Involvement of Salicylic Acid on Antioxidant and Anticancer Properties, Anthocyanin Production and Chalcone Synthase Activity in Ginger (Zingiber officinale Roscoe) Varieties
Abstract
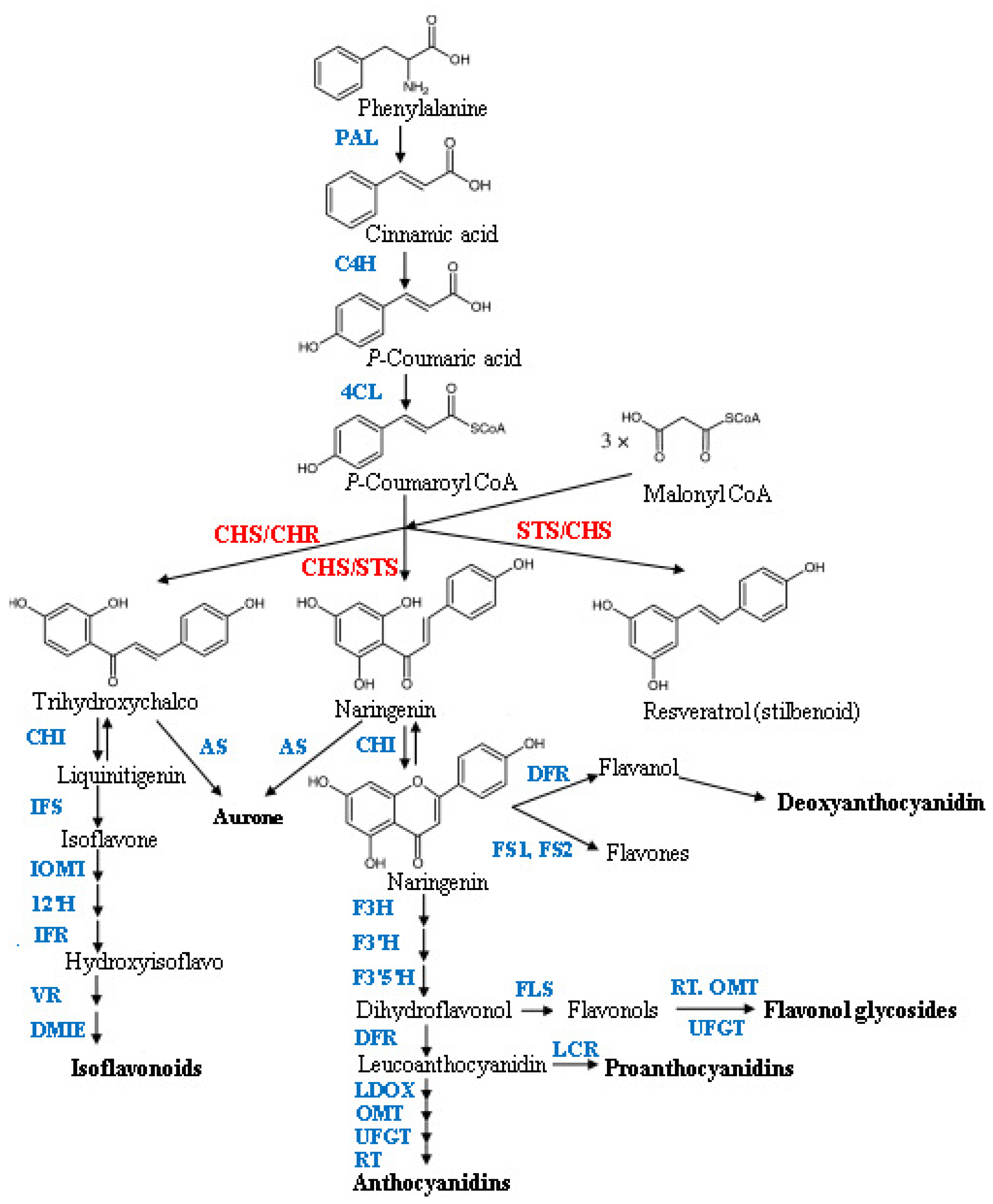
:1. Introduction
2. Results and Discussion
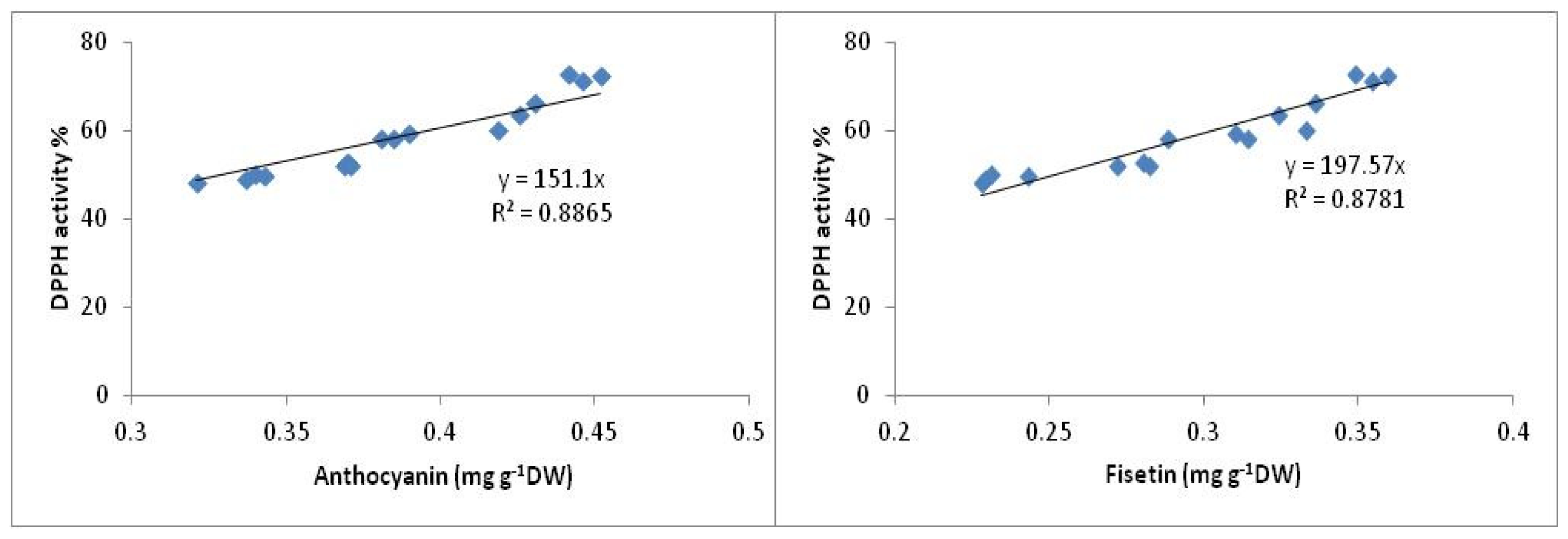
2.1. HPLC Analysis of Flavonoid Compounds
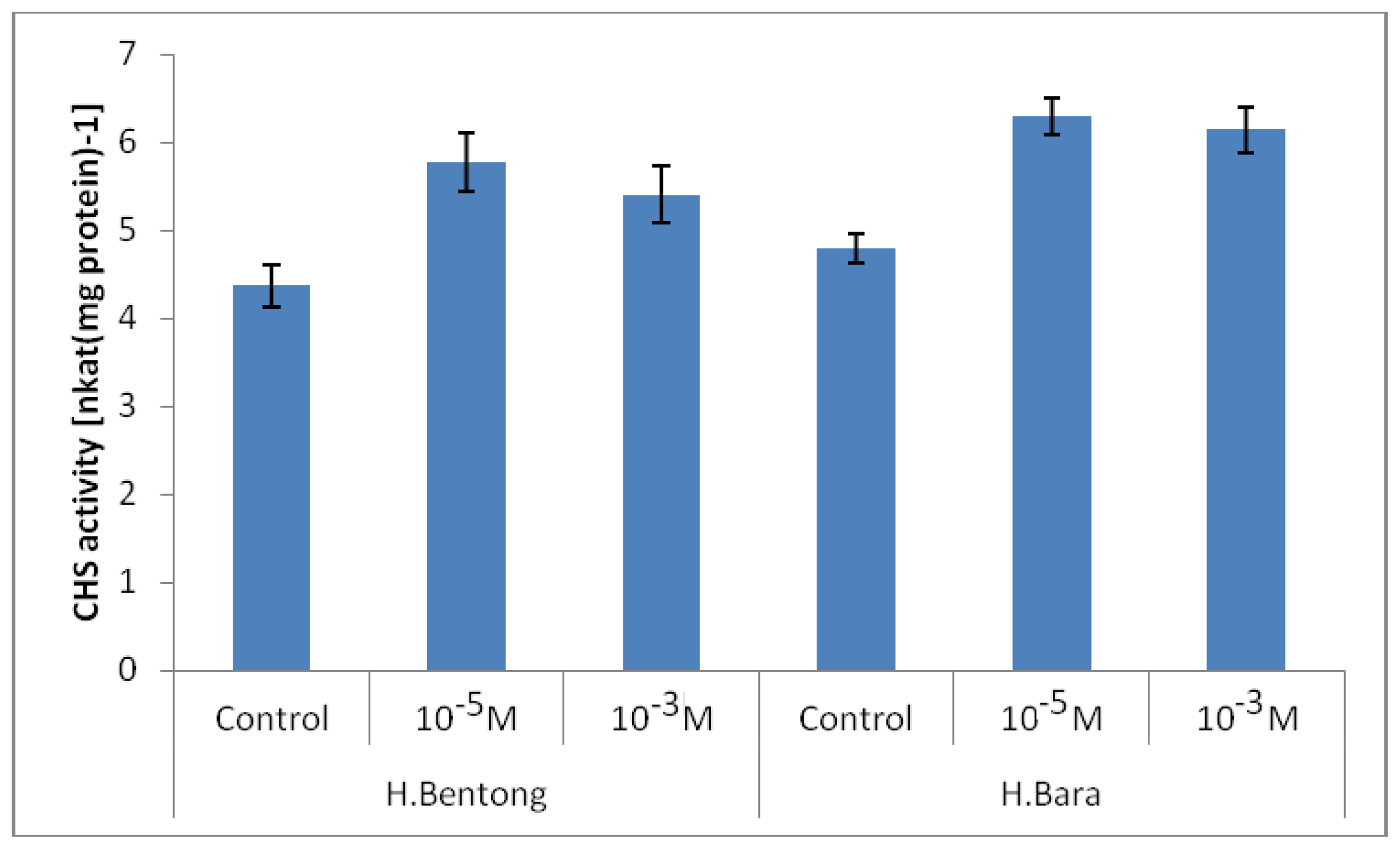
2.2. Chalcone Synthase Enzyme (CHS) Activity
2.3. Antioxidant Activity
2.3.1. Ferric Reducing Antioxidant Potential (FRAP) Assay
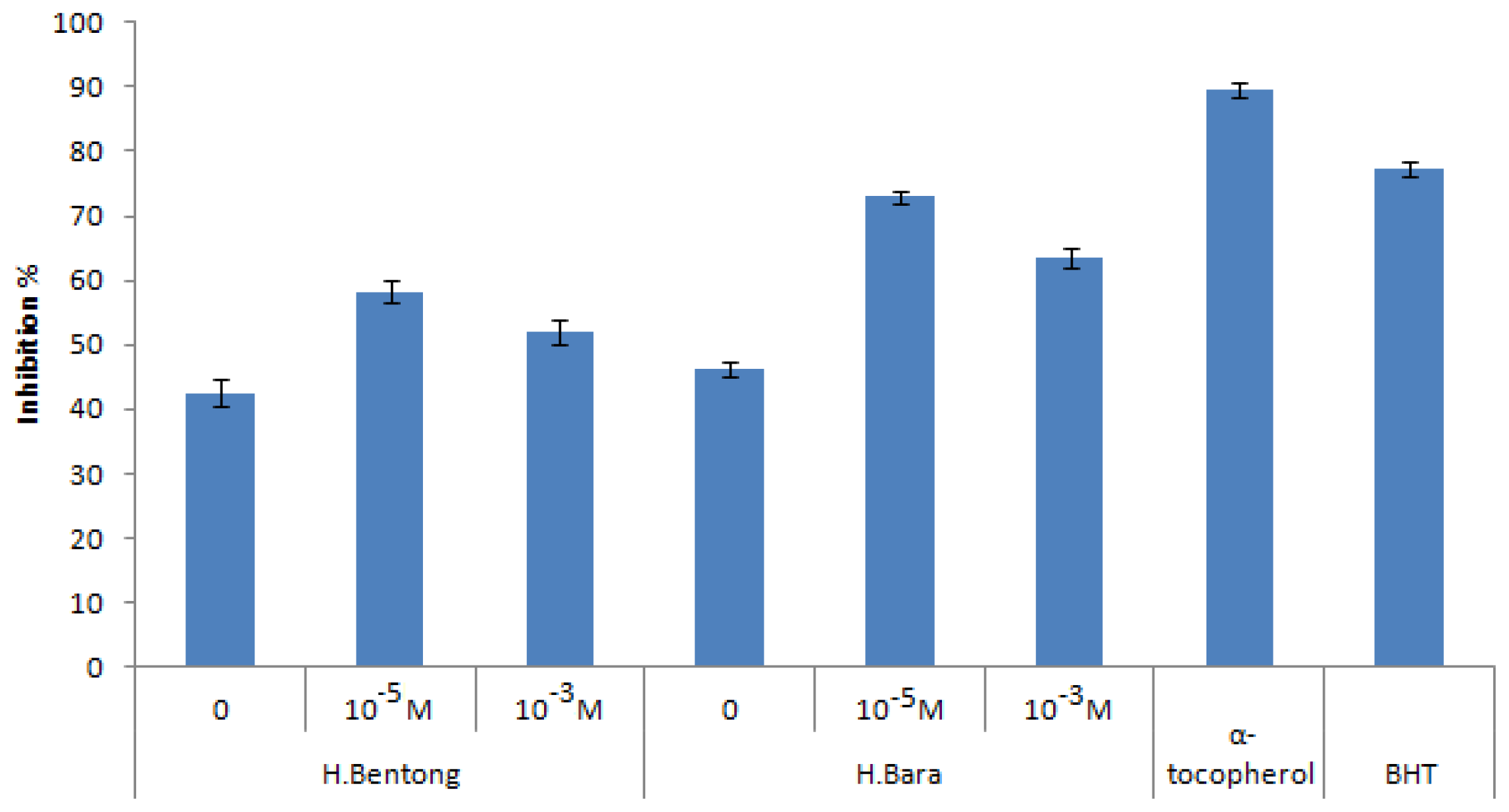
2.3.2. DPPH Antioxidant Activity
2.4. Anticancer Activity
3. Experimental Section
3.1. Plant Material and Maintenance
3.2. Extract Preparation
3.3. High Performance Liquid Chromatography (HPLC) Analysis
3.3.1. Standard Curve Preparation
3.3.2. Chromatography Conditions
3.4. Chalcone Synthase (CHS) Assay
3.5. Determination of Antioxidant Activities
3.5.1. Ferric Reducing Antioxidant Potential (FRAP) Assay
3.5.2. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
3.6. Determination of Anticancer Activity
3.6.1. Cell Culture and Treatment
3.6.2. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Amanullah, M.M.; Sekar, S.; Vincent, S. Plant growth substances in crop production. Asian J. Plant Sci 2010, 9, 215–222. [Google Scholar]
- Karimi, E.; Jaafar, H.Z.; Ahmad, S. Phytochemical analysis and antimicrobial activities of methanolic extracts of leaf, stem and root from different varieties of Labisa pumila Benth. Molecules 2011, 16, 4438–4450. [Google Scholar]
- Park, S.J.; Myoung, H.; Kim, Y.Y.; Paeng, J.Y.; Park, J.W.; Kim, M.J.; Hong, S.M. Anticancer effects of genistein, green tea catechins, and cordycepin on oral squamous cell carcinoma. J. Korean Oral Maxillofac. Surg 2008, 34, 1–10. [Google Scholar]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem 2008, 109, 477–483. [Google Scholar]
- Xie, J.; Zhu, L.; Luo, H.; Zhou, L.; Li, C.; Xu, X. Direct extraction of specific pharmacophoric flavonoids from gingko leaves using a molecularly imprinted polymer for quercetin. J. Chromatogr. A 2001, 934, 1–11. [Google Scholar]
- Liao, H.B.; He, Z.E. Research and prospect of kudzu root. Natl. Food Assoc 2003, 24, 81–83. [Google Scholar]
- Chen, H.G.; Yu, Y.G.; Zeng, O.X. Study on extraction of flavonoids and alkaloids from lotus leaf. Food Sci 2002, 23, 69–71. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar]
- Dao, T.T.; Linthorst, H.J.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev 2011, 10, 397–412. [Google Scholar]
- Campos, A.D.; Ferreira, A.G.; Hampe, M.M.V.; Antunes, I.F.; Brancão, N.; Silveira, E.P.; da Silva, J.B.; Osório, V.A. Induction of chalcone synthase and phenylalanine ammonia-lyase by salicylic acid and Colletotrichum lindemuthianum in common bean Determination of Antioxidant Activities. Braz. J. Plant Physiol 2003, 15, 129–134. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Elevated Carbon Dioxide Increases Contents of Flavonoids and Phenolic Compounds, and Antioxidant Activities in Malaysian Young Ginger (Zingiber officinale Roscoe) Varieties. Molecules 2010, 15, 7907–7922. [Google Scholar]
- Tang, Y.; Zhao, A. Study on the flavone content of buckwheat. J. Sichuan Agric. Univ 2001, 19, 352–354. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E. Anticancer and antioxidant activities of Malaysian young ginger (Zingiber officinale Roscoe) varieties grown under different CO2 concentration. J. Med. Plant Res 2011, 5, 3247–3255. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E. Effect of CO2 Enrichment on Some Primary and Secondary Metabolites synthesis in Ginger (Zingiber officinale Roscoe). Int. J. Mol. Sci 2011, 12, 1101–1114. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A.; Megat Wahab, P.E.; Halim, M.R. Effect of Different Light Intensities on Total Phenolics and Flavonoids Synthesis and Anti-oxidant Activities in Young Ginger Varieties (Zingiber officinale Roscoe). Int. J. Mol. Sci 2010, 11, 3885–3897. [Google Scholar]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol 2001, 126, 485–493. [Google Scholar]
- Obinata, N.; Yamakawat, T.; Takamiya, M.; Tanaka, N.; Ishimaru, K.; Kodama, T. Effects of Salicylic Acid on the Production of Procyanidin and Anthocyanin in Cultured Grape Cell. Plant Biotechnol 2003, 20, 105–111. [Google Scholar]
- Hayder, N.; Bouhlel, I.; Skandrani, I.; Kadri, M.; Steiman, R.; Guiraud, P.; Mariotte, A.M.; Ghedira, K.; Dijoux-Franca, M.G.; Chekir-Ghedira, L. In vitro antioxidant and antigenotoxic potentials of myricetin-3-O-galactoside and myricetin-3-O-rhamnoside from Myrtus communis: Modulation of expression of genes involved in cell defence system using cDNA microarray. Toxicol. In Vitro 2008, 22, 567–581. [Google Scholar]
- Brown, J.; Prey, J.; Harrison, P.R. Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: Involvement of the Akt and stress kinase pathways. Carcinogenesis 2003, 24, 171–177. [Google Scholar]
- Kawabata, K.; Tanaka, T.; Honjo, S.; Kakumoto, M.; Hara, A.; Makita, H.; Tatematsu, N.; Ushida, J.; Tsuda, H.; Mori, H. Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int. J. Cancer 1999, 83, 381–386. [Google Scholar]
- Song, Y.M.; Kang, J.W.; Zhou, J.; Wang, Z.H.; Lua, X.Q.; Wang, L.F.; Gao, J.Z. Study on the fluorescence spectra and electrochemical behavior of ZnL2 and morin with DNA. Spectrochim. Acta A 2000, 56, 2491–2497. [Google Scholar]
- Muzika, R.M. Terpenes and phenolics in response to nitrogen fertilization: A test of the carbon/nutrient balance hypothesis. Chemoecology 1993, 4, 3–7. [Google Scholar]
- Fajer, E.D.; Bowers, M.D.; Bazaaz, F.A. The effects of nutrients and enriched CO2 environment on the production of carbon based allelochemicals in Plantago: A test of the carbon/nutrient balance hypothesis. Am. Nat 1992, 140, 707–723. [Google Scholar]
- Winger, A.; Purdy, S.; Maclean, A.; Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. New Phytol 2006, 161, 781–789. [Google Scholar]
- Ibrahim, M.H.; Jaafar, H.Z.E. Impact of Elevated Carbon Dioxide on Primary, Secondary Metabolites and Antioxidant Responses of Eleais guineensis Jacq. (Oil Palm) Seedlings. Molecules 2012, 17, 5195–5211. [Google Scholar]
- Ozeki, Y.; Komamine, A.; Noguchi, H.; Sankawa, U. Changes in activities of enzymes involved in flavonoid metabolism during the initiation and suppression of anthocyanin synthesis in carrot suspension cultures regulated by 2,4-dichlorophenoxyacetic acid. Physiol. Plant 2006, 69, 123–128. [Google Scholar]
- Takeda, J. Ligh induced synthesis of anthocyanin in carrot cells in suspension. J. Exp. Bot 1990, 41, 749–755. [Google Scholar]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Jaafar, H.Z. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 2010, 15, 6244–6256. [Google Scholar]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, A.M.; Aruoma, O.I. Antioxidant activities of phenolic, proanthocyanidin and flavonoid components in extracts of Acacia fistula. J. Agric. Food Chem 2005, 50, 5042–5047. [Google Scholar]
- Wojdy, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 2006, 105, 940–947. [Google Scholar]
- Karimi, E.; Jaafar, H.Z. HPLC and GC-MS determination of bioactive compounds in microwave obtained extracts of three varieties of Labisia pumila Benth. Molecules 2011, 16, 6791–6805. [Google Scholar]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem 2009, 115, 994–998. [Google Scholar]
- Astadi, I.R.; Astuti, M.; Santoso, U.; Nugraheni, P.S. In vitro antioxidant activity of anthocyanins of black soybean seed coat in human low density lipoprotein (LDL). Food Chem 2009, 112, 652–663. [Google Scholar]
- Khanduja, K.L.; Bhardwaj, A. Stable free radical scavenging and antiperoxidative properties of resveratrol compared in vitro with some other bioflavonoids. Indian J. Biochem. Biophys 2003, 40, 416–422. [Google Scholar]
- Shih, P.H.; Chan, Y.C.; Liao, J.W.; Wang, M.F.; Yen, G.C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer's disease. J. Nutr. Biochem 2010, 21, 598–605. [Google Scholar]
- Sengupta, B.; Banerjee, A.; Sengupta, P.K. Investigations on the binding and antioxidant properties of the plant flavonoid fisetin in model biomembranes. FEBS Lett 2004, 570, 77–81. [Google Scholar]
- Cragg, G.B.; Newman, D.G. Plants as a source of anti-cancer and anti-HIV agents. Ann. Appl. Biol 2003, 143, 127–133. [Google Scholar]
- Davis, W.; Lamson, M.S.; Matthew, S.; Brignall, N.D. Antioxidants and Cancer III: Quercetin. Alter. Med. Rev 2000, 5, 196–204. [Google Scholar]
- Gibellini, L.; Pinti, M.; Nasi, M.; De, B.S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar]
- Verschoyle, R.D.; Steward, W.P.; Gescher, A.J. Putative cancer chemopreventive agents of dietary origin-how safe are they? Nutr. Cancer 2007, 59, 152–162. [Google Scholar]
- Itharat, A.; Houghton, P.J.; Eno, A.E.; Burke, P.J.; Sampson, J.H.; Raman, A. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J. Ethnopharm 2004, 90, 33–38. [Google Scholar]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and anti-inflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod 1999, 62, 294–296. [Google Scholar]
- Pool-Zobel, B.L.; Bub, A.; Schroder, N.; Rechkemmer, G. Anthocyanins are potent antioxidants in model systems but do not reduce endogenous oxidative DNA damage in human colon cells. Eur. J. Nutr 1999, 38, 227–234. [Google Scholar]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar]
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem 2002, 50, 7731–7737. [Google Scholar]
- Koide, T.; Kamei, H.; Hashimoto, Y.; Kojima, T.; Hasegawa, M. Antitumor effect of hydrolyzed anthocyanin from grape rinds and red rice. Cancer Biother. Radiopharm 1996, 11, 273–277. [Google Scholar]
- Kamei, H.; Kojima, T.; Hasegawa, M.; Koide, T.; Umeda, T.; Yukawa, T.; Terabe, K. Suppression of tumor cell growth by anthocyanins in vitro. Cancer Invest 1995, 13, 590–594. [Google Scholar]
- Bomser, J.; Madhavi, D.L.; Singletary, K.; Smith, M.A. In vitro anticancer activity of fruit extracts from Vaccinium species. Planta Med 1996, 62, 212–216. [Google Scholar]
- Koide, T.; Hashimoto, Y.; Kamei, H.; Kojima, T.; Hasegawa, M.; Terabe, K. Antitumor effect of anthocyanin fractions extracted from red soybeans and red beans in vitro and in vivo. Cancer Biother. Radiopharm 1997, 12, 277–280. [Google Scholar]
- Harris, G.K.; Gupta, A.; Nines, R.G.; Kresty, L.A.; Habib, S.G.; Frankel, W.L.; LaPerle, K.; Gallaher, D.D.; Schwartz, S.J.; Stoner, G.D. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr. Cancer 2001, 40, 125–133. [Google Scholar]
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of apoptosis in cancer cells by bilberry (Vaccinium myrtillus) and the anthocyanins. J. Agric. Food Chem 2003, 51, 68–75. [Google Scholar]
- Kang, S.Y.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Tart cherry anthocyanins inhibit tumor development in Apc(Min) mice and reduce proliferation of human colon cancer cells. Cancer Lett 2003, 194, 13–19. [Google Scholar]
- Hagiwara, A.; Miyashita, K.; Nakanishi, T.; Sano, M.; Tamano, S.; Kadota, T.; Koda, T.; Nakamura, M.; Imaida, K.; Ito, N. Anthocyanin-Rich Extracts and Colonic Cell Growth. J. Agric. Food Chem 2004, 52, 6120–6127. [Google Scholar]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr 2000, 130, 2243–2250. [Google Scholar]
- Park, H.H.; Lee, S.; Oh, J.M.; Lee, M.S.; Yoon, K.H.; Park, B.H.; Kim, J.W.; Song, H.; Kim, S.H. Anti-inflammatory activity of fisetin in human mast cells (HMC-1). Pharmacol. Res 2007, 55, 31–37. [Google Scholar]
- Woodman, O.L.; Chan, E.C. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharmacol. Physiol 2004, 31, 786–790. [Google Scholar]
- Fotsis, T.; Pepper, M.S.; Aktas, E.; Breit, S.; Rasku, S.; Adlercreutz, H.; Wahala, K.; Montesano, R.; Schweigerer, L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res 1997, 57, 2916–2921. [Google Scholar]
- Touil, Y.S.; Fellous, A.; Scherman, D.; Chabot, G.G. Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization. Nutr. Cancer 2009, 6, 310–321. [Google Scholar]
- Tripathi, R.; Samadder, T.; Gupta, S.; Surolia, A.; Shaha, C. Anticancer activity of a combination of cisplatin and fisetin in embryonal carcinoma cells and xenograft tumors. Mol. Cancer Ther 2011, 10, 255–268. [Google Scholar]
- Khan, N.; Asim, M.; Afaq, F.; Abu, Z.M.; Mukhtar, H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res 2008, 68, 8555–8563. [Google Scholar]
- Chien, C.S.; Shen, K.H.; Huang, J.S.; Ko, S.C.; Shih, Y.W. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol. Cell Biochem 2010, 333, 169–180. [Google Scholar]
- Crozier, A.; Jensen, E.; Lean, M.E.J.; Mc Donald, M.S. Quantitative analysis of flavonoids by reversed-phase high performance liquid chromatography. J. Chromatogr 1997, 761, 315–321. [Google Scholar]
- Wang, T.C.; Chuang, Y.C.; Ku, Y.H. Quantification of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chem 2007, 102, 1163–1171. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem 1996, 239, 70–76. [Google Scholar]
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; Santos, T.S.; Coube, C.S. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res 2001, 15, 127–130. [Google Scholar]
- Lau, C.S.; Ho, C.Y.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.L.; Chow, M.S. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci 2004, 75, 797–808. [Google Scholar]
- Doares, S.H.; Narvaez-Vasquez, J.; Conconi, A.; Ryan, C.A. Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol 1995, 108, 1741–1746. [Google Scholar]
- Niccholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role disease resistance. Ann. Rev. Phytopathol 1992, 30, 369–389. [Google Scholar]
| Compounds | Halia Bentong | Halia Bara | ||||
|---|---|---|---|---|---|---|
| Control | SA 10−5 | SA 10−3 | Control | SA 10−5 | SA 10−3 | |
| Rutin | 0.893 ± 0.03 b,c | 0.736 ± 0.09 c | 0.79 ± 0.06 c | 1.13 ± 0.12 a | 0.883 ± 0.07 b,c | 0.993 ± 0.07 a,b |
| Apigenin | 0.384 ± 0.049 c | 0.276 ± 0.08 d | 0.305 ± 0.09 d | 0.553 ± 0.06 a | 0.45 ± 0.06 b | 0.55 ± 0.04 a |
| Myricetin | 0.04 ± 0.009 b | 0.088 ± 0.009 a,b | 0.059 ± 0.018 b | 0.06 ± 0.001 b | 0.112 ± 0.004 a | 0.074 ± 0.006 a,b |
| Naringenin | 0.227 ± 0.049 a | 0.29 ± 0.1 a | 0.259 ± 0.045 a | 0.259 ± 0.033 a | 0.303 ± 0.097 a | 0.304 ± 0.02 a |
| Fisetin | ND | 0.237 ± 0.017 c | 0.228 ± 0.03 c | ND | 0.359 ± 0.046 a | 0.304 ± 0.01 b |
| Morien | 0.117 ± 0.02 a,b | 0.173 ± 0.055 a,b | 0.158 ± 0.042 a,b | 0.102 ± 0.042 b | 0.193 ± 0.03 a | 0.182 ± 0.017 a |
| Anthocyanin | ND | 0.381 ± 0.05 b | 0.369 ± 0.053 b | ND | 0.442 ± 0.041 a | 0.426 ± 0.122 a |
| Variety | SA (M) | FRAP (μmol Fe (II)/g) |
|---|---|---|
| H. Bentong | 0 | 476.22 + 10.19 e |
| 10−5 | 644.7 + 32.7 c | |
| 10−3 | 521.3 + 27.1 d | |
| 0 | 509.4 + 14.26 d | |
| H. Bara | 10−5 | 793.26 + 33.2 a |
| 10−3 | 716.62 + 32.5 b | |
| Positive controls | BHT | 611.82 + 15.2 |
| α-tocopherol | 887.34 + 29.5 |
| Variety | SA (M) | MCF-7 | MDA-MB-231 | Inhibition % (MCF-7) | Inhibition % (MDA-MB-231) |
|---|---|---|---|---|---|
| H. Bentong | 0 | 54.7 ± 2.17 a | 59.36 ± 2.2 a | 45.3 | 40.64 |
| 10−5 | 41.08 ± 1.56 b | 45.3 ± 1.63 b | 58.92 | 54.7 | |
| 10−3 | 47.44 ± 1.64 b | 48.69 ± 1.77 b | 52.56 | 51.31 | |
| H. Bara | 0 | 51.63 ± 1.92 a | 56.2 ± 2.11 a | 48.37 | 43.8 |
| 10−5 | 38.47 ± 1.15 c | 40.12 ± 1.4 c | 61.53 | 59.88 | |
| 10−3 | 42.28 ± 1.68 b | 47.18 ± 1.58 c | 58.72 | 52.82 | |
| Positive control | Tamoxifen | 22.56 ± 1.07 | 26.18 ± 1.27 | 77.44 | 73.82 |
| Variety | SA (M) | MCF-7 | MDA-MB-231 |
|---|---|---|---|
| H. Bentong | 0 | 50.6 ± 1.45 a | 54.7 ± 1.74 a |
| 10−5 | 42.5 ± 1.33 b | 48.8 ± 1.3b c | |
| 10−3 | 44.4 ± 1.72 b | 50.6 ± 1.28 b | |
| H. Bara | 0 | 39.1 ± 1.18 c | 45.2 ± 1.14 d |
| 10−5 | 31.5 ± 1.66 d | 40.6 ± 1.06 f | |
| 10−3 | 33.6 ± 1.2 d | 44.5 ± 1.66 e | |
| Tamoxifen | 17.4 ± 2.16 | 19.5 ± 1.88 | |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ghasemzadeh, A.; Jaafar, H.Z.E.; Karimi, E. Involvement of Salicylic Acid on Antioxidant and Anticancer Properties, Anthocyanin Production and Chalcone Synthase Activity in Ginger (Zingiber officinale Roscoe) Varieties. Int. J. Mol. Sci. 2012, 13, 14828-14844. https://doi.org/10.3390/ijms131114828
Ghasemzadeh A, Jaafar HZE, Karimi E. Involvement of Salicylic Acid on Antioxidant and Anticancer Properties, Anthocyanin Production and Chalcone Synthase Activity in Ginger (Zingiber officinale Roscoe) Varieties. International Journal of Molecular Sciences. 2012; 13(11):14828-14844. https://doi.org/10.3390/ijms131114828
Chicago/Turabian StyleGhasemzadeh, Ali, Hawa Z. E. Jaafar, and Ehsan Karimi. 2012. "Involvement of Salicylic Acid on Antioxidant and Anticancer Properties, Anthocyanin Production and Chalcone Synthase Activity in Ginger (Zingiber officinale Roscoe) Varieties" International Journal of Molecular Sciences 13, no. 11: 14828-14844. https://doi.org/10.3390/ijms131114828






