A Novel LMP1 Antibody Synergizes with Mitomycin C to Inhibit Nasopharyngeal Carcinoma Growth in Vivo Through Inducing Apoptosis and Downregulating Vascular Endothelial Growth Factor
Abstract
:1. Introduction
2. Results and Discussion
2.1. MMC in Combination with Anti-LMP1 Fab Exhibits Synergistic Effect to Inhibit HNE2 Tumor Growth in Vivo
2.2. MMC in Combination with Anti-LMP1 Fab Exhibits Synergistic Effect to Induce the Apoptosis of HNE2 Cells in Vivo
2.3. MMC in Combination with Anti-LMP1 Fab Exhibits Synergistic Effect to Inhibit VEGF Expression in HNE2 Cells
2.4. Discussion
3. Materials and Methods
3.1. Reagents
3.2. Cell Culture
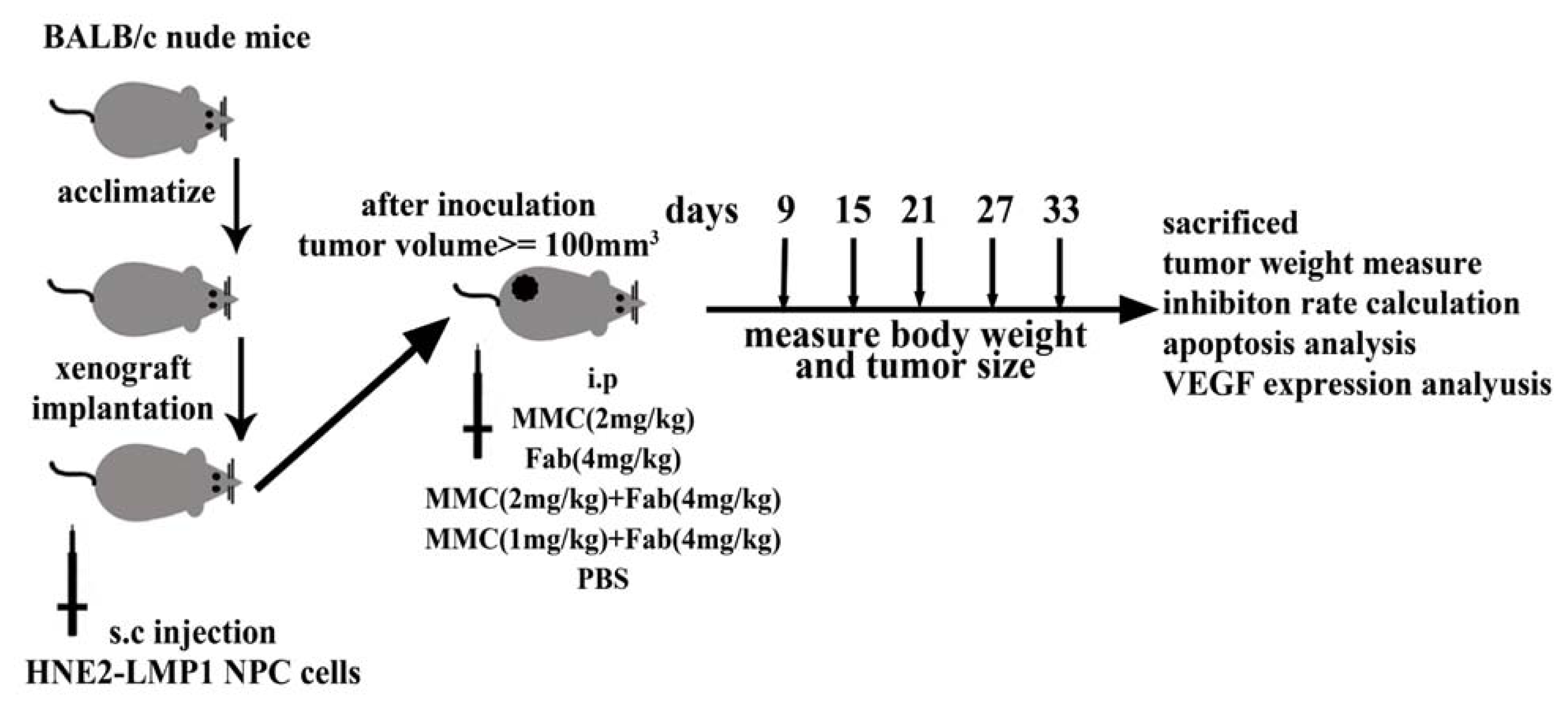
3.3. In Vivo Tumor Xenograft Model
3.4. Annexin V/PI Assay for Apoptosis
3.5. Immunohistochemistry
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Chang, E.T.; Adami, H.O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev 2006, 15, 1765–1777. [Google Scholar]
- Guigay, J. Advances in nasopharyngeal carcinoma. Curr. Opin. Oncol 2008, 20, 264–269. [Google Scholar]
- Tao, Q.; Chan, A.T. Nasopharyngeal carcinoma: Molecular pathogenesis and therapeutic developments. Expert. Rev. Mol. Med 2007, 9, 1–24. [Google Scholar]
- Agulnik, M.; Epstein, J.B. Nasopharyngeal carcinoma: Current management, future directions and dental implications. Oral. Oncol 2008, 44, 617–627. [Google Scholar]
- Ma, B.B.; Hui, E.P.; Chan, A.T. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: Current and future directions. Cancer Sci 2008, 99, 1311–1318. [Google Scholar]
- Thompson, M.P.; Kurzrock, R. Epstein-barr virus and cancer. Clin. Cancer Res 2004, 10, 803–821. [Google Scholar]
- Gullo, C.; Low, W.K.; Teoh, G. Association of epstein-barr virus with nasopharyngeal carcinoma and current status of development of cancer-derived cell lines. Ann. Acad. Med 2008, 37, 769–777. [Google Scholar]
- Krishna, S.M.; James, S.; Balaram, P. Expression of VEGF as prognosticator in primary nasopharyngeal cancer and its relation to EBV status. Virus Res 2006, 115, 85–90. [Google Scholar]
- Morris, M.A.; Dawson, C.W.; Young, L.S. Role of the Epstein-Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncol 2009, 5, 811–825. [Google Scholar]
- Ma, B.B.; Kam, M.K.; Leung, S.F.; Hui, E.P.; King, A.D.; Chan, S.L.; Mo, F.; Loong, H.; Yu, B.K.; Ahuja, A.; Chan, A.T. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann. Oncol 2011. [Google Scholar] [CrossRef]
- You, B.L.; Tourneau, C.; Chen, E.X.; Wang, L.; Jarvi, A.; Bharadwaj, R.R.; Kamel-Reid, S.; Perez-Ordonez, B.; Mann, V.; Siu, L.L. A Phase II trial of erlotinib as maintenance treatment after gemcitabine plus platinum-based chemotherapy in patients with recurrent and/or metastatic nasopharyngeal carcinoma. Am. J. Clin. Oncol 2011. [Google Scholar] [CrossRef]
- Renjie, C.; Dawei, Z.; Yuan, M.; Jin, Z.; Hao, M.; Juan, W.; Jun, M.; Qing, C.; Hong, L.; Qi, T.; et al. A human Fab-based immunoconjugate specific for the LMP1 extracellular domain inhibits nasopharyngeal carcinoma growth in vitro and in vivo. Mol. Cancer Ther. 2011. [Google Scholar] [CrossRef]
- Volpato, M.; Seargent, J.; Loadman, P.M.; Phillips, R.M. Formation of DNA interstrand cross-links as a marker of Mitomycin C bioreductive activation and chemosensitivity. Eur. J. Cancer 2005, 41, 1331–1338. [Google Scholar]
- Cao, Y.; Chen, D.; Zhao, P.; Liu, L.; Huang, X.; Qi, C.; Liu, Y.; He, H.; Wang, Q.; Liu, Y.; Chen, S. Intracellular delivery of mitomycin C with targeted polysaccharide conjugates against multidrug resistance. Ann. Biomed. Eng 2011, 39, 2456–2465. [Google Scholar]
- Ming, H.; Zhang, D.; Lin, H.; Chen, R.; Feng, Z.; Zhu, J. Inhibiting effect of mitomycin C on human nasopharyngeal carcinoma cell line HNE2, HNE2/lmp1 and its mechanism. J. Clin. Med. Prac 2009, 13, 21–32. [Google Scholar]
- Li, M.; Zhang, J.; Wang, D.; Zhong, B.; Tucker, S.; Lu, C.; Cheng, J.; Cao, C.; Xu, J.; Xu, J.; Pan, H. A phase II study of intra-arterial chemotherapy of 5-fluorouracil, cisplatin, and mitomycin C for advanced nonresectable gastric cancer. Anticancer 2009, 20, 941–945. [Google Scholar]
- Xu, Y.; Kolesar, J.M.; Schaaf, L.J.; Drengler, R.; Duan, W.; Otterson, G.; Shapiro, C.; Kuhn, J.; Villalona-Calero, M.A. Phase I and pharmacokinetic study of mitomycin C and celecoxib as potential modulators of tumor resistance to irinotecan in patients with solid malignancies. Cancer Chemother. Pharmacol 2009, 63, 1073–1082. [Google Scholar]
- Lu, Z.X.; Ma, X.Q.; Yang, L.F.; Wang, Z.L.; Zeng, L.; Li, Z.J.; Li, X.N.; Tang, M.; Yi, W.; Gong, J.P.; et al. DNAzymes targeted to EBV-encoded latent membrane protein-1 induce apoptosis and enhance radiosensitivity in nasopharyngeal carcinoma. Cancer Lett 2008, 265, 226–238. [Google Scholar]
- Louis, C.U.; Straathof, K.; Bollard, C.M.; Gerken, C.; Huls, M.H.; Gresik, M.V.; Wu, M.F.; Weiss, H.L.; Gee, A.P.; Brenner, M.K.; et al. Enhancing the in vivo expansion of adoptively transferred EBV specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood 2009, 113, 2442–2450. [Google Scholar]
- Ho, C.H.; Chen, C.L.; Li, W.Y.; Chen, C.J. Decoy receptor 3, upregulated by Epstein-Barr virus latent membrane protein 1, enhances nasopharyngeal carcinoma cell migration and invasion. Carcinogenesis 2009, 30, 1443–1451. [Google Scholar]
- Pallis, A.G.; Agelaki, S.; Agelidou, A.; Varthalitis, I.; Syrigos, K.; Kentepozidis, N.; Pavlakou, G.; Kotsakis, A.; Kontopodis, E.; Georgoulias, V. A randomized phase III study of the docetaxel/carboplatin combination versus docetaxel single-agent as second line treatment for patients with advanced/metastatic non-small cell lung cancer. BMC Cancer 2010, 10. [Google Scholar] [CrossRef]
- Akao, Y.; Nakagawa, Y.; Iinuma, M.; Nozawa, Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Int. J. Mol. Sci 2008, 9, 355–370. [Google Scholar]
- El-Ghazal, R.; Podoltsev, N.; Marks, P.; Chu, E.; Saif, M.W. Mitomycin-C-induced thrombotic thrombocytopenic purpura/hemolytic uremic syndrome: Cumulative toxicity of an old drug in a new era. Clin. Colorectal. Cancer 2011, 10, 142–145. [Google Scholar]
- Zhou, Q.M.; Zhang, H.; Lu, Y.Y.; Wang, X.F.; Su, S.B. Curcumin reduced the side effects of mitomycin C by inhibiting GRP58-mediated DNA cross-linking in MCF-7 breast cancer xenografts. Cancer Sci 2009, 100, 2040–2045. [Google Scholar]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar]
- Toomey, D.P.; Murphy, J.F.; Conlon, K.C. COX-2, VEGF and tumour angiogenesis. Surgeon 2009, 7, 174–180. [Google Scholar]
- Pircher, A.; Hilbe, W.; Heidegger, I.; Drevs, J.; Tichelli, A.; Medinger, M. Biomarkers in tumor angiogenesis and anti-angiogenic therapy. Int. J. Mol. Sci 2011, 12, 7077–7099. [Google Scholar]
- Tan, Y.N.; Tao, Y.G.; Song, X.; Tang, M.; Ai, M.D.; Cao, Y. Expression of JAK3 in nasopharyngeal carcinoma cell line associated with STAT activation regulated by EB virus encoded protein LMP1. Prog. Biochem. Biophys 2003, 30, 560–565. [Google Scholar]
- Wang, Z.; Luo, F.; Li, L.; Yang, L.; Hu, D.; Ma, X.; Lu, Z.; Sun, L.; Cao, Y. STAT3 activation induced by Epstein-Barr virus latent membrane protein1 causes vascular endothelial growth factor expression and cellular invasiveness via JAK3 And ERK signaling. Eur. J. Cancer 2009, 46, 2996–3006. [Google Scholar]
- Friedrich, M.; Villena-Heinsen, C.; Reitnauer, K.; Schmidt, W.; Tilgen, W.; Reichrath, J. Malignancies of the uterine corpus and immunoreactivity score of the DNA “mismatch-repair” enzyme human Mut-S-homologon-2. J. Histochem. Cytochem 1999, 47, 113–118. [Google Scholar]



| Treatment groups | Tumor volume (mm3) | Tumor weight (g) | Inhibition rate |
|---|---|---|---|
| Dosage (mg/kg) | |||
| I: MMC (2 mg/kg) | 557.88 ± 67.68 (*,**,***) | 0.446 ± 0.054 (*,**) | 20.1% |
| II: Fab (4 mg/kg) | 646.69 ± 59.18 (*,**,***) | 0.517 ± 0.047 (*,**,***) | 7.3% |
| III: MMC (2 mg/kg) + Fab (4 mg/kg) | 398.67 ± 64.87 (*) | 0.321 ± 0.054 (*) | 42.5% |
| IV: MMC (1 mg/kg) + Fab (4 mg/kg) | 419.44 ± 53.93 (*) | 0.332 ± 0.043 (*) | 40.5% |
| V: PBS | 697.56 ± 77.48 | 0.558 ± 0.062 | - |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mao, Y.; Zhang, D.-W.; Wen, J.; Cao, Q.; Chen, R.-J.; Zhu, J.; Feng, Z.-Q. A Novel LMP1 Antibody Synergizes with Mitomycin C to Inhibit Nasopharyngeal Carcinoma Growth in Vivo Through Inducing Apoptosis and Downregulating Vascular Endothelial Growth Factor. Int. J. Mol. Sci. 2012, 13, 2208-2218. https://doi.org/10.3390/ijms13022208
Mao Y, Zhang D-W, Wen J, Cao Q, Chen R-J, Zhu J, Feng Z-Q. A Novel LMP1 Antibody Synergizes with Mitomycin C to Inhibit Nasopharyngeal Carcinoma Growth in Vivo Through Inducing Apoptosis and Downregulating Vascular Endothelial Growth Factor. International Journal of Molecular Sciences. 2012; 13(2):2208-2218. https://doi.org/10.3390/ijms13022208
Chicago/Turabian StyleMao, Yuan, Da-Wei Zhang, Juan Wen, Qing Cao, Ren-Jie Chen, Jin Zhu, and Zhen-Qing Feng. 2012. "A Novel LMP1 Antibody Synergizes with Mitomycin C to Inhibit Nasopharyngeal Carcinoma Growth in Vivo Through Inducing Apoptosis and Downregulating Vascular Endothelial Growth Factor" International Journal of Molecular Sciences 13, no. 2: 2208-2218. https://doi.org/10.3390/ijms13022208




