Modulation of MDR1 and MRP3 Gene Expression in Lung Cancer Cells after Paclitaxel and Carboplatin Exposure
Abstract
:1. Introduction
2. Results
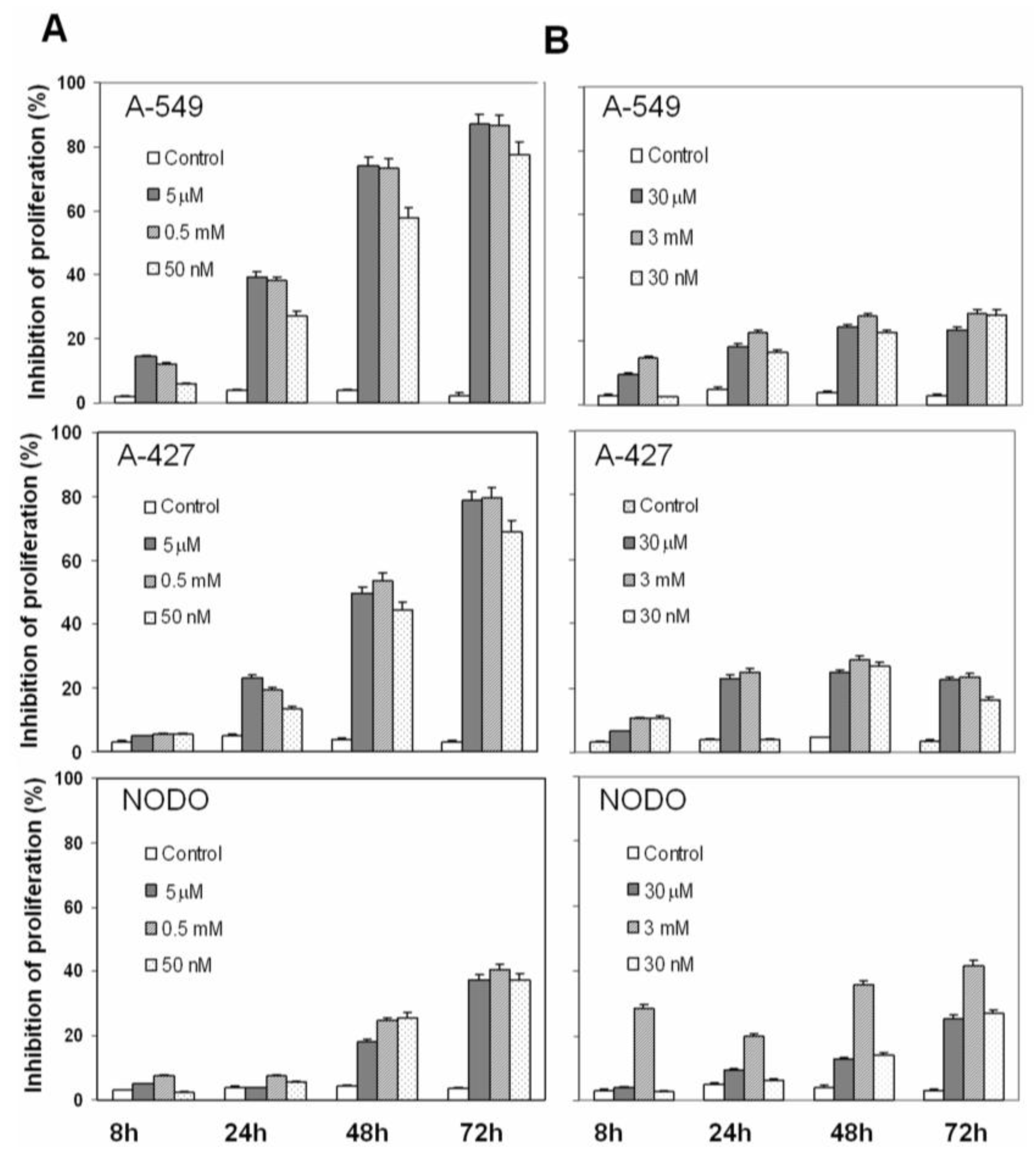
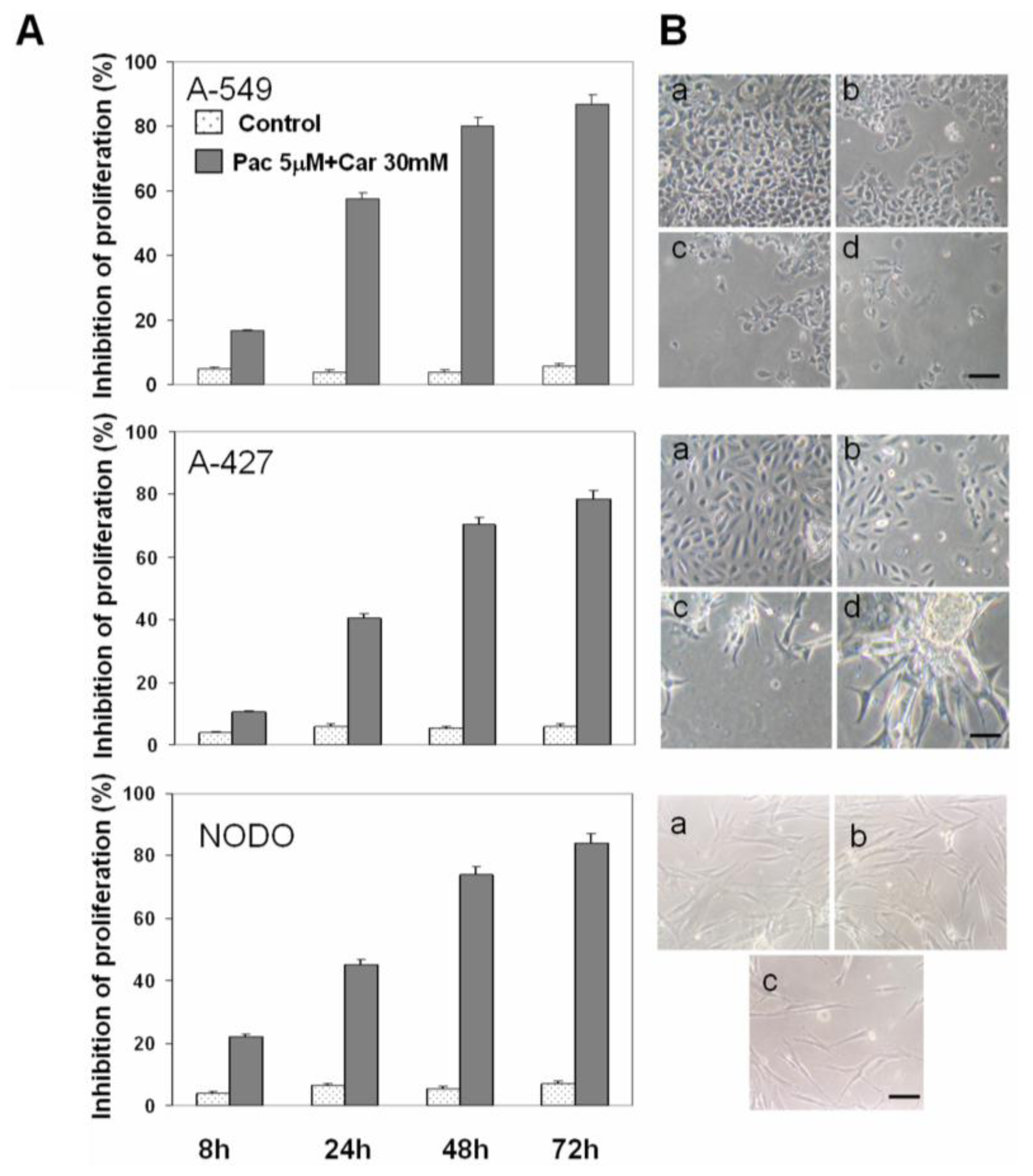
2.1. Drug Cytotoxicity in NSCLC Cells
2.2. Apoptosis Analysis
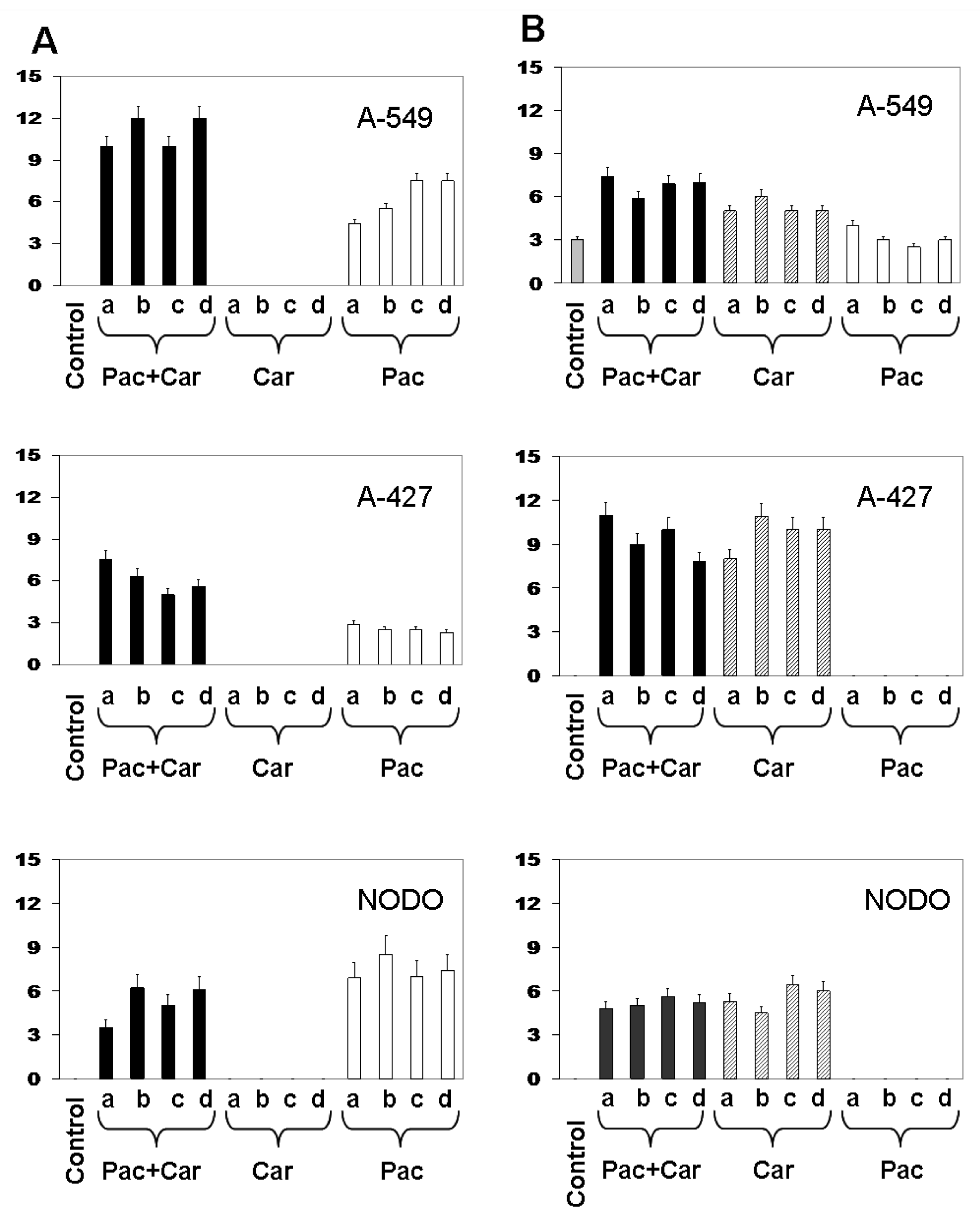
2.3. Modulation of MDR1 and MRP3 Gene Expression in NSCLC Cell Lines
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. In Vitro Cell Proliferation Assay
4.3. Apoptosis Analysis
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Schiller, J.H.; Harrington, D.; Belani, C.P.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H. Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar]
- Monzo, M.; Rosell, R.; Taron, M. Drug resistance in non-small cell lung cancer. Lung Cancer 2001, 34, S91–S94. [Google Scholar]
- Ikuta, K.; Takemura, K.; Sasaki, K.; Kihara, M.; Nishimura, M.; Ueda, N.; Naito, S.; Lee, E.; Shimizu, E.; Yamauchi, A. Expression of multidrug resistance proteins and accumulation of cisplatin in human non-small cell lung cancer cells. Biol. Pharm. Bull 2005, 28, 707–712. [Google Scholar]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev 2012, 55, 3–29. [Google Scholar]
- Amiri-Kordestani, L.; Basseville, A.; Kurdziel, K.; Fojo, A.T.; Bates, S.E. Targeting MDR in breast and lung cancer: Discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist. Updat 2012, 15, 50–61. [Google Scholar]
- Horwitz, S.B.; Cohen, D.; Rao, S.; Ringel, I.; Shen, H.J.; Yang, C.P. Taxol: Mechanisms of action and resistance. J. Natl. Cancer Inst. Monogr 1993, 15, 55–61. [Google Scholar]
- Kamazawa, S.; Kigawa, J.; Minagawa, Y.; Itamochi, H.; Shimada, M.; Takahashi, M.; Sato, S.; Akeshima, R.; Terakawa, N. Cellular efflux pump and interaction between cisplatin and paclitaxel in ovarian cancer cells. Oncology 2000, 59, 329–335. [Google Scholar]
- Hilgeroth, A.; Hemmer, M.; Coburger, C. The impact of the induction of multidrug resistance transporters in therapies by used drugs: Recent studies. Mini Rev. Med. Chem 2012, 12, 1127–1134. [Google Scholar]
- Dingemans, A.M.; van Ark-Otte, J.; van der Valk, P.; Apolinario, R.M.; Scheper, R.J.; Postmus, P.E.; Giaccone, G. Expression of the human major vault protein LRP in human lung cancer samples and normal lung tissues. Ann. Oncol 1996, 7, 625–630. [Google Scholar]
- Young, L.C.; Campling, B.G.; Voskoglou-Nomikos, T.; Cole, S.P.C.; Deeley, R.G.; Gerlach, J.H. Expression of multidrug resistance protein-related genes in lung cancer: Correlation with drug response. Clin. Cancer Res 1999, 5, 673–680. [Google Scholar]
- Young, L.C.; Campling, B.G.; Cole, S.P.; Deeley, R.G.; Gerlach, J.H. Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: Correlation of protein levels with drug response and messenger RNA levels. Clin. Cancer Res 2001, 7, 1798–1804. [Google Scholar]
- Wang, M.; Hong, X.; Sun, Q.; Li, R.; Yang, Z.; Chen, G. Establishment of animal model of a human lung adenocarcinoma drug-resistant cell line Anip973/NVB and investigation on mechanism of drug resistance. Zhongguo Fei Ai Za Zhi 2012, 15, 146–151. [Google Scholar]
- Chen, Z.J.; Le, H.B.; Zhang, Y.K.; Qian, L.Y.; Sekhar, K.R.; Li, W.D. Lung resistance protein and multidrug resistance protein in non-small cell lung cancer and their clinical significance. J. Int. Med. Res 2011, 39, 1693–1700. [Google Scholar]
- Scheper, R.J.; Broxterman, H.J.; Scheffer, G.L.; Kaaijk, P.; Dalton, W.S.; van Heijningen, T.H.; van Kalken, C.K.; Slovak, M.L.; de Vries, E.G.; van der Valk, P.; et al. Overexpression of a M(r) 110,000 vesicular protein in non-Pglycoprotein-mediated multidrug resistance. Cancer Res 1993, 53, 1475–1479. [Google Scholar]
- Thatcher, N.; Heighway, J. Maintenance and consolidation therapy in patients with unresectable stage III/IV non-small cell lung cancer. Oncologist 2010, 15, 1034–1042. [Google Scholar]
- Kaye, S.; Aamdal, S.; Jones, R.; Freyer, G.; Pujade-Lauraine, E.; de Vries, E.G.; Barriuso, J.; Sandhu, S.; Tan, D.S.; Hartog, V.; et al. Phase I study of saracatinib (AZD0530) in combination with paclitaxel and/or carboplatin in patients with solid tumours. Br. J. Cancer 2012, 106, 1728–1734. [Google Scholar]
- Tiseo, M.; Franciosi, V.; Grossi, F.; Ardizzoni, A. Adjuvant chemotherapy for non-small cell lung cancer: Ready for clinical practice. Eur. J. Cancer 2006, 42, 8–16. [Google Scholar]
- Li, X.-Q.; Li, J.; Chen, P. Expression and clinical significance of MRP1 and LRP mRNA in nonsmall cell lung cancer. Pract. J. Cancer 2008, 23, 145–147. [Google Scholar]
- Ueda, K.; Cardarelli, C.; Gottesman, M.M.; Pastan, I. Expression of a full-length cDNA for the human “MDR1” gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc. Natl. Acad. Sci. USA 1987, 84, 3004–3008. [Google Scholar]
- Ling, V. Charles F. Kettering Prize. P-glycoprotein and resistance to anticancer drugs. Cancer 1992, 69, 2603–2609. [Google Scholar]
- Lorico, A.; Rappa, G.; Flavell, R.A.; Sartorelli, A.C. Double knockout of the MRP gene leads to increased drug sensitivity in vitro. Cancer Res 1996, 56, 5351–5355. [Google Scholar]
- Kawanobe, T.; Kogure, S.; Nakamura, S.; Sato, M.; Katayama, K.; Mitsuhashi, J.; Noguchi, K.; Sugimoto, Y. Expression of human ABCB5 confers resistance to taxanes and anthracyclines. Biochem. Biophys. Res. Commun 2012, 418, 736–741. [Google Scholar]
- Wang, Y.; Chen, Q.; Jin, S.; Deng, W.; Li, S.; Tong, Q.; Chen, Y. Up-regulation of P-glycoprotein is involved in the increased paclitaxel resistance in human esophageal cancer radioresistant cells. Scand. J. Gastroenterol 2012, 47, 802–808. [Google Scholar]
- Podolski-Renić, A.; Andelković, T.; Banković, J.; Tanić, N.; Ruždijić, S.; Pešić, M. The role of paclitaxel in the development and treatment of multidrug resistant cancer cell lines. Biomed. Pharmacother 2011, 65, 345–353. [Google Scholar]
- Kars, M.D.; Işeri, O.D.; Gündüz, U. A microarray based expression profiling of paclitaxel and vincristine resistant MCF-7 cells. Eur. J. Pharmacol 2011, 657, 4–9. [Google Scholar]
- Rolff, J.; Dorn, C.; Merk, J.; Fichtner, I. Response of patient-derived non-small cell lung cancer xenografts to classical and targeted therapies is not related to multidrug resistance markers. J. Oncol 2009, 2009, 814140. [Google Scholar]
- Volm, M.; Koomägi, R.; Mattern, J.; Efferth, T. Protein expression profiles indicative for drug resistance of non-small cell lung cancer. Br. J. Cancer 2002, 87, 251–257. [Google Scholar]
- Meschini, S.; Marra, M.; Calcabrini, A.; Monti, E.; Gariboldi, M.; Dolfini, E.; Arancia, G. Role of the lung resistance-related protein (LRP) in the drug sensitivity of cultured tumor cells. Toxicol. In Vitro 2002, 16, 389–398. [Google Scholar]
- Chiou, J.F.; Liang, J.A.; Hsu, W.H.; Wang, J.J.; Ho, S.T.; Kao, A. Comparing the relationship of Taxol-based chemotherapy response with P-glycoprotein and lung resistance-related protein expression in non-small cell lung cancer. Lung 2003, 181, 267–273. [Google Scholar]
- Shimomura, M.; Yaoi, T.; Itoh, K.; Kato, D.; Terauchi, K.; Shimada, J.; Fushiki, S. Drug resistance to paclitaxel is not only associated with ABCB1 mRNA expression but also with drug accumulation in intracellular compartments in human lung cancer cell lines. Int. J. Oncol 2012, 40, 995–1004. [Google Scholar]
- Maráz, A.; Furák, J.; Pálföldi, R.; Eller, J.; Szántó, E.; Kahán, Z.; Thurzó, L.; Molnár, J.; Tiszlavicz, L.; Hideghéty, K. Roles of BCL-2 and MDR1 expression in the efficacy of paclitaxel-based lung cancer chemoradiation. Anticancer Res 2011, 31, 1431–1436. [Google Scholar]
- Boyerinas, B.; Park, S.M.; Murmann, A.E.; Gwin, K.; Montag, A.G.; Zillhardt, M.; Hua, Y.J.; Lengyel, E.; Peter, M.E. Let-7 modulates acquired resistance of ovarian cancer to Taxanes via IMP-1-mediated stabilization of multidrug resistance 1. Int. J. Cancer 2012, 130, 1787–1797. [Google Scholar]
- Parker, R.J.; Eastman, A.; Bostick-Bruton, F.; Reed, E. Acquired cisplatin resistance in human ovarian cancer cells is associated with enhanced repair of cisplatin-DNA lesions and reduced drug accumulation. J. Clin. Invest 1991, 87, 772–777. [Google Scholar]
- Bogush, T.A.; Konukhova, A.V.; Ravcheeva, A.B.; Zabotina, T.N.; Kadagidze, Z.G.; Bogush, E.A.; Komov, D.V.; Polotskiĭ, B.E.; Laktionov, K.K.; Davydov, M.I. Inhibition of ABC-transporter(s)’ function in non-small cell lung cancer cells by platinum drugs. Antibiot. Khimioter 2003, 48, 11–15. [Google Scholar]
- Marsh, R.E.; Tuszyński, J.A.; Sawyer, M.B.; Vos, K.J. Emergence of power laws in the pharmacokinetics of paclitaxel due to competing saturable processes. J. Pharm. Pharm. Sci 2008, 11, 77–96. [Google Scholar]
- Fukuda, M.; Oka, M.; Soda, H.; Terashi, K.; Kawabata, S.; Nakatomi, K.; Takatani, H.; Tsurutani, J.; Tsukamoto, K.; Noguchi, Y.; et al. Phase I study of irinotecan combined with carboplatin in previously untreated solid cancers. Clin. Cancer Res 1999, 5, 3963–3969. [Google Scholar]
- Prados, J.; Melguizo, C.; Marchal, J.A.; Vélez, C.; Alvarez, L.; Aránega, A. Therapeutic differentiation in a human rhabdomyosarcoma cell line selected for resistance to actinomycin D. Int. J. Cancer 1998, 75, 379–383. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Melguizo, C.; Prados, J.; Luque, R.; Ortiz, R.; Caba, O.; Álvarez, P.J.; Gonzalez, B.; Aranega, A. Modulation of MDR1 and MRP3 Gene Expression in Lung Cancer Cells after Paclitaxel and Carboplatin Exposure. Int. J. Mol. Sci. 2012, 13, 16624-16635. https://doi.org/10.3390/ijms131216624
Melguizo C, Prados J, Luque R, Ortiz R, Caba O, Álvarez PJ, Gonzalez B, Aranega A. Modulation of MDR1 and MRP3 Gene Expression in Lung Cancer Cells after Paclitaxel and Carboplatin Exposure. International Journal of Molecular Sciences. 2012; 13(12):16624-16635. https://doi.org/10.3390/ijms131216624
Chicago/Turabian StyleMelguizo, Consolación, Jose Prados, Raquel Luque, Raúl Ortiz, Octavio Caba, Pablo J. Álvarez, Beatriz Gonzalez, and Antonia Aranega. 2012. "Modulation of MDR1 and MRP3 Gene Expression in Lung Cancer Cells after Paclitaxel and Carboplatin Exposure" International Journal of Molecular Sciences 13, no. 12: 16624-16635. https://doi.org/10.3390/ijms131216624



