Pharmacokinetic Comparison of Ferulic Acid in Normal and Blood Deficiency Rats after Oral Administration of Angelica sinensis, Ligusticum chuanxiong and Their Combination
Abstract
:1. Introduction
2. Results
2.1. General and Hemogram Observation of Rats
2.2. HPLC Chromatograms
2.3. Validation of Chromatographic Methods
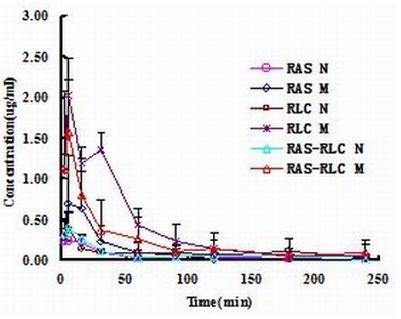
2.4. Determination of FA in Plasma and Pharmacokinetic Analysis
3. Discussion
4. Experimental Section
4.1. Materials and Extraction
4.2. Animals
4.3. Chemicals and Reagents
4.4. Drug Administration and Collection of Rat Plasma Samples
4.5. Plasma Sample Preparation
4.6. Chromatographic Conditions
4.6.1. Linearity
4.6.2. Recovery and Accuracy
4.7. Pharmacokinetic Analysis
5. Conclusion
Acknowledgments
References
- Yi, L.; Liang, Y.; Wu, H.; Yuan, D. The analysis of Radix Angelicae Sinensis (Danggui). J. Chromatogr. A 2009, 1216, 1991–2001. [Google Scholar]
- Nanjing University of Chinese Medicine, Dictionary of Chinese Herbal Medicines (in Chinese), 2nd ed; Shanghai Science and Technology Press: Shanghai, China, 2006.
- Huang, S.H.; Lin, C.M.; Chiang, B.H. Protective effects of Angelica sinensis extract on amyloid β-peptide-induced neurotoxicity. Phytomedicine 2008, 15, 710–721. [Google Scholar]
- Lao, S.C.; Li, S.P.; Kan, K.K.W.; Li, P.; Wan, J.B.; Wang, Y.T.; Dong, T.T.X.; Tsim, K.W.K. Identification and quantification of 13 components in Angelica sinensis (Danggui) by gas chromatography-mass spectrometry coupled with pressurized liquid extraction. Anal. Chim. Acta 2004, 526, 131–137. [Google Scholar]
- Wang, Y.L.; Liang, Y.Z.; Chen, B.M. High-performance liquid chromatography with atmospheric pressure chemical ionization and electrospray ionization mass spectrometry for analysis of Angelica sinensis. Phytochem. Anal 2007, 18, 265–274. [Google Scholar]
- Lee, T.F.; Lin, Y.L.; Huang, Y.T. Studies on antiproliferative effects of phthalides from Ligusticum chuanxiong in hepatic stellate cells. Planta Med 2007, 73, 527–534. [Google Scholar]
- Wang, H.; Tang, Y.P.; Ding, A.W.; Ye, L.; Duan, J.A. Data analysis of Radix Angelicae Sinensis and Rhizoma Ligusticum of different proportions in the TCM clinic application. Chin. J. Exp. Tradit. Med. Formulae 2009, 15, 44–47. [Google Scholar]
- Bi, C.W.; Xie, H.Q.; Xu, L.; Li, J.; Cheung, A.W.; Zhu, J.T.; Zheng, Y.Z.; Chen, V.P.; Lau, D.T.; Choi, R.C.; et al. Fo Shou San, an ancient herbal decoction prepared from Rhizoma Chuanxiong and Radix Angelicae Sinensis, stimulates the production of hemoglobin and erythropoietin in cultured cells. Planta Med 2010, 76, 1525–1529. [Google Scholar]
- Li, W.X.; Hua, Y.Q.; Tang, Y.P.; Wang, H.; Qian, L.; Gao, M.; Duan, J.A. Effects of Radix Angelicae Sinensis and Rhizoma Chuanxiong on mouse uterine contractions in vitro. J. Nanjing Univ. Tradit. Chin. Med 2010, 26, 120–122. [Google Scholar]
- Tang, Y.P.; Zhu, M.; Yu, S.; Hua, Y.Q.; Duan, J.A.; Su, S.L.; Zhang, X.; Lu, Y.; Ding, A.W. Identification and comparative quantification of bioactive phthalides in essential oils from Si-Wu-Tang, Fo-Shou-San, Radix Angelica and Rhizoma Chuanxiong. Molecules 2010, 15, 341–351. [Google Scholar]
- Zhu, M.; Tang, Y.P.; Duan, J.A.; Guo, J.M.; Guo, S.; Su, S.L.; Shang, E.X.; Qian, D.W.; Ding, A.W. Roles of paeoniflorin and senkyunolide I in Si-Wu decoction on antiplatelet and anticoagulation activities. J. Sep. Sci 2010, 33, 3335–3340. [Google Scholar]
- Hua, Y.Q.; Su, S.L.; Duan, J.A.; Wang, Q.J.; Lu, Y.; Chen, L. Danggui-Shaoyao-San, a traditional Chinese prescription, suppresses PGF2α production in endometrial epithelial cells by inhibiting COX-2 expression and activity. Phytomedicine 2008, 15, 1046–1052. [Google Scholar]
- Su, S.L.; Guo, J.M.; Duan, J.A.; Wang, T.J.; Shang, E.X.; Qian, D.W.; Tang, Y.P.; Ding, A.W. Ultra-performance liquid chromatography-tandem mass spectrometry analysis of the bioactive components and their metabolites of ShaofuZhuyu decoction active extract in rat plasma. J. Chromatogr. B 2010, 878, 355–362. [Google Scholar]
- Li, X.C.; Wu, X.T.; Huang, L. Correlation between antioxidant activities and phenolic contents of Radix Angelicae Sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Budak, U. Phenolic compounds, antioxidant and antimicrobial properties of Helichrysum species collected from Eastern Anatolia, Turkey. Turk. J. Biol 2010, 34, 463–473. [Google Scholar]
- Hyogo, A.; Kobayashi, T.; Saz, E.G.; Seguchi, H. Antioxidant effects of protocatechuic acid, ferulic acid, and caffeic acid in human neutrophils using a fluorescent substance. Int. J. Morphol 2010, 28, 911–920. [Google Scholar]
- Kobayashi, S.; Mimura, Y.; Naitoh, T.; Kimura, I.; Kimura, M. Chemical structure-activity of Cnidium rhizome-derived phthalides for the competence inhibition of proliferation in primary cultures of mouse aorta smooth muscle cells. Jpn. J. Pharmacol 1993, 63, 353–359. [Google Scholar]
- Hou, Y.Z.; Zhao, G.R.; Yang, J.; Yuan, Y.J.; Zhu, G.G.; Hiltunen, R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci 2004, 75, 1775–1786. [Google Scholar]
- Xie, J.J.; Lu, J.; Qian, Z.M.; Yu, Y.; Duan, J.A.; Li, S.P. Optimization and comparison of five methods for extraction of coniferyl ferulate from Angelica sinensis. Molecules 2009, 14, 555–565. [Google Scholar]
- Hou, Y.Z.; Yang, J.; Zhao, G.R.; Yuan, Y.J. Ferulic acid inhibits vascular smooth muscle cell proliferation induced by angiotensin II. Eur. J. Pharmacol 2004, 499, 85–90. [Google Scholar]
- Mathew, S.; Abraham, T.E. Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol 2004, 24, 59–83. [Google Scholar]
- Lin, Z.; Zhu, D.; Yan, Y.; Yu, B. Neuroprotection by herbal formula FBD and its active compounds. Pharm. Boil 2009, 47, 608–614. [Google Scholar]
- Barone, E.; Calabrese, V.; Mancuso, C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 2009, 10, 97–108. [Google Scholar]
- Madhujith, T.; Shahidi, F. Antioxidant and antiproliferative potential of pearled barley (Hordeum vulgarae). Pharm. Boil 2008, 46, 88–95. [Google Scholar]
- Chawla, A.S.; Singh, M.; Murthy, M.S.; Gupta, M.; Singh, H. Anti-inflammatory action of ferulic acid and its esters in carrageen induced rat paw edema model. Indian J. Exp. Biol 1987, 25, 187–189. [Google Scholar]
- Chotimarkorn, C.; Ushio, H. The effect of trans-ferulic acid and gamma-oryzanol on ethanol-induced liver injury in C57BL mouse. Phytomedicine 2008, 15, 951–958. [Google Scholar]
- Nakashima, H.; Murakami, T.; Yamamoto, N.; Naoe, T.; Kawazoe, Y.; Konno, K.; Sakagami, H. Lignified materials as medicinal resources V. anti-HIV (human immunodeficiency virus) activity of some synthetic lignins. Chem. Pharm. Bull 1992, 40, 2102–2105. [Google Scholar]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem 2008, 109, 691–702. [Google Scholar]
- Guo, X.R.; Chen, X.H.; Cheng, W.M.; Yang, K.Y.; Ma, Y.F.; Bi, K.S. RP-LC determination and pharmacokinetic study of ferulic acid and isoferulic acid in rat plasma after taking traditional Chinese medicinal-preparation: Guanxinning Lyophilizer. Chromatographia 2008, 67, 1007–1011. [Google Scholar]
- Wen, X.D.; Qi, L.W.; Li, P.; Bao, K.D.; Yan, X.W.; Yi, L.; Li, C.Y. Simultaneous determination of calycosin-7-O-β-d-glucoside, ononin, astragaloside IV, astragaloside I and ferulic acid in rat plasma after oral administration of Danggui Buxue Tang extract for their pharmacokinetic studies by liquid chromatography-mass spectrometry. J. Chromatogr. B 2008, 865, 99–105. [Google Scholar]
- Li, W.X.; Tang, Y.P.; Guo, J.M.; Huang, M.Y.; Li, W.; Qian, D.W.; Duan, J.A. Enriching blood effect comparison in three kinds of blood deficiency model after oral administration of the drug pair of Angelicae Sinensis Radix and Chuanxiong Rhizoma and each single herb. Zhongguo Zhong Yao Za Zhi 2011, 36, 26–33. [Google Scholar]
- Ben, C.E. The creation and experimental study on the animal model of blood deficiency. Beijing Shiyan Dongwu Kexue Yu Guanli 1994, 11, 5–10. [Google Scholar]
- Li, Y.K. Methodology of Pharmacological Experiments for the Traditional Chinese Medicine (in Chinese); Shanghai Science and Technology Press: Shanghai, China, 1991; p. 283. [Google Scholar]
- Chen, Q. Methodology of Pharmacological Studies for the Traditional Chinese Medicine (in Chinese); The People’s Medical Publishing House: Beijing, China, 1993; p. 1010. [Google Scholar]
- Ben, C.E.; Li, S.G.; Yang, M.J. Further investigation on the anminal model of “Blood” deficiency—observation of the influence of hemolytic anemia on cytochemistry of blood cells and histochemistry of the liver of rat. J. Tradit. Chin. Med 1981, 1, 99–104. [Google Scholar]
- Peng, X.; Qin, L.; Deng, H.L.; Xu, Y.F.; Miao, Y.L. The blood flow and coagulation funtion effect of siwutang on the rat model of blood deficiency. Shandong Zhongyiyao Daxue Xuebao 2008, 32, 163–165. [Google Scholar]
- Reza, M. Effect of experimental diabetes mellitus on the pharmacokinetics of atenolol enantiomers in rats. J. Pharmacol. Sci 1991, 80, 207–211. [Google Scholar]
- He, X.; Xing, D.; Ding, Y.; Li, Y.; Xu, L.; Du, L. Effects of cerebral ischemia-reperfusion on pharmacokinetic fate of paeoniflorin after intravenous administration of Paeoniae Radix extract in rats. J. Ethnopharmacol 2004, 94, 339–344. [Google Scholar]
- Kim, Y.C.; Oh, E.Y.; Kim, S.H.; Lee, M.G. Pharmacokinetics of diclofenac in rat model of diabetes mellitus induced by alloxan or steptozotocin. Biopharm. Drug Dispos 2006, 27, 85–92. [Google Scholar]
- Huang, X.; Ren, P.; Wen, A.D.; Wang, L.L.; Zhang, L.; Gao, F. Pharmacokinetics of traditional Chinese syndrome and recipe: a hypothesis and its verification (I). World J. Gastroenterol 2000, 6, 384–391. [Google Scholar]
- Tian, Y.; Yang, Z.F.; Li, Y.; Qiao, Y.; Yang, J.; Jia, Y.Y.; Wen, A.D. Pharmacokinetic comparisons of hydroxysafflower yellow A in normal and blood stasis syndrome rats. J. Ethnopharmacol 2010, 129, 1–4. [Google Scholar]
- Qi, J.; Jin, X.; Huang, L.; Ping, Q. Simultaneous determination of hydroxysafflor yellow A and ferulic acid in rat plasma after oral adiministration of the co-extractum of Rhizoma Chuanxiong and Flos Carthami by HPLC-diode array detector. Biomed. Chromatogr 2007, 21, 816–822. [Google Scholar]
- Liu, X.F.; Wu, X.A.; Wei, Y.H.; Luo, J.Y. Study on absorption pharmacokinetic of ferulic acid and freulic acid of Chuanxiong decoction in rat. Zhongyaocai 2007, 30, 831–833. [Google Scholar]
- Zhao, Z.H.; Egashira, Y.; Sanada, H. Ferulic acid sugar esters are recovered in rat plasma and urine mainly as the sulfoglucuronide of ferulic acid. J. Nutr 2004, 134, 3083–3088. [Google Scholar]
- Peng, X.; Xia, L.; Li, J.G.; Qin, L.; Li, H.; Song, Y.Q. The investigation on variation of the haemorheology and coagulation function in blood defieieney rats. Zhongguo Xueye Liubianxue Zazhi 2006, 16, 174–177. [Google Scholar]
- Xu, S.Y.; Bian, R.L.; Chen, X. Methodology of Pharmacological Experiments (in Chinese); The People’s Medical Publishing House: Beijing, China, 2001; p. 1861. [Google Scholar]




| Group | WBC/109·L−1 | RBC/1012·L−1 | HGB/g·L−1 | HCT/% |
|---|---|---|---|---|
| Normal | 14.78 ± 3.31 | 7.01 ± 1.59 | 140.87 ± 10.22 | 0.42 ± 0.04 |
| Model | 133.25 ± 10.04 ** | 3.72 ± 0.88 ** | 92.33 ± 5.28 ** | 0.33 ± 0.05 ** |
| Parameters | Normal | Blood deficiency | ||||
|---|---|---|---|---|---|---|
| RAS | RLC | RAS-RLC | RAS | RLC | RAS-RLC | |
| t1/2α (min) | 3.902 ± 0.783 | 28.728 ± 5.813 Δ | 28.797 ± 5.208 | 5.342 ± 0.802 | 31.231 ± 3.493 | 28.984 ± 3.066 # |
| t1/2β (min) | 45.785 ± 6.224 | 53.125 ± 7.663 | 37.126 ± 2.545 | 21.787 ± 2.693 | 23.121 ± 4.005 | 30.117 ± 2.056 |
| Vd (L/mg) | 7.965 ± 1.002 | 50.882 ± 8.135 ΔΔ | 23.005 ± 3.031 | 4.318 ± 0.228 | 9.633 ± 2.209 | 14.972 ± 0.206 # |
| CL (L/min/kg) | 0.886 ± 0.237 | 0.905 ± 0.218 | 0.760 ± 0.186 | 0.732 ± 0.056 | 0.327 ± 0.093 | 0.114 ± 0.002 * |
| AUC(0–t) (mg/L·min) | 21.649 ± 5.292 | 16.925 ± 3.368 | 20.194 ± 5.000 | 38.504 ± 4.912 | 88.685 ± 5.589 | 118.336 ± 5.876 * ## |
| AUC(0–∞) (mg/L·min) | 25.689 ± 2.664 | 29.013 ± 2.276 | 27.412 ± 3.638 | 51.487 ± 4.240 | 94.457 ± 5.898 | 175.970 ± 3.437 ## |
| K10 (1/min) | 0.759 ± 0.018 | 0.018 ± 0.004 Δ | 0.035 ± 0.015 | 0.309 ± 0.043 | 0.077 ± 0.000 | 0.008 ± 0.000 |
| K12 (1/min) | 0.615 ± 0.187 | 0.002 ± 0.000 ΔΔ | 0.043 ± 0.003 | 0.318 ± 0.059 | 0.030 ± 0.000 | 0.013 ± 0.001 |
| K21 (1/min) | 0.040 ± 0.015 | 0.022 ± 0.001 | 0.013 ± 0.003 | 0.070 ± 0.024 | 0.012 ± 0.000 | 0.014 ± 0.001 |
| Tmax (min) | 9.898 ± 1.025 | 5.000 ± 0.000 | 8.775 ± 1.202 | 9.996 ± 0.889 | 16.667 ± 2.583 | 15.000 ± 0.010 |
| Cmax (mg/L) | 0.444 ± 0.112 | 0.321 ± 0.026 | 0.582 ± 0.124 | 0.918 ± 0.320 | 1.962 ± 0.042 | 1.088 ± 0.202 * |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, W.; Guo, J.; Tang, Y.; Wang, H.; Huang, M.; Qian, D.; Duan, J.-A. Pharmacokinetic Comparison of Ferulic Acid in Normal and Blood Deficiency Rats after Oral Administration of Angelica sinensis, Ligusticum chuanxiong and Their Combination. Int. J. Mol. Sci. 2012, 13, 3583-3597. https://doi.org/10.3390/ijms13033583
Li W, Guo J, Tang Y, Wang H, Huang M, Qian D, Duan J-A. Pharmacokinetic Comparison of Ferulic Acid in Normal and Blood Deficiency Rats after Oral Administration of Angelica sinensis, Ligusticum chuanxiong and Their Combination. International Journal of Molecular Sciences. 2012; 13(3):3583-3597. https://doi.org/10.3390/ijms13033583
Chicago/Turabian StyleLi, Weixia, Jianming Guo, Yuping Tang, Huan Wang, Meiyan Huang, Dawei Qian, and Jin-Ao Duan. 2012. "Pharmacokinetic Comparison of Ferulic Acid in Normal and Blood Deficiency Rats after Oral Administration of Angelica sinensis, Ligusticum chuanxiong and Their Combination" International Journal of Molecular Sciences 13, no. 3: 3583-3597. https://doi.org/10.3390/ijms13033583