. Ther. Health Med 2003, 9, 62–66. [Google Scholar]
- Wang, D.Y.; Zhang, H.Q.; Li, X. Apoptosis induced by the C21 sterols in Baishouwu and its mechanism of action in hepatoma (In Chinese). Yao Xue Xue Bao 2007, 42, 366–370. [Google Scholar]
- Gao, L.J.; Wang, J.H.; Cui, J.H.; Wang, H.Z. Studies on immunoregulation of polysaccharides-la from Radix Cynanchi Bungei (In Chinese). Zhongguo Zhong Yao Za Zhi 2005, 30, 1352–1355. [Google Scholar]
- Ding, H.Y.; Chang, T.S.; Shen, H.C.; Tai, S.S. Murine tyrosinase inhibitors from Cynanchum bungei and evaluation of in vitro and in vivo depigmenting activity. Exp. Dermatol 2011, 20, 720–724. [Google Scholar]
- Sun, Y.; Lin, H.; Wang, J.; Hu, J.; Liu, Z.; Gao, A. An application of high-speed counter-current chromatography for separation and purification of bungeiside-A bungeiside-B and baishouwubenzophenone from Cynanchum bungei Decne. Phytochem. Anal 2011, 22, 526–531. [Google Scholar]
- Liu, Z.B.; Sun, Y.S.; Wang, J.H.; Zhu, H.F.; Zhou, H.Y.; Hu, J.N.; Wang, J. Preparative isolation and purification of acetophenones from the Chinese medicinal plant Cynanchum bungei Decne. by high-speed counter-current chromatography. Sep. Purif. Technol 2008, 64, 247–252. [Google Scholar]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem 2002, 373, 23–30. [Google Scholar]
- Coelho, J.P.; Cristino, A.F.; Matos, P.G.; Rauter, A.P.; Nobre, B.P.; Mendes, R.L.; Barroso, J.G.; Mainar, A.; Urieta, J.S.; Fareleira, J.M.; et al. Extraction of volatile oil from aromatic plants with supercritical carbon dioxide: experiments and modeling. Molecules 2012, 17, 10550–10573. [Google Scholar]
- Wan, J.B.; Li, P.; Li, S.; Wang, Y.; Dong, T.T.; Tsim, K.W. Simultaneous determination of 11 saponins in Panax notoginseng using HPLC-ELSD and pressurized liquid extraction. J. Sep. Sci 2006, 29, 2190–2196. [Google Scholar]
- Zhang, J.; Liang, Z.; Li, S.; Li, Y.; Peng, B.; Zhou, W.; Gao, H. In-situ metathesis reaction combined with ultrasound-assisted ionic liquid dispersive liquid-liquid microextraction method for the determination of phenylurea pesticides in water samples. Talanta 2012, 98, 145–151. [Google Scholar]
- Kim, H.K.; Do, J.R.; Lim, T.S.; Akram, K.; Yoon, S.R.; Kwon, J.H. Optimisation of microwave-assisted extraction for functional properties of Vitis coignetiae extract by response surface methodology. J. Sci. Food Agric 2012, 92, 1780–1785. [Google Scholar]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar]
- Carabias-Martinez, R.; Rodriguez-Gonzalo, E.; Revilla-Ruiz, P.; Hernandez-Mendez, J. Pressurized liquid extraction in the analysis of food and biological samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar]
- Li, W.; Wang, Z.; Chen, L.; Zhang, J.; Han, L.; Hou, J.; Zheng, Y. Pressurized liquid extraction followed by LC-ESI/MS for analysis of four chromones in Radix Saposhnikoviae. J. Sep. Sci 2010, 33, 2881–2887. [Google Scholar]
- Li, W.; Liu, Z.B.; Wang, Z.; Chen, L.; Sun, Y.S.; Hou, J.G.; Zheng, Y.N. Application of accelerated solvent extraction to the investigation of saikosaponins from the roots of Bupleurum falcatum. J. Sep. Sci 2010, 33, 1870–1876. [Google Scholar]
- Wan, J.B.; Lai, C.M.; Li, S.P.; Lee, M.Y.; Kong, L.Y.; Wang, Y.T. Simultaneous determination of nine saponins from Panax notoginseng using HPLC and pressurized liquid extraction. J. Pharm. Biomed. Anal 2006, 41, 274–279. [Google Scholar]
- Jiang, Y.; Li, P.; Li, S.P.; Wang, Y.T.; Tu, P.F. Optimization of pressurized liquid extraction of five major flavanoids from Lysimachia clethroide. J. Pharm. Biomed. Anal 2007, 43, 341–345. [Google Scholar]
- Peres, V.F.; Saffi, J.; Melecchi, M.I.; Abad, F.C.; de Assis, J.R.; Martinez, M.M.; Oliveira, E.C.; Caramao, E.B. Comparison of soxhlet ultrasound-assisted and pressurized liquid extraction of terpenes fatty acids and Vitamin E from Piper gaudichaudianum Kunth. J. Chromatogr. A 2006, 1105, 115–118. [Google Scholar]
- Zhao, J.; Li, S.P.; Yang, F.Q.; Li, P.; Wang, Y.T. Simultaneous determination of saponins and fatty acids in Ziziphus jujuba Suanzaoren by high performance liquid chromatography-evaporative light scattering detection and pressurized liquid extraction. J. Chromatogr. A 2006, 1108, 188–194. [Google Scholar]
- Barbero, G.F.; Palma, M.; Barroso, C.G. Pressurized liquid extraction of capsaicinoids from peppers. J. Agric. Food Chem 2006, 54, 3231–3236. [Google Scholar]
- Kim, S.H.; Kim, H.K.; Yang, E.S.; Lee, K.Y.; Kim, S.D.; Kim, Y.C.; Sung, S.H. Optimization of pressurized liquid extraction for spicatoside A in Liriope platyphylla. Sep. Purif. Technol 2010, 71, 168–172. [Google Scholar]
- Chen, D.; Cao, X.; Tao, Y.; Wu, Q.; Pan, Y.; Huang, L.; Wang, X.; Wang, Y.; Peng, D.; Liu, Z.; et al. Development of a sensitive and robust liquid chromatography coupled with tandem mass spectrometry and a pressurized liquid extraction for the determination of aflatoxins and ochratoxin A in animal derived foods. J. Chromatogr. A 2012, 1253, 110–119. [Google Scholar]
- Rodrigues, R.C.; Kenealy, W.R.; Dietrich, D.; Jeffries, T.W. Response surface methodology RSM to evaluate moisture effects on corn stover in recovering xylose by DEO hydrolysis. Bioresour. Technol 2011, 108, 134–139. [Google Scholar]
- Li, W.; Wang, Z.; Sun, Y.S.; Chen, L.; Han, L.K.; Zheng, Y.N. Application of response surface methodology to optimise ultrasonic-assisted extraction of four chromones in Radix Saposhnikoviae. Phytochem. Anal 2011, 22, 313–321. [Google Scholar]
- Kwak, T.Y.; Kim, N.H.; Rhee, M.S. Response surface methodology-based optimization of decontamination conditions for Escherichia coli O157:H7 and Salmonella Typhimurium on fresh-cut celery using thermoultrasound and calcium propionate. Int. J. Food Microbiol 2011, 150, 128–135. [Google Scholar]
- Zhao, L.C.; Liang, J.; Li, W.; Cheng, K.M.; Xia, X.; Deng, X.; Yang, G.L. The use of response surface methodology to optimize the ultrasound-assisted extraction of five anthraquinones from Rheum palmatum L. Molecules 2011, 16, 5928–5937. [Google Scholar]
| Run | Coded variables levels | Yield of acetophenones (μg/g) | |||
|---|---|---|---|---|---|
| X1, ethanol (%) | X2, time (min) | X3, temperature (°C) | Actual values | Predicted values | |
| 1 | 30 | 8 | 140 | 850.4 | 850.5 |
| 2 | 100 | 8 | 140 | 772.8 | 771.3 |
| 3 | 30 | 20 | 140 | 665.4 | 666.9 |
| 4 | 100 | 20 | 140 | 848.8 | 848.7 |
| 5 | 30 | 14 | 100 | 812.3 | 804.4 |
| 6 | 100 | 14 | 100 | 844.4 | 838.1 |
| 7 | 30 | 14 | 180 | 732.4 | 738.7 |
| 8 | 100 | 14 | 180 | 799.6 | 807.5 |
| 9 | 65 | 8 | 100 | 774.2 | 782.0 |
| 10 | 65 | 20 | 100 | 795.6 | 802.0 |
| 11 | 65 | 8 | 180 | 813.3 | 806.9 |
| 12 | 65 | 20 | 180 | 688.7 | 680.9 |
| 13 | 65 | 14 | 140 | 846.5 | 856.7 |
| 14 | 65 | 14 | 140 | 857.8 | 856.7 |
| 15 | 65 | 14 | 140 | 858.5 | 856.7 |
| 16 | 65 | 14 | 140 | 860.3 | 856.7 |
| 17 | 65 | 14 | 140 | 860.4 | 856.7 |
| Source | SS | DF | MS | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 58991.25 | 9 | 6554.58 | 83.52 | <0.0001 | significant |
| Residual | 549.33 | 7 | 78.48 | |||
| Lack of fit | 414.19 | 3 | 138.06 | 4.09 | 0.1037 | insignificant |
| Experimental error | 135.14 | 4 | 33.79 |
| Variables | DF | SS | MS | F-values | p-value |
|---|---|---|---|---|---|
| X1 | 1 | 5258.25 | 5258.25 | 67.01 | <0.0001 |
| X2 | 1 | 5628.61 | 5628.61 | 71.72 | <0.0001 |
| X3 | 1 | 4632.03 | 4632.03 | 59.03 | 0.0001 |
| X1X2 | 1 | 17030.25 | 17030.25 | 217.01 | <0.0001 |
| X1X3 | 1 | 308.00 | 308.00 | 3.92 | 0.0880 |
| X2X3 | 1 | 5329.00 | 5329.00 | 67.90 | <0.0001 |
| X12 | 1 | 1957.65 | 1957.65 | 24.95 | 0.0016 |
| X22 | 1 | 10860.51 | 10860.51 | 138.39 | <0.0001 |
| X32 | 1 | 6068.01 | 6068.01 | 77.32 | <0.0001 |
| Ethanol (%) | Extraction time (min) | Temperature (°C) | Yield of acetophenones (μg/g) | |
|---|---|---|---|---|
| Optimum conditions | 100 | 17.29 | 121.39 | 874.9 (predicted) |
| Modified conditions | 100 | 17.00 | 120.00 | 877.8 ± 21.11 (actual) |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, W.; Zhao, L.-C.; Sun, Y.-S.; Lei, F.-J.; Wang, Z.; Gui, X.-B.; Wang, H. Optimization of Pressurized Liquid Extraction of Three Major Acetophenones from Cynanchum bungei Using a Box-Behnken Design. Int. J. Mol. Sci. 2012, 13, 14533-14544. https://doi.org/10.3390/ijms131114533
Li W, Zhao L-C, Sun Y-S, Lei F-J, Wang Z, Gui X-B, Wang H. Optimization of Pressurized Liquid Extraction of Three Major Acetophenones from Cynanchum bungei Using a Box-Behnken Design. International Journal of Molecular Sciences. 2012; 13(11):14533-14544. https://doi.org/10.3390/ijms131114533
Chicago/Turabian StyleLi, Wei, Li-Chun Zhao, Yin-Shi Sun, Feng-Jie Lei, Zi Wang, Xiong-Bin Gui, and Hui Wang. 2012. "Optimization of Pressurized Liquid Extraction of Three Major Acetophenones from Cynanchum bungei Using a Box-Behnken Design" International Journal of Molecular Sciences 13, no. 11: 14533-14544. https://doi.org/10.3390/ijms131114533
APA StyleLi, W., Zhao, L.-C., Sun, Y.-S., Lei, F.-J., Wang, Z., Gui, X.-B., & Wang, H. (2012). Optimization of Pressurized Liquid Extraction of Three Major Acetophenones from Cynanchum bungei Using a Box-Behnken Design. International Journal of Molecular Sciences, 13(11), 14533-14544. https://doi.org/10.3390/ijms131114533






