The Characterization of SaPIN2b, a Plant Trichome-Localized Proteinase Inhibitor from Solanum americanum
Abstract
:1. Introduction
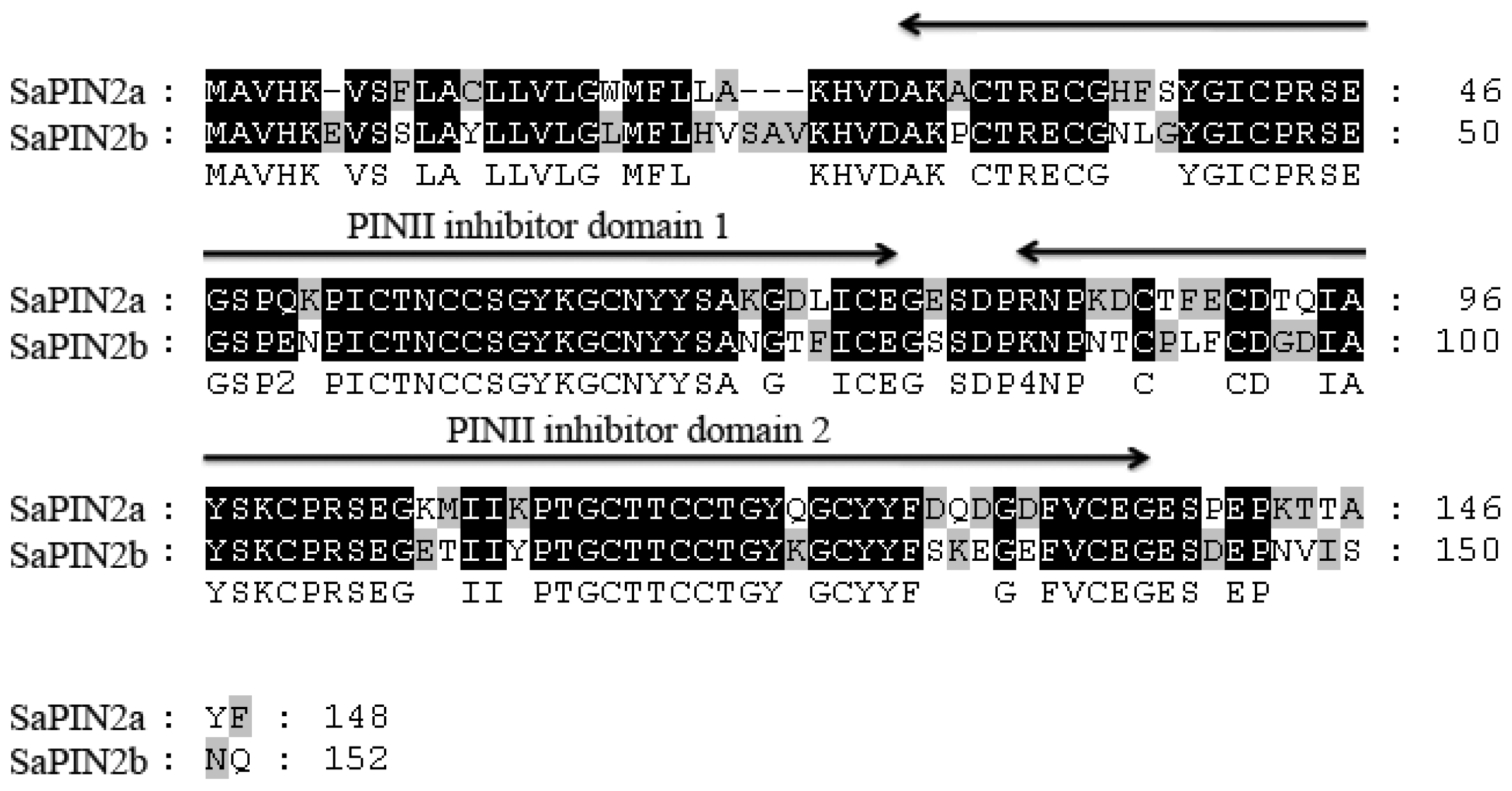
2. Results and Discussion
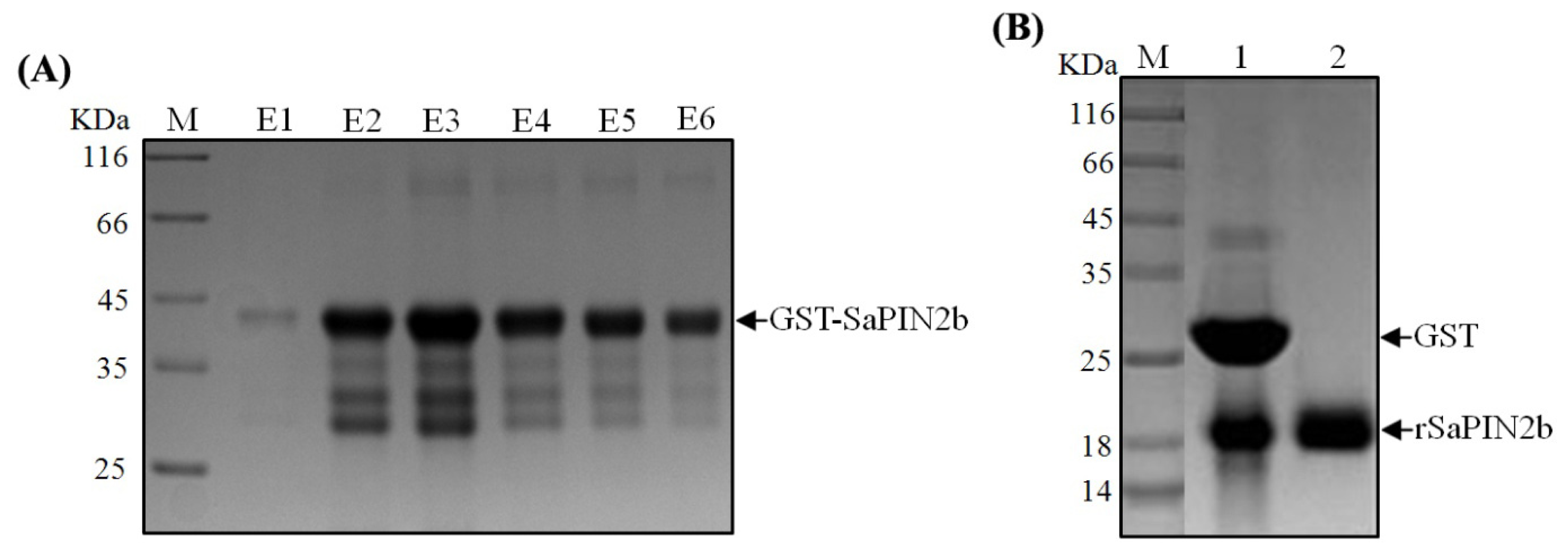
2.1. The Expression and Purification of Recombinant SaPIN2b
2.2. Recombinant SaPIN2b as a Potent Serine Proteinase Inhibitor
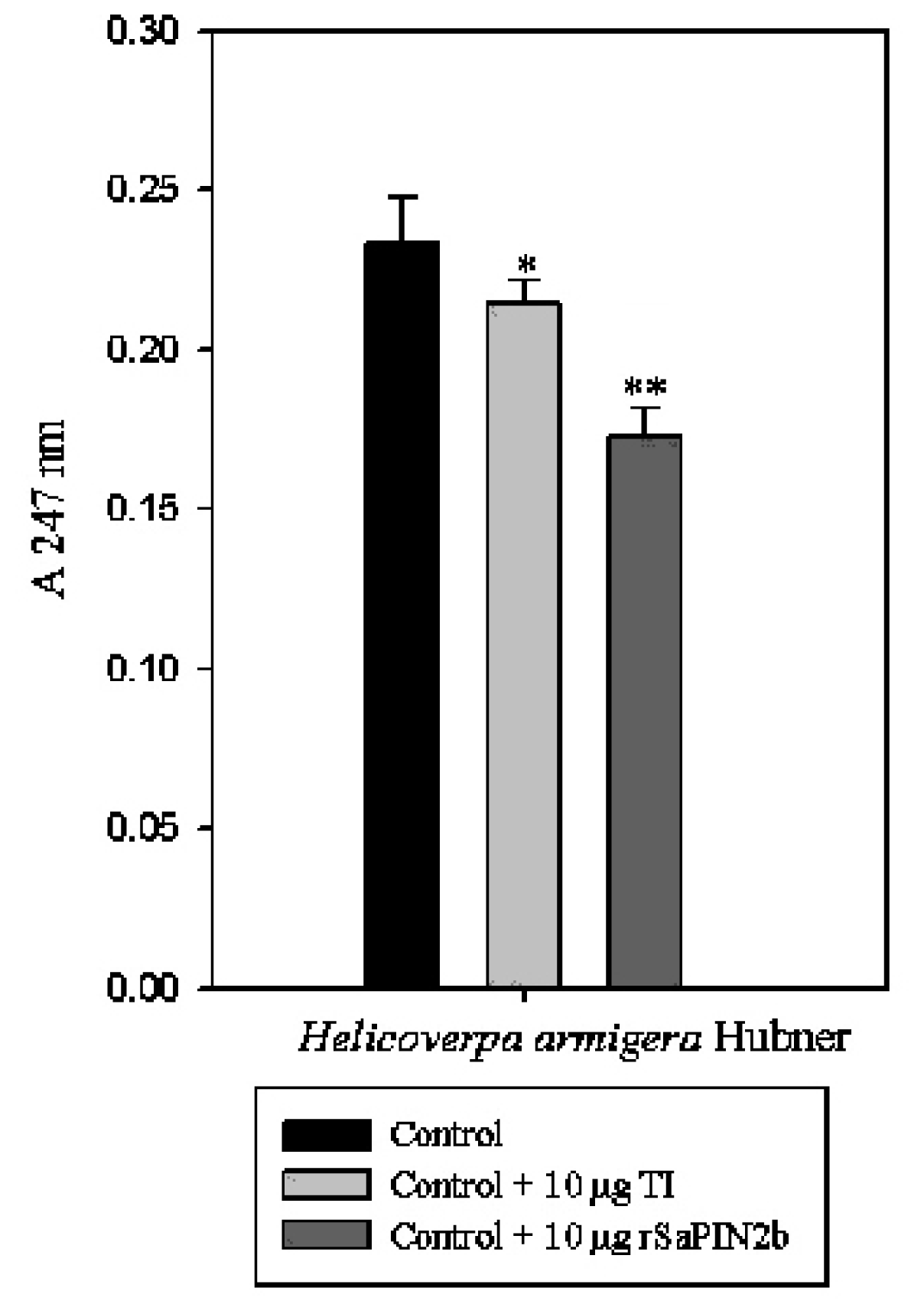
2.3. Enzymatic Assays to Test rSaPIN2b Against Digestive Proteinases That Have Been Obtained from the Midgut of Helicoverpa armigera Larvae
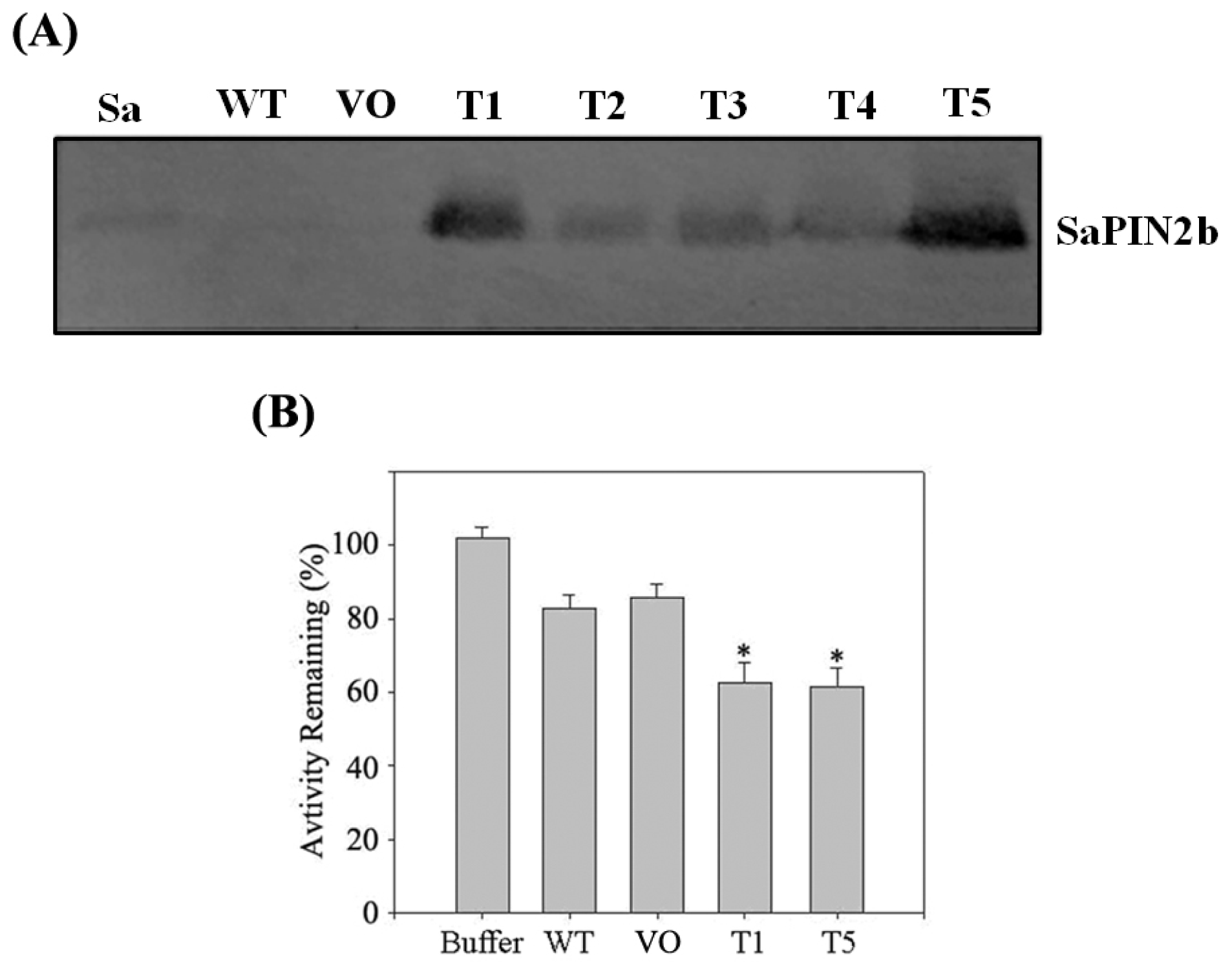
2.4. The Overexpression of SaPIN2b in Transgenic Tobacco Plants Enhanced the Resistance of These Plants to Helicoverpa armigera
2.5. Discussion
3. Experimental Methods
3.1. RNA Isolation and RT-PCR
3.2. The Expression and Purification of Recombinant SaPIN2b
3.3. The Production of SaPIN2b Polyclonal Antibody
3.4. The Determination of the Protein Concentration
3.5. Proteinase Inhibition Assays and Kinetic Analysis
3.6. The Extraction and Activity Assay of Proteinases from Insect Midguts
3.7. The Generation of SaPIN2b-Overexpressing Transgenic Plants
3.8. Western Blot Analysis
3.9. Insect Feeding Trials
4. Conclusions
Acknowledgements
References
- Chung, S.H.; Felton, G.W. Specificity of induced resistance in tomato against specialist Lepidopteran and Coleopteran species. J. Chem. Ecol 2011, 37, 378–386. [Google Scholar]
- Kouzuma, Y.; Kawano, K.; Kimura, M.; Yamasaki, N.; Kadowaki, T.; Yamamoto, K. Purification characterization, and sequencing of two cysteine proteinase inhibitors, Sea and Scb, from sunflower (Helianthus annuus) seeds. J. Biochem 1996, 119, 1106–1113. [Google Scholar]
- Ryan, S.N.; McManus, M.T.; Laing, W.A. Identification and characterisation of proteinase inhibitors and their genes from seeds of apple (Malus domestica). J. Biochem 2003, 134, 31–42. [Google Scholar]
- Pearce, G.; Johnson, S.; Ryan, C.A. Purification and characterization from tobacco (Nicotiana-tabacum) leaves of six small, wound-inducible, proteinase isoinhibitors of the potato inhibitor-II family. Plant Physiol 1993, 102, 639–644. [Google Scholar]
- Coffeen, W.C.; Wolpert, T.J. Purification and characterization of serine proteases that exhibit caspase-like activity and are associated with programmed cell death in Avena sativa. Plant Cell 2004, 16, 857–873. [Google Scholar]
- Palmieri, G.; Bianco, C.; Cennamo, G.; Giardina, P.; Marino, G.; Monti, M.; Sannia, G. Purification, characterization, and functional role of a novel extracellular protease from Pleurotus ostreatus. Appl. Environ. Microbiol 2001, 67, 2754–2759. [Google Scholar]
- Oliveira, A.S.; Migliolo, L.; Aquino, R.O.; Ribeiro, J.K.; Macedo, L.L.; Bemquerer, M.P.; Santos, E.A.; Kiyota, S.; de Sales, M.P. Two Kunitz-type inhibitors with activity against trypsin and papain from Pithecellobium dumosum seeds: Purification, characterization, and activity towards pest insect digestive enzyme. Protein Pept. Lett 2009, 16, 1526–1532. [Google Scholar]
- Turra, D.; Lorito, M. Potato type I and II proteinase inhibitors: Modulating plant physiology and host resistance. Curr. Protein Pept. Sci 2011, 12, 374–385. [Google Scholar]
- Li, S.; Guo, D.; Zhao, B.; Ye, J.; Tian, J.; Ren, W.; Ju, Y.; Cui, P.; Li, R. Cloning and expression of cDNA encoding a cysteine protease inhibitor from clamworm and its possible use in managing Anoplophora glabripennis Motschulsky (Coleoptera: Cerambycidae). J. Microbiol. Biotechnol 2010, 20, 1243–1250. [Google Scholar]
- Schluter, U.; Benchabane, M.; Munger, A.; Kiggundu, A.; Vorster, J.; Goulet, M.C.; Cloutier, C.; Michaud, D. Recombinant protease inhibitors for herbivore pest control: A multitrophic perspective. J. Exp. Bot 2010, 61, 4169–4183. [Google Scholar]
- Lawrence, P.K.; Koundal, K.R. Plant protease inhibitors in control of phytophagous insects. Electron. J. Biotechnol 2002, 5, 93–109. [Google Scholar]
- Mosolov, V.; Valueva, T. Proteinase inhibitors and their function in plants: A review. Appl. Biochem. Microbiol 2005, 41, 227–246. [Google Scholar]
- Majeed, A.; Makhdoom, R.; Husnain, T.; Riazuddin, S. Assessment of potato proteinase inhibitor-II gene as an antifungal and insecticidal agent. Acta Agric. Scand. Sect. B 2011, 61, 92–96. [Google Scholar]
- Carrillo, L.; Martinez, M.; Alvarez-Alfageme, F.; Castanera, P.; Smagghe, G.; Diaz, I.; Ortego, F. A barley cysteine-proteinase inhibitor reduces the performance of two aphid species in artificial diets and transgenic Arabidopsis plants. Transgenic Res 2011, 20, 305–319. [Google Scholar]
- Dunse, K.M.; Stevens, J.A.; Lay, F.T.; Gaspar, Y.M.; Heath, R.L.; Anderson, M.A. Coexpression of potato type I and II proteinase inhibitors gives cotton plants protection against insect damage in the field. Proc. Natl. Acad. Sci. USA 2010, 107, 15011–15015. [Google Scholar]
- Hartl, M.; Giri, A.P.; Kaur, H.; Baldwin, I.T. Serine protease inhibitors specifically defend Solanum nigrum against generalist herbivores but do not influence plant growth and development. Plant Cell 2010, 22, 4158–4175. [Google Scholar]
- Gatehouse, J.A. Prospects for using proteinase inhibitors to protect transgenic plants against attack by herbivorous insects. Curr. Protein Pept. Sci 2011, 12, 409–416. [Google Scholar]
- Altpeter, F.; Diaz, I.; McAuslane, H.; Gaddour, K.; Carbonero, P.; Vasil, I.K. Increased insect resistance in transgenic wheat stably expressing trypsin inhibitor CMe. Mol. Breed 1999, 5, 53–63. [Google Scholar]
- Lee, I.; Lee, S.H.; Koo, C.; Jin, C.H.; Lim, C.O.; Mun, H.; Han, S.Y.; Cho, J. Soybean Kunitz trypsin inhibitor (SKTI) confers resistance to the brown planthopper (Nilaparvata lugens Stal) in transgenic rice. Mol. Breed 1999, 5, 1–9. [Google Scholar]
- Vila, L.; Quilis, J.; Meynard, D.; Breitler, J.C.; Marfa, V.; Murillo, I.; Vassal, J.M.; Messeguer, J.; Guiderdoni, E.; San Segundo, B. Expression of the maize proteinase inhibitor (mpi) gene in rice plants enhances resistance against the striped stem borer (Chilo suppressalis): Effects on larval growth and insect gut proteinases. Plant Biotechnol. J 2005, 3, 187–202. [Google Scholar]
- Jongsma, M.A.; Bakker, P.L.; Peters, J.; Bosch, D.; Stiekema, W.J. Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition. Proc. Natl. Acad. Sci. USA 1995, 92, 8041–8045. [Google Scholar]
- Cloutier, C.; Jean, C.; Fournier, M.; Yelle, S.; Michaud, D. Adult Colorado potato beetles, Leptinotarsa decemlineata compensate for nutritional stress on oryzacystatin I-transgenic potato plants by hypertrophic behavior and over-production of insensitive proteases. Arch. Insect. Biochem. Physiol 2000, 44, 69–81. [Google Scholar]
- Harsulkar, A.M.; Giri, A.P.; Patankar, A.G.; Gupta, V.S.; Sainani, M.N.; Ranjekar, P.K.; Deshpande, V.V. Successive use of non-host plant proteinase inhibitors required for effective inhibition of Helicoverpa armigera gut proteinases and larval growth. Plant Physiol 1999, 121, 497–506. [Google Scholar]
- Xu, Z.F.; Qi, W.Q.; Ouyang, X.Z.; Yeung, E.; Chye, M.L. A proteinase inhibitor II of Solanum americanum is expressed in phloem. Plant Mol. Biol 2001, 47, 727–738. [Google Scholar]
- Liu, J.; Xia, K.F.; Zhu, J.C.; Deng, Y.G.; Huang, X.L.; Hu, B.L.; Xu, X.; Xu, Z.F. The nightshade proteinase inhibitor IIb gene is constitutively expressed in glandular trichomes. Plant Cell Physiol 2006, 47, 1274–1284. [Google Scholar]
- Luo, M.; Wang, Z.; Li, H.; Xia, K.F.; Cai, Y.; Xu, Z.F. Overexpression of a weed (Solanum americanum) proteinase inhibitor in transgenic tobacco results in increased glandular trichome density and enhanced resistance to Helicoverpa armigera and Spodoptera litura. Int. J. Mol. Sci 2009, 10, 1896–1910. [Google Scholar]
- Wang, Z.Y.; Ding, L.W.; Ge, Z.J.; Wang, Z.; Wang, F.; Li, N.; Xu, Z.F. Purification and characterization of native and recombinant SaPIN2a, a plant sieve element-localized proteinase inhibitor. Plant Physiol. Biochem 2007, 45, 757–766. [Google Scholar]
- Bown, D.P.; Wilkinson, H.S.; Gatehouse, J.A. Differentially regulated inhibitor-sensitive and insensitive protease genes from the phytophagous insect pest, Helicoverpa armigera, are members of complex multigene families. Insect Biochem. Mol. Biol 1997, 27, 625–638. [Google Scholar]
- ClustalX software, version 2.1; European Bioinformatics Institute: Dublin, Ireland, 2010.
- Pfam databases. Available online: http://pfam.sanger.ac.uk/ accessed on 1 August 2012.
- Beekwilder, J.; Schipper, B.; Bakker, P.; Bosch, D.; Jongsma, M. Characterization of potato proteinase inhibitor II reactive site mutants. Eur. J. Biochem 2000, 267, 1975–1984. [Google Scholar]
- Volpicella, M.; Schipper, A.; Jongsma, M.A.; Spoto, N.; Gallerani, R.; Ceci, L.R. Characterization of recombinant mustard trypsin inhibitor 2 (MTI2) expressed in Pichia pastoris. FEBS Lett 2000, 468, 137–141. [Google Scholar]
- Johnson, R.; Narvaez, J.; An, G.; Ryan, C. Expression of proteinase inhibitors I and II in transgenic tobacco plants: Effects on natural defense against Manduca sexta larvae. Proc. Natl. Acad. Sci. USA 1989, 86, 9871–9875. [Google Scholar]
- Chen, H.; Gonzales-Vigil, E.; Wilkerson, C.G.; Howe, G.A. Stability of plant defense proteins in the gut of insect herbivores. Plant Physiol 2007, 143, 1954–1967. [Google Scholar]
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol 2002, 156, 145–169. [Google Scholar]
- Jongsma, M.A.; Bolter, C. The adaptation of insects to plant protease inhibitors. J. Insect Physiol 1997, 43, 885–895. [Google Scholar]
- Tian, M.; Huitema, E.; da Cunha, L.; Torto-Alalibo, T.; Kamoun, S. A Kazal-like extracellular serine protease inhibitor from Phytophthora infestans targets the tomato pathogenesis-related protease P69B. J. Biol. Chem 2004, 279, 26370–26377. [Google Scholar]
- Ryan, C.A. Protease inhibitors in plants: Genes for improving defenses against insects and pathogens. Annu. Rev. Phytopathol 1990, 28, 425–449. [Google Scholar]
- Li, L.; Zhao, Y.; McCaig, B.C.; Wingerd, B.A.; Wang, J.; Whalon, M.E.; Pichersky, E.; Howe, G.A. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 2004, 16, 126–143. [Google Scholar]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 2012, 17, 250–259. [Google Scholar]
- Wagner, G.; Wang, E.; Shepherd, R. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot 2004, 93, 3–11. [Google Scholar]
- Peiffer, M.; Tooker, J.F.; Luthe, D.S.; Felton, G.W. Plants on early alert: glandular trichomes as sensors for insect herbivores. New Phytol 2009, 184, 644–656. [Google Scholar]
- Murray, C.; Christeller, J.T. Genomic nucleotide sequence of a proteinase inhibitor II gene. Plant Physiol 1994, 106, 1681. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Hatakeyama, T.; Kohzaki, H.; Yamasaki, N. A microassay for proteases using succinylcasein as a substrate. Anal. Biochem 1992, 204, 181–184. [Google Scholar]
- Kollipara, K.P.; Hymowitz, T. Characterization of trypsin and chymotrypsin inhibitors in the wild perennial Glycine species. J. Agric. Food Chem 1992, 40, 2356–2363. [Google Scholar]
- Yasuda, Y.; Kageyama, T.; Akamine, A.; Shibata, M.; Kominami, E.; Uchiyama, Y.; Yamamoto, K. Characterization of new fluorogenic substrates for the rapid and sensitive assay of cathepsin E and cathepsin D. J. Biochem 1999, 125, 1137–1143. [Google Scholar]
- Rickauer, M.; Fournier, J.; Esquerre-Tugaye, M.T. Induction of proteinase inhibitors in tobacco cell suspension culture by elicitors of Phytophthora parasitica var nicotianae. Plant Physiol 1989, 90, 1065–1070. [Google Scholar]
- Wesley, S.V.; Helliwell, C.A.; Smith, N.A.; Wang, M.B.; Rouse, D.T.; Liu, Q.; Gooding, P.S.; Singh, S.P.; Abbott, D.; Stoutjesdijk, P.A.; et al. Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 2001, 27, 581–590. [Google Scholar]
- Gleave, A.P. A versatile binary vector system with a T-DNA organizational-structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol. Biol 1992, 20, 1203–1207. [Google Scholar]
- Hofgen, R.; Willmitzer, L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 1988, 16, 9877. [Google Scholar]
- Horsch, R.B.; Fry, J.E.; Hoffmann, N.L.; Eichholtz, D.; Rogers, S.G.; Fraley, R.T. A simple and general-method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar]
- Xu, Z.F.; Teng, W.L.; Chye, M.L. Inhibition of endogenous trypsin-and chymotrypsin-like activities in transgenic lettuce expressing heterogeneous proteinase inhibitor SaPIN2a. Planta 2004, 218, 623–629. [Google Scholar]


| Proteinase (final concentration) | rSaPIN2b (nM) | I/E a | Inhibition (nM) b | Substrate (final concentration) | IC50 |
|---|---|---|---|---|---|
| Subtilisin A (83 nM) | 74 | 0.9 | 74.3 | Succinylcasein (14 nM) | 7.3 |
| Chymotrypsin (25 nM) | 41.6 | 1.7 | 54 | BTEE (0.5 mM) | 34.9 |
| Trypsin (7.5 nM) | 131.2 | 17.5 | 61 | TAME (1 mM) | 126.7 |
| Mortality (%) | Pupation rate (%) | |
|---|---|---|
| WT | 23.3 ± 5.8 | 78.6 ± 6.2 |
| VO | 30.0 ± 13.3 | 81.5 ± 3.2 |
| T1 | 56.7 ± 5.8 ** | 38.3 ± 12.6 ** |
| T5 | 50.0 ± 10 ** | 41.1 ± 8.4 ** |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luo, M.; Ding, L.-W.; Ge, Z.-J.; Wang, Z.-Y.; Hu, B.-L.; Yang, X.-B.; Sun, Q.-Y.; Xu, Z.-F. The Characterization of SaPIN2b, a Plant Trichome-Localized Proteinase Inhibitor from Solanum americanum. Int. J. Mol. Sci. 2012, 13, 15162-15176. https://doi.org/10.3390/ijms131115162
Luo M, Ding L-W, Ge Z-J, Wang Z-Y, Hu B-L, Yang X-B, Sun Q-Y, Xu Z-F. The Characterization of SaPIN2b, a Plant Trichome-Localized Proteinase Inhibitor from Solanum americanum. International Journal of Molecular Sciences. 2012; 13(11):15162-15176. https://doi.org/10.3390/ijms131115162
Chicago/Turabian StyleLuo, Ming, Ling-Wen Ding, Zhi-Juan Ge, Zhen-Yu Wang, Bo-Lun Hu, Xiao-Bei Yang, Qiao-Yang Sun, and Zeng-Fu Xu. 2012. "The Characterization of SaPIN2b, a Plant Trichome-Localized Proteinase Inhibitor from Solanum americanum" International Journal of Molecular Sciences 13, no. 11: 15162-15176. https://doi.org/10.3390/ijms131115162





