An Electrochemical Method to Detect Gamma Glutamyl Transpeptidase
Abstract
:1. Introduction
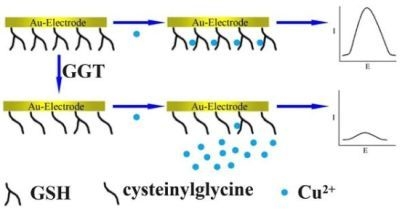
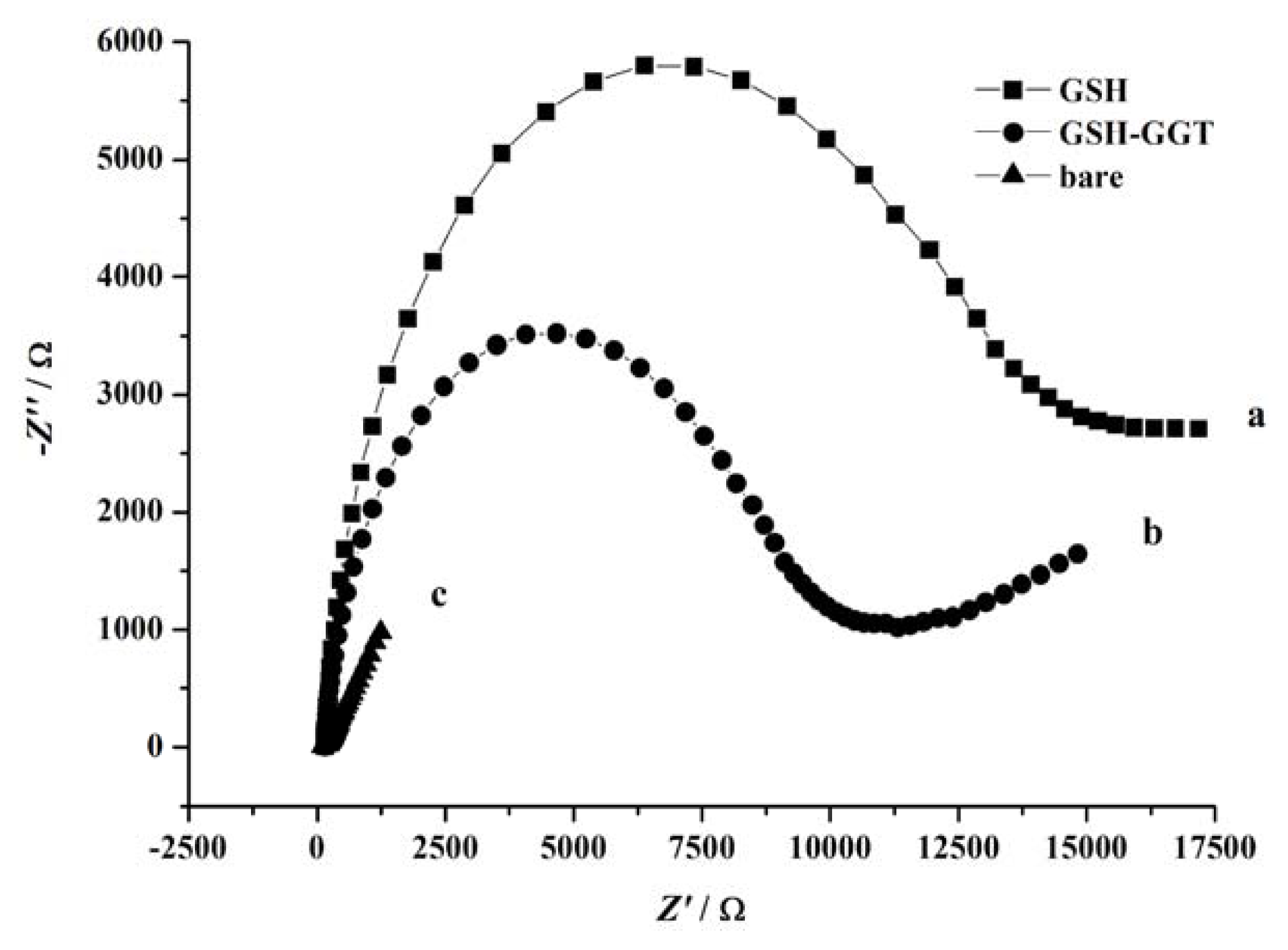
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Preparation of GSH Modified Electrode
3.3. GGT Catalyzed Reaction on the GSH Modified Electrode
3.4. Electrochemical Measurements
4. Conclusions
Acknowledgments
References
- Griffith, O.W.; Bridges, R.J.; Meister, A. Transport of gamma-glutamyl amino acids: Role of glutathione and gamma-glutamyl transpeptidase. Proc. Natl. Acad. Sci. USA 1979, 76, 6319–6322. [Google Scholar]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem 1983, 52, 711–760. [Google Scholar]
- Pero, M.E.; Pelagalli, A.; Lombardi, P.; Avallone, L. Glutathione concentration and gamma-glutamyltransferase activity in water buffalo colostrums. J. Anim. Physiol. Anim. Nutr 2010, 94, 549–551. [Google Scholar]
- Jenderny, S.; Lin, H.; Garrett, T.; Tew, K.D.; Townsend, D.M. Protective effects of a glutathione disulfide mimetic (NOV-002) against cisplatin induced kidney toxicity. Biomed. Pharmacother 2010, 64, 73–76. [Google Scholar]
- Reuter, S.; Schnekenburger, M.; Cristofanon, S.; Buck, I.; Teiten, M.H.; Daubeuf, S.; Eifes, S.; Dicato, M.; Aggarwal, B.B.; Visvikis, A.; et al. Tumor necrosis factor α induces γ-glutamyltransferase expression via nuclear factor-κB in cooperation with Sp1. Biochem. Pharmacol 2009, 77, 397–411. [Google Scholar]
- Pompella, A.; de Tata, V.; Paolicchi, A.; Zunino, F. Expression of γ-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem. Pharmacol 2006, 71, 231–238. [Google Scholar]
- Giommarelli, C.; Corti, A.; Supino, R.; Favini, E.; Paolicchi, A.; Pompella, A.; Zunino, F. Cellular response to oxidative stress and ascorbic acid in melanoma cells overexpressing γ-glutamyltransferase. Eur. J. Cancer 2008, 44, 750–759. [Google Scholar]
- Pompella, A.; Corti, A.; Paolicchi, A.; Giommarelli, C.; Zunino, F. γ-Glutamyltransferase, redox regulation and cancer drug resistance. Curr. Opin. Pharmacol 2007, 7, 360–366. [Google Scholar]
- Whitfield, J.B. Gamma glutamyl transferase. Crit. Rev. Clin. Lab. Sci 2001, 38, 263–355. [Google Scholar]
- Hann, H.W.L.; Lee, J.; Bussard, A.; Liu, C.; Jin, Y.R.; Guha, K.; Clayton, M.M.; Ardlie, K.; Pellini, M.J.; Feitelson, M.A. Preneoplastic markers of hepatitis B virus-associated hepatocellular carcinoma. Cancer Res 2004, 64, 7329–7335. [Google Scholar]
- Gao, Q.; Qiu, S.J.; Fan, J.; Zhou, J.; Wang, X.Y.; Xiao, Y.S.; Xu, Y.; Li, Y.W.; Tang, Z.Y. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol 2007, 25, 2586–2593. [Google Scholar]
- Infante, J.R.; Matsubayashi, H.; Sato, N.; Tonascia, J.; Klein, A.P.; Riall, T.A.; Yeo, C.; Iacobuzio-Donahue, C.; Goggins, M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J. Clin. Oncol 2007, 25, 319–325. [Google Scholar]
- Tarao, K.; Takemiya, S.; Tamai, S.; Sugimasa, Y.; Ohkawa, S.; Akaike, M.; Tanabe, H.; Shimizu, A.; Yoshida, M.; Kakita, A. Relationship between the recurrence of hepatocellular carcinoma (HCC) and serum alanine aminotransferase levels in hepatectomized patients with hepatitis C virus-associated cirrhosis and HCC. Cancer 1997, 79, 688–694. [Google Scholar]
- Nagasue, N.; Kohno, H.; Tachibana, M.; Yamanoi, A.; Ohmori, H.; El-Assal, O.N. Prognostic factors after hepatic resection for hepatocellular carcinoma associated with child-turcotte class B and C cirrhosis. Ann. Surg 1999, 229, 84–90. [Google Scholar]
- Zhou, X.D.; Tang, Z.Y.; Yang, B.H.; Lin, Z.Y.; Ma, Z.C.; Ye, S.L.; Wu, Z.Q.; Fan, J.; Qin, L.X.; Zheng, B.H. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer 2001, 91, 1479–1486. [Google Scholar]
- Silva, I.S.; Ferraz, M.L.; Perez, R.M.; Lanzoni, V.P.; Figueiredo, V.M.; Silva, A.E. Role of γ-glutamyl transferase activity in patients with chronic hepatitis C virus infection. J. Gastroenterol. Hepatol 2004, 19, 314–318. [Google Scholar]
- Yao, D.F.; Jiang, D.R.; Huang, Z.W.; Lu, J.X.; Tao, Q.Y.; Yu, Z.J.; Meng, X.Y. Abnormal expression of hepatoma specific γ-glutamyl transferase and alteration of γ-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer 2000, 88, 761–769. [Google Scholar]
- Stefaniuk, P.; Cianciara, J.; Wiercinska-Drapalo, A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J. Gastroenterol 2010, 16, 418–424. [Google Scholar]
- Ikeda, Y.; Taniguchi, N. Gene expression of γ-glutamyltranspeptidase. Methods Enzymol 2005, 401, 408–425. [Google Scholar]
- Pettersen, I.; Andersen, J.H.; Bjornland, K.; Mathisen, O.; Bremnes, R.; Wellman, M.; Visvikis, A.; Huseby, N.E. Heterogeneity in γ-glutamyltransferase mRNA expression and glycan structures. Search for tumor-specific variants in human liver metastases and colon carcinoma cells. Biochim. Biophys. Acta 2003, 1648, 210–218. [Google Scholar]
- Huseby, N.E.; Stromme, J.H. Practical points regarding routine determination of γ-glutamyl transferase (γ-GT) in serum with a kinetic method at 37 °C. Scand. J. Clin. Lab. Invest 1974, 34, 357–361. [Google Scholar]
- Masia, A.; Destroa, T.; Turettaa, L.; Varottob, S.; Caporalec, G.; Ferrettia, M. Localization of γ-glutamyl transferase activity and protein in Zea mays organs and tissues. J. Plant Physiol 2007, 164, 1527–1535. [Google Scholar]
- Urano, Y.; Sakabe, M.; Kosaka, N.; Ogawa, M.; Mitsunaga, M.; Asanuma, D.; Kamiya, M.; Young, M.R.; Nagano, T.; Choyke, P.L.; et al. Rapid cancer detection by topically spraying a γ-glutamyltranspeptidase-activated fluorescent probe. Sci. Transl. Med 2011, 3. [Google Scholar] [CrossRef]
- Kiuchi, K.; Kiuchi, K.; Nagatsu, T.; Togari, A.; Kumagai, H. Highly sensitive assay for γ-glutamyltranspeptidase activity by high-performance liquid chromatography with electrochemical detection. J. Chromatogr 1986, 357, 191–198. [Google Scholar]
- Yao, D.F.; Huang, Z.W.; Chen, S.Z.; Huang, J.F.; Lu, J.X.; Xiao, M.B.; Meng, X.Y. Diagnosis of hepatocellular carcinoma by quantitative detection of hepatoma-specific bands of serum γ-glutamyltransferase. Am. J. Clin. Pathol 1998, 110, 743–749. [Google Scholar]
- Fang, C.; Zhou, X.Y. Voltammetry and EQCM investigation of glutathione monolayer and its complexation with Cu2+. Electroanalysis 2003, 15, 20–26. [Google Scholar]
- Yang, W.R.; Gooding, J.J.; Hibbert, D.B. Redox voltammetry of sub-parts per billion levels of Cu2+ at polyaspartate-modified gold electrodes. Analyst 2001, 126, 1573–1577. [Google Scholar]
- Liu, A.C.; Chen, D.C.; Lin, C.C.; Chou, H.H.; Chen, C.H. Application of cysteine monolayers for electrochemical determination of sub-ppb copper(II). Anal. Chem 1999, 71, 1549–1552. [Google Scholar]



© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, G.; Ni, S.; Zhu, S.; Yang, J.; Yin, Y. An Electrochemical Method to Detect Gamma Glutamyl Transpeptidase. Int. J. Mol. Sci. 2012, 13, 2801-2809. https://doi.org/10.3390/ijms13032801
Chen G, Ni S, Zhu S, Yang J, Yin Y. An Electrochemical Method to Detect Gamma Glutamyl Transpeptidase. International Journal of Molecular Sciences. 2012; 13(3):2801-2809. https://doi.org/10.3390/ijms13032801
Chicago/Turabian StyleChen, Guifang, Shengfa Ni, Sha Zhu, Jinghua Yang, and Yongmei Yin. 2012. "An Electrochemical Method to Detect Gamma Glutamyl Transpeptidase" International Journal of Molecular Sciences 13, no. 3: 2801-2809. https://doi.org/10.3390/ijms13032801