Healing, Antioxidant and Cytoprotective Properties of Indigofera truxillensis in Different Models of Gastric Ulcer in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analyses of Secondary Metabolites
2.2. Ethanol-Induced Gastric Lesions
2.3. Gastric Secretion in Lesions Induced by Pylorus Ligature
2.4. Prostaglandin Production Determination
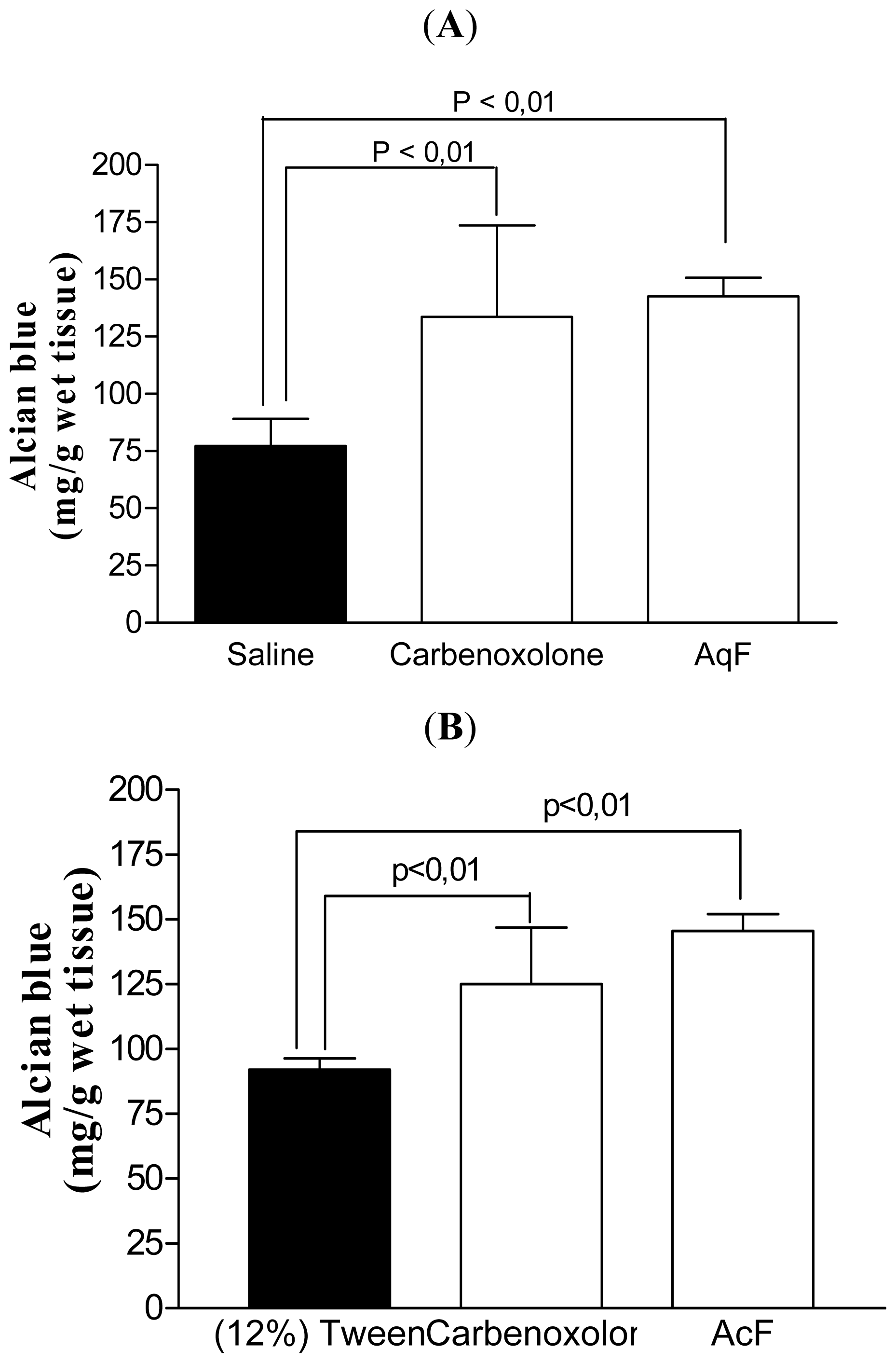
2.5. Determination of the Gastric Mucus Content
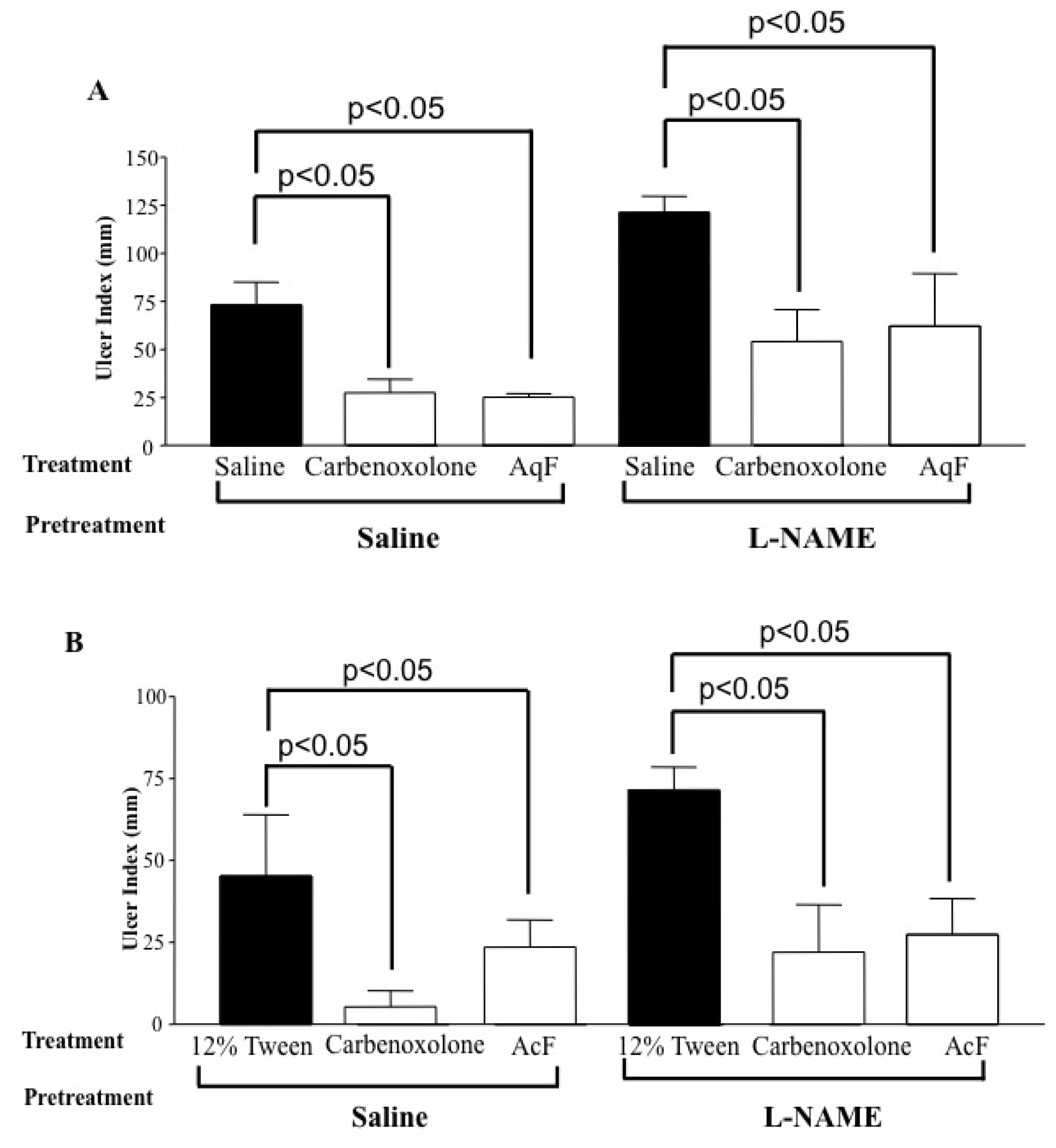
2.6. Determination of the Role of Nitric Oxide (NO) and Sulfhydryl Compounds (SH) in Gastric Protection
2.7. Gastric Ischemia–Reperfusion
2.8. Glutathione Peroxidase (GSH-Px), Glutathione Reductase (GSH-Gr) and Superoxide Dismutase (SOD) Activities
2.9. Healing in Acetic-Induced Gastric Lesions
3. Experimental Section
3.1. Animals
3.2. Drugs
3.3. Plant Material
3.4. Preparation of Fractions
3.5. Identification of Fraction Constituents AcF and AqF
3.6. Antiulcer Activity
3.6.1. Ethanol-Induced Gastric Lesions
3.6.2. Gastric Secretion in Lesions Induced by Pylorus Ligature
3.6.3. Prostaglandin Production Determination
3.6.4. Determination of the Gastric Mucus Content
3.6.5. Determination of the Role of Nitric Oxide (NO) and Sulfhydryl Compounds (SH) in Gastric Protection
3.6.6. Gastric Ischemia–Reperfusion
3.7. Enzymatic Assays
3.8. Healing in Acetic-Induced Gastric Lesion
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Moreira, J.L.A.; Azevedo-Tozzi, A.M.G. Indigofera L. (Leguminosae, Papilionoideae) no estado de São Paulo, Brasil. Revta. Brasil. Bot 1997, 20, 97–117. [Google Scholar]
- Roig, J.T. Plantas Medicinales, Aromaticas o Venenosas de Cuba; Editorial Cientifica-Tecnica: La Havana, Cuba, 1988; p. 164. [Google Scholar]
- Cola-Miranda, M.; Barbastefano, V.; Hiruma-Lima, C.A.; Calvo, T.R.; Vilegas, W.; Brito, A.R.M.S. Atividade altiulcerogênica de Indigofera truxillensis Kunth. Biota Neotrop 2006, 6, 1–9. [Google Scholar]
- Carli, C.B.A.; Quilles, M.B.; Maia, D.C.; Lopes, F.C.; Santos, R.; Pavan, F.R.; Fujimura Leite, C.Q.; Calvo, T.R.; Vilegas, W.; Carlos, I.Z. Antimycobacterial activity of Indigofera suffruticosa with activation potential of the innate immune system. Pharm. Biol 2010, 48, 878–882. [Google Scholar]
- Luiz-Ferreira, A.; Cola, M.; Barbastefano, V.; Farias-Silva, E.; Calvo, T.R.; de Almeida, A.B.; Pellizzon, C.H.; Hiruma-Lima, C.A.; Vilegas, W.; Souza-Brito, A.R. Indigofera suffruticosa Mill as new source of healing agent: Involvement of prostaglandin and mucus and heat shock proteins. J. Ethnopharmacol 2011, 137, 192–198. [Google Scholar]
- Calvo, T.R.; Cardoso, C.R.; da Silva Moura, A.C.; dos Santos, L.C.; Colus, I.M.; Vilegas, W.; Varanda, E.A. Mutagenic activity of Indigofera truxillensis and I. suffruticosa aerial parts. Evid Based Complement. Alternat. Med. 2009. [Google Scholar] [CrossRef]
- Farias-Silva, E.; Cola, M.; Calvo, T.R.; Barbastefano, V.; Ferreira, A.L.; de Paula Michelatto, D.; de Almeida, A.C.A.; Hiruma-Lima, C.A.; Vilegas, W.; Brito, A.R. Antioxidant activity of indigo and its preventive effect against ethanol-induced DNA damage in rat gastric mucosa. Planta Med 2007, 73, 1241–1246. [Google Scholar]
- Kwiecień, S.; Brzozowski, T.; Konturek, S.J. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J. Physiol. Pharmacol 2002, 53, 39–50. [Google Scholar]
- Loomis, T.A.; Hayes, A.W. Essentials of Toxicology; Academic Press Limited: London, UK, 1996; pp. 33–46. [Google Scholar]
- Robaszkiewicz, A.; Balcerczyk, A.; Bartosz, G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol. Int 2007, 10, 1245–1250. [Google Scholar]
- Martinsen, T.C.; Bergh, K.; Waldum, H.L. Gastric juice: A barrier against infectious diseases. Basic Clin. Pharmacol. Toxicol 2005, 96, 94–102. [Google Scholar]
- Schubert, M.L. Gastric secretion. Curr. Opin. Gastroenterol 2008, 24, 659–664. [Google Scholar]
- Yeomans, N.D. The ulcer sleuths: The search for the cause of peptic ulcers. J. Gastroenterol. Hepatol 2011, 1, 35–41. [Google Scholar]
- Canani, R.B.; Terrin, G. Gastric acidity inhibitors and the risk of intestinal infections. Curr. Opin. Gastroenterol 2010, 26, 31–35. [Google Scholar]
- deFoneska, A.; Kaunitz, J.D. Gastroduodenal mucosal defense. Curr. Opin. Gastroenterol 2010, 26, 604–610. [Google Scholar]
- Takeuchi, K.; Kato, S.; Amagase, K. Prostaglandin EP receptors involved in modulating gastrointestinal mucosal integrity. J. Pharmacol. Sci 2010, 114, 248–261. [Google Scholar]
- Alanko, J.; Riutta, A.; Holm, P.; Mucha, I.; Vapaatalo, H.; Metsä-Ketelä, T. Modulation of arachidonic acid metabolism by phenols: Relation to their structure and antioxidant/prooxidant properties. Free Radic. Biol. Med 1999, 26, 193–201. [Google Scholar]
- Toma, W.; Trigo, J.R.; de Paula, A.C.B.; Brito, A.R.M.S. Preventive activity of pyrrolizidine alkaloids from Senecio brasiliensis (Asteraceae) on gastric and duodenal induced ulcer on mice and rats. J. Ethnopharmacol 2004, 95, 345–351. [Google Scholar]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol 2011, 9, 265–278. [Google Scholar]
- Gottke, M.; Chadee, K. Exogenous nitric oxide stimulates mucin secretion from LS174T colonic adenocarcinoma cells. Inflamm. Res 1996, 45, 209–212. [Google Scholar]
- Song, J.S.; Kang, C.M.; Yoo, M.B.; Kim, S.J.; Yoon, H.K.; Kim, Y.K.; Kim, K.H.; Moon, H.S.; Park, S.H. Nitric oxide induces MUC5AC mucin in respiratory epithelial cells through PKC and ERK dependent pathways. Respir. Res 2007, 8, 1–12. [Google Scholar]
- Wallace, J.L. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn’t the stomach digest itself? Physiol. Rev 2008, 88, 1547–1565. [Google Scholar]
- Wallace, J.L.; Miller, M.J. Nitric oxide in mucosal defense: A little goes a long way. Gastroenterology 2000, 119, 512–520. [Google Scholar]
- Konturek, P.K.; Brzozowski, T.; Konturek, S.J.; Dembinski, A. Role of epidermal growth factor, prostaglandin, and sulfhydryls in stress-induced gastric lesions. Gastroenterology 1990, 99, 1607–1615. [Google Scholar]
- Szabo, S.; Pihan, G.; Dupuy, D. The Biochemical Pharmacology of Sulphydryl Compounds in Gastric Mucosal Injury and Protection. In New Pharmacology of Ulcer Disease; Szabo, S., Mózsik, G., Eds.; Elsevier Science: New York, NY, USA, 1987; pp. 424–446. [Google Scholar]
- Kimura, M.; Goto, S.; Ihara, Y.; Wada, A.; Yahiro, K.; Niidome, T.; Aoyagi, H.; Hirayama, T.; Kondo, T. Impairment of glutathione metabolism in human gastric epithelial cells treated with vacuolating cytotoxin from Helicobacter pylori. Microb. Pathog 2001, 31, 29–36. [Google Scholar]
- Rao, C.V.; Vijayakumar, M. Protective effect of (+)-catechin against gastric mucosal injury induced by ischaemia-reperfusion in rats. J. Pharm. Pharmacol 2007, 59, 1103–1107. [Google Scholar]
- Smith, G.S.; Mercer, D.W.; Cross, J.M.; Barreto, J.C.; Miller, T.A. Gastric injury induced by ethanol and ischemia-reperfusion in the rat. Differing roles for lipid peroxidation and oxygen radicals. Dig. Dis. Sci 1996, 41, 1157–1164. [Google Scholar]
- La Casa, C.; Villegas, I.; Alarcón de la Lastra, C.; Motilva, V.; Calero, M.J.M. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol 2000, 71, 45–53. [Google Scholar]
- Tariq, M.; Khan, H.A.; Elfaki, I.; Arshaduddin, M.; Al Moutaery, M.; Al Rayes, H.; Al Swailam, R. Gastric antisecretory and antiulcer effects of simvastatin in rats. J. Gastroenterol. Hepatol 2007, 22, 2316–2323. [Google Scholar]
- Rodríguez, Z.B.Z.; Alvarez, R.G.; Guanche, D.; Merino, N.; Rosales, F.H.; Cepero, S.M.; González, Y.A.; Schulz, S. Antioxidant mechanism is involved in the gastroprotective effects of ozonized sunflower oil in ethanol-induced ulcers in rats. Mediators Inflamm 2007. [Google Scholar] [CrossRef]
- Repetto, M.G.; Llesuy, S.F. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz. J. Med. Biol. Res 2002, 35, 523–534. [Google Scholar]
- Barry, H. Antioxidant effects a basis for drug selection? Drugs 1991, 42, 569–605. [Google Scholar]
- Bafna, P.A.; Balaraman, R. Anti-ulcer and antioxidant activity of DHC-1, a herbal formulation. J. Ethnopharmacol 2004, 90, 123–127. [Google Scholar]
- Takagi, K.; Okabe, S.; Saziki, R. A new method for the production of chronic gastric ulcer in rats and the effect of several drugs on its healing. Jpn. J. Pharmacol 1969, 19, 418–426. [Google Scholar]
- Okabe, S.; Amagase, K. An overview of acetic acid ulcer models—The history and state of the art of peptic ulcer research. Biol. Pharm. Bull 2005, 28, 1321–1341. [Google Scholar]
- Tarnawski, A.; Stachura, J.; Krause, W.J.; Douglass, T.G.; Gergely, H. Quality of gastric ulcer healing—A new, emerging concept. J. Clin. Gastroenterol 1991, 13, S42–S47. [Google Scholar]
- Tarnawski, A. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig. Dis. Sci 2005, 50, S24–S33. [Google Scholar]
- Toma, W.; Trigo, J.R.; de Paula, A.C.B.; Brito, A.R.M.S. Modulation of gastrin and epidermal growth factor by pyrrolizidine alkaloids obtained from Senecio brasiliensis in acute and chronic induced gastric ulcers. Can. J. Physiol. Pharmacol 2004, 82, 319–325. [Google Scholar]
- Tatematsu, M.; Tsukamoto, T.; Inada, K. Stem cells and gastric cancer: Role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci 2003, 94, 135–141. [Google Scholar]
- Morimoto, Y.; Shimohara, K.; Oshima, S.; Sukamoto, T. Effects of the new anti-ulcer agent KB-5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of teprenone and cimetidine. Jpn. J. Pharmacol 1991, 57, 495–505. [Google Scholar]
- Szelenyi, I.; Thiemer, K. Distention ulcer as a model for testing of drugs for ulcerogenic side-effects. Arch. Toxicol 1978, 41, 99–105. [Google Scholar]
- Shay, H.; Komarov, S.A.; Fels, S.E.; Meraze, D.; Gruentein, M.; Siplet, H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 1945, 5, 43–61. [Google Scholar]
- Curtis, G.H.; MacNaughton, W.K.; Gall, D.G.; Wallace, J.L. Intraluminal pH modulates gastric prostaglandin synthesis. Can. J. Physiol. Pharmacol 1995, 73, 130–134. [Google Scholar]
- Rafatullah, S.; Tariq, M.; Al-Yahya, M.A.; Mossa, J.S.; Ageel, A.M. Evaluation of turmeric (Curcuma longa) for gastric and duodenal antiulcer activity in rats. J. Ethnopharmacol 1990, 29, 25–34. [Google Scholar]
- Arrieta, J.; Benitez, J.; Flores, E.; Castillo, C.; Navarrete, A. Purification of gastroprotective triterpenoids from stem bark of Amphipterygium adstringens; roles of prostaglandins, sulfhydryls, nitric oxide and capsaicin neurons. Planta Med 2003, 69, 905–909. [Google Scholar]
- Ueda, S.; Yoshikawa, T.; Takahashi, S.; Ichikawa, H.; Yasuda, M.; Oyamada, H.; Tanigawa, T.; Sugino, S.; Kondo, M. Role of free radicals and lipid peroxidation in gastric mucosal injury induced by ischemia-reperfusion in rats. Scand. J. Gastroenterol. Suppl 1989, 162, 55–58. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Yoshikawa, T.; Naito, Y.; Kishi, A.; Tomii, T.; Kaneko, T.; Iinuma, S.; Ichikawa, H.; Yasuda, M.; Takahashi, S.; Kondo, M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 1993, 34, 732–737. [Google Scholar]
- Worthington, D.J.; Rosemeyer, M.A. Human glutathione reductase: Purification of the crystalline enzyme from erythrocytes. Eur. J. Biochem 1974, 48, 167–177. [Google Scholar]
- Winterbourn, C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med 1975, 85, 337–341. [Google Scholar]


| Treatments (p.o.) | N | Dose (mg/kg) | Ulcer index | Inhibition (%) |
|---|---|---|---|---|
| Saline | 5 | 10 mL/kg | 66.4 ± 15.4 | - |
| Lanzoprazole | 5 | 30 | 19.8 ± 6.4 ** | 70 |
| AqF–I. truxillensis | 5 | 50 | 43.4 ± 14.6 * | 35 |
| AqF–I. truxillensis | 5 | 100 | 13.8 ± 6.3 ** | 79 |
| AqF–I. truxillensis | 5 | 200 | 25.4 ± 9.0 ** | 61 |
| 12% Tween | 5 | 10 mL/kg | 33.8 ± 13.1 | - |
| Lansoprazole | 5 | 30 | 1 ± 0.7 ** | 97 |
| AcF–I. truxillensis | 5 | 50 | 6.4 ± 2.7 ** | 81 |
| AcF–I. truxillensis | 5 | 100 | 6.2 ± 4.0 ** | 81 |
| AcF–I. truxillensis | 5 | 200 | 6.0 ± 2.5 ** | 82 |
| Treatments (i.d.) | N | Dose (mg/kg) | pH units | Gastric juice (mg) |
|---|---|---|---|---|
| Saline | 5 | 10 mL/kg | 2.7 ± 0.2 | 0.5 ± 0.1 |
| Cimetidine | 5 | 100 | 3.2 ± 0.1 ** | 0.5 ± 0.0 |
| AqF–I. truxillensis | 5 | 100 | 2.8 ± 0.1 | 0.5 ± 0.1 |
| 12% Tween | 5 | 10 mL/kg | 3.2 ± 0.1 | 0.6 ± 0.1 |
| Cimetidine | 5 | 100 | 3.7 ± 0.2 ** | 0.5 ± 0.1 |
| AcF–I. truxillensis | 5 | 50 | 3.3 ± 0.2 | 0.4 ± 0.1 * |
| Treatment (p.o) | N | Dose (mg/kg) | Ulcer area (mm2) | Inhibition (%) |
|---|---|---|---|---|
| Sham | 5 | - | 0 ± 0 ** | - |
| Saline | 5 | 10 mL/kg | 1.7 ± 0.8 | - |
| Rutin | 5 | 200 | 0.3 ± 0.1 ** | 82 |
| AqF–I. truxillensis | 5 | 100 | 0.3 ± 0.1 ** | 82 |
| 12% Tween | 5 | 10 mL/kg | 15.6 ± 5.3 | - |
| Rutin | 5 | 200 | 5.3 ± 3.2 ** | 66 |
| AcF–I. truxillensis | 5 | 50 | 0.8 ± 0.1 ** | 95 |
| Treatment | Dose (mg/kg) | SOD (U/mg of protein) | GSH-Px (pmol/min/mg of protein) | GSH-Rd (pmol/min/mg of protein) |
|---|---|---|---|---|
| Sham | - | 10.6 ± 1.9 | 36.9 ± 8.7 | 27.6 ± 2.8 |
| Saline | 10 mL/kg | 3.7 ± 0.5 | 23.8 ± 1.5 | 12.8 ± 0.1 |
| Rutin | 200 | 19.0 ± 4.3 ** | 19.2 ± 4.0 | 19.3 ± 3.2 |
| AqF–I. truxillensis | 100 | 8.8 ± 0.9 * | 26.5 ± 3.9 | 109.2 ± 28.0 ** |
| 12% Tween | 10 mL/kg | 3.8 ± 0.7 | 13.9 ± 0.9 | 8.6 ± 0.5 |
| Rutin | 200 | 7.8 ± 1.5 | 16.2 ± 1.6 | 15.2 ± 2.4 |
| AcF–I. truxillensis | 50 | 8.9 ± 1.9 * | 33.7 ± 5.6 * | 22.4 ± 3.5 * |
| Treatment | N | Dose (mg/kg) | Ulcer area (mm2) | Cure rate (%) |
|---|---|---|---|---|
| Sham | 5 | - | 0 ± 0 ** | - |
| Saline | 5 | 10 mL/kg | 3.9 ± 0.9 | - |
| Cimetidine | 5 | 100 | 1.6 ± 0.6 ** | 57 |
| AqF–I. truxillensis | 5 | 100 | 3.6 ± 0.8 | - |
| 12% Tween | 5 | 10 mL/kg | 4.5 ± 0.4 | |
| Cimetidine | 5 | 100 | 1.4 ± 0.5 ** | 68 |
| AcF–I. truxillensis | 5 | 50 | 2.8 ± 1.4 * | 38 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luiz-Ferreira, A.; Cola, M.; Barbastefano, V.; De-Faria, F.M.; Almeida, A.B.A.d.; Farias-Silva, E.; Calvo, T.R.; Hiruma-Lima, C.A.; Vilegas, W.; Souza-Brito, A.R.M. Healing, Antioxidant and Cytoprotective Properties of Indigofera truxillensis in Different Models of Gastric Ulcer in Rats. Int. J. Mol. Sci. 2012, 13, 14973-14991. https://doi.org/10.3390/ijms131114973
Luiz-Ferreira A, Cola M, Barbastefano V, De-Faria FM, Almeida ABAd, Farias-Silva E, Calvo TR, Hiruma-Lima CA, Vilegas W, Souza-Brito ARM. Healing, Antioxidant and Cytoprotective Properties of Indigofera truxillensis in Different Models of Gastric Ulcer in Rats. International Journal of Molecular Sciences. 2012; 13(11):14973-14991. https://doi.org/10.3390/ijms131114973
Chicago/Turabian StyleLuiz-Ferreira, Anderson, Maira Cola, Victor Barbastefano, Felipe Meira De-Faria, Ana Beatriz A. de Almeida, Elisângela Farias-Silva, Tamara Regina Calvo, Clélia A. Hiruma-Lima, Wagner Vilegas, and Alba Regina M. Souza-Brito. 2012. "Healing, Antioxidant and Cytoprotective Properties of Indigofera truxillensis in Different Models of Gastric Ulcer in Rats" International Journal of Molecular Sciences 13, no. 11: 14973-14991. https://doi.org/10.3390/ijms131114973







