Photosensized Controlling Benzyl Methacrylate-Based Matrix Enhanced Eu3+ Narrow-Band Emission for Fluorescence Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Studies of Ligand Properties
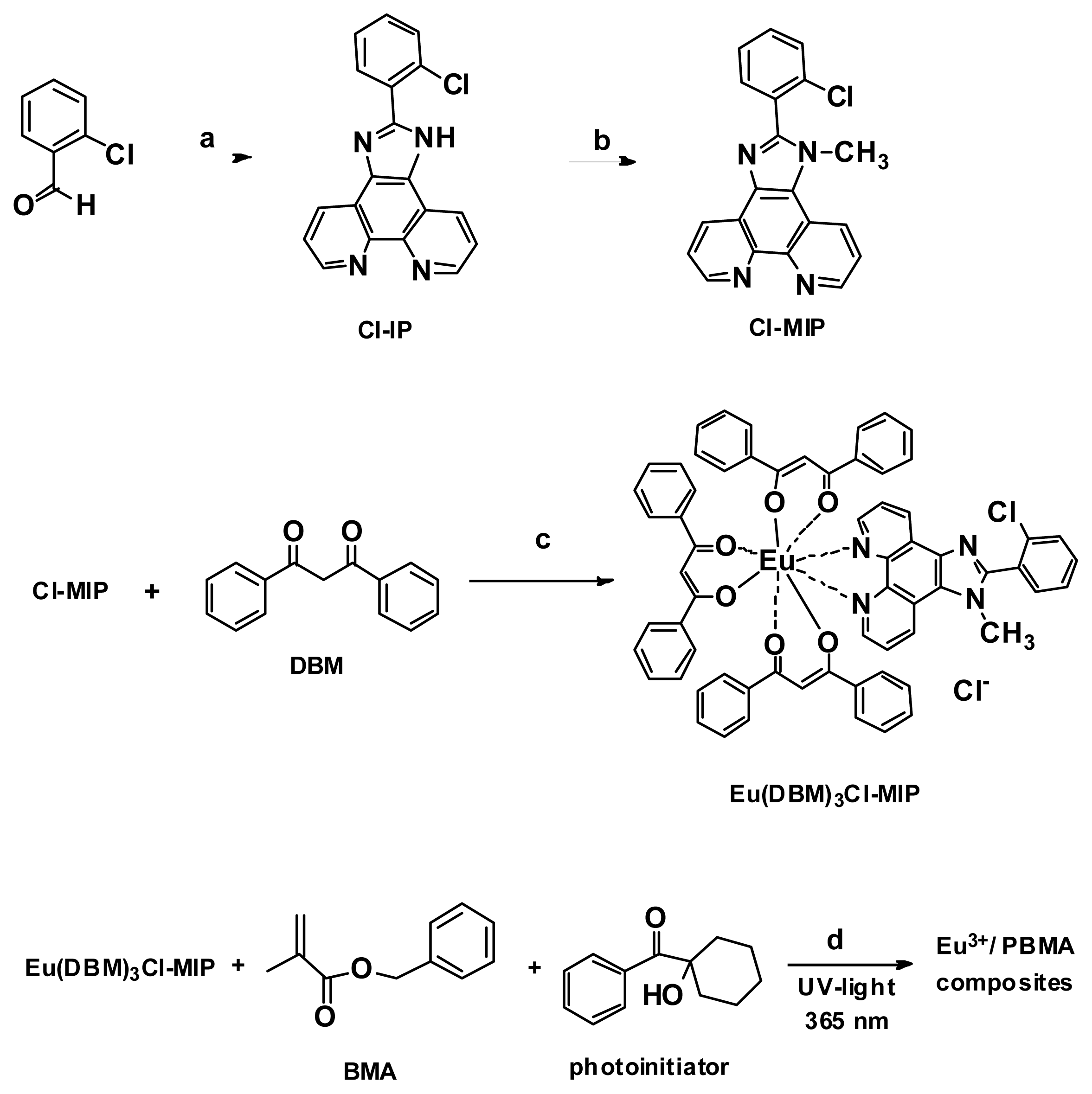
2.2. Single Crystals of Cl-MIP and Eu(DBM)3Cl-MIP
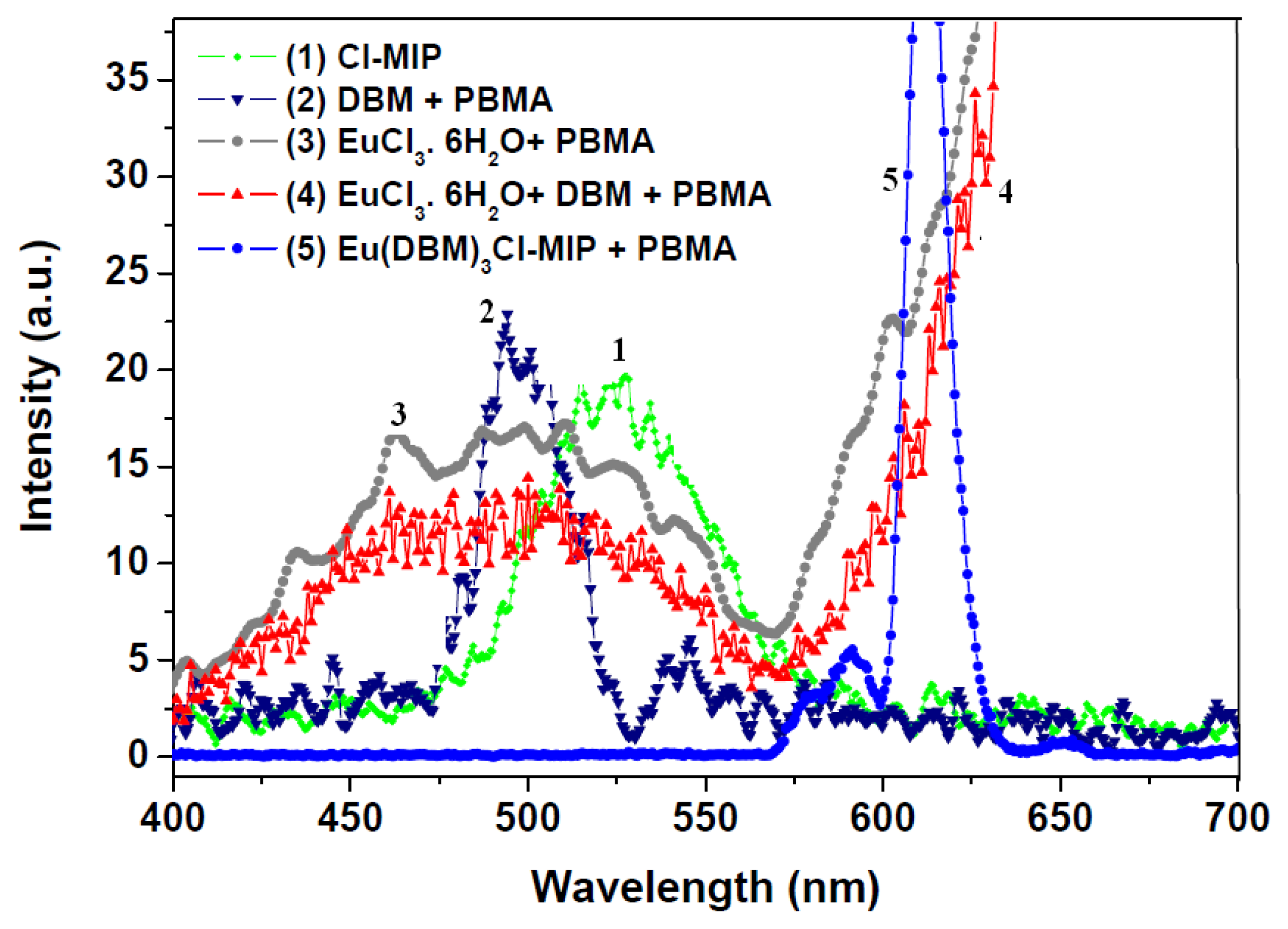
2.3. Photophysical Properties of Europium/PBMA Composites
2.4. Aggregated Nature of Europium/PBMA Composites
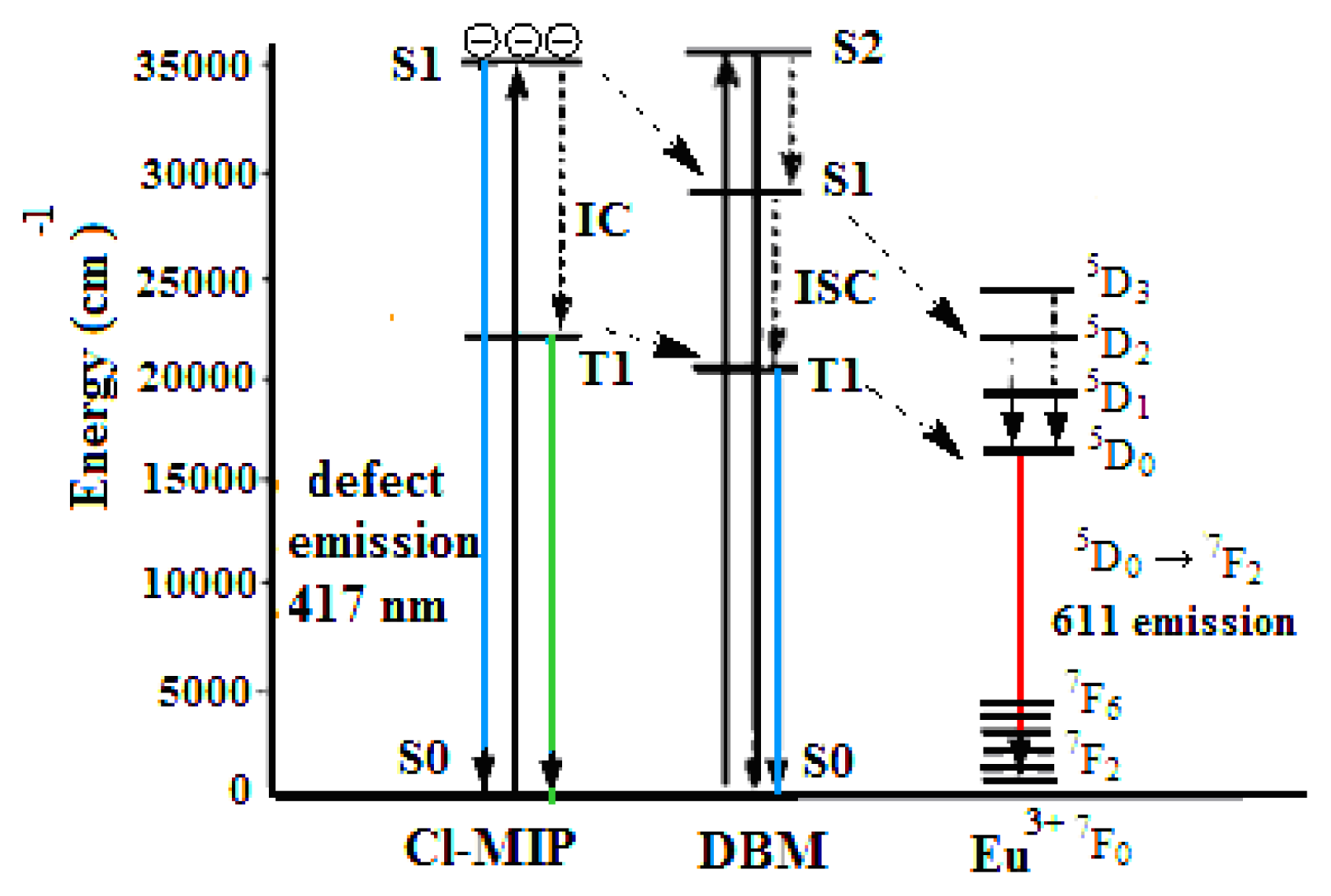
2.5. Emissive Transitional States of Europium/PBMA Composite
2.6. Emission Lifetime Analyses of Eu3+/PBMA Composites
2.7. Controlling Optimized Fluorescence Response of Europium/PBMA Composite
2.8. Energy Transfer to Metal Ions within Europium/PBMA Composites
3. Experimental Section
3.1. Materials
3.2. Methods
3.3. Synthesis of 2-(2-Chlorophenyl)-1-methyl-1H-imidazo[4,5-f][1,10]phenanthroline (Cl-MIP)
3.4. Synthesis of Eu(DBM)3Cl-MIP Complex
3.5. Eu(DBM)3Cl-MIP/PBMA Composite Sample Results
3.6. Polymer Sample Results
4. Conclusions
Supplementary Information
ijms-13-03718-s001.pdfAcknowledgements
References
- Lenaerts, P.; Driesen, K.; Van Deun, R.; Binnemans, K. Covalent coupling of luminescent tris(2-thenoyl-trifluoroacetonato)lanthanide(III) complexes on a merrifield resin. Chem. Mater 2005, 17, 2148–2154. [Google Scholar]
- Bergstedt, M.D.T.; Zhang, C.; Saab, A.P.; O’Regan, M.B; Bazan, G.C.; Srdanov, V.I.; Heeger, A.J. Narrow bandwidth luminescence from blends with energy transfer from semiconducting conjugated polymers to europium complexes. Adv. Mater. 1999, 11, 1349–1354. [Google Scholar]
- Hong, Z.R.; Liang, C.J.; Li, R.G.; Li, W.L.; Zhao, D.; Fan, D.; Wang, D.Y.; Chu, B.; Zang, F.X.; Hong, L.S.; Lee, S.T. Rare earth complex as a high-efficiency emitter in an electroluminescent device. Adv. Mater 2001, 13, 1241–1245. [Google Scholar]
- Shunmugam, R.; Tew, G.N. Polymers that contain ligand metals in their side chain: building foundation for functional materials in optoelectronic applications with an emphasis on lanthanide ions. Macromol. Rapid Commun 2008, 29, 1355–1362. [Google Scholar]
- Xin, H.; Li, F.Y.; Guan, M.; Huang, C.H.; Sun, M.; Wang, K.Z.; Zhang, Y.A.; Jin, L.P. Carbazole-functionalized europium complex and its high-efficiency organic electroluminescent properties. J. Appl. Phys 2003, 94, 4729–4731. [Google Scholar]
- Moynihan, S.; Iacopino, D.; O’Carroll, D.; Doyle, H.; Tanner, D.A.; Redmond, G. Emission colour tuning in semiconducting polymer nanotubes by energy transfer to organo-lanthanide dopants. Adv. Mater 2007, 19, 2474–2479. [Google Scholar]
- Carlos, L.D.; Sá Ferreira, R.A.; Rainho, J.P.; de Zea Bermudez, V. Fine-tuning of the chromaticity of the emission color of organic–inorganic hybrids co-doped with EuIII, TbIII, and TmIII. Adv. Funct. Mater 2002, 12, 819–823. [Google Scholar]
- Kido, J.; Okamoto, Y. Organo Lanthanide metal complexes for electroluminescent materials. Chem. Rev 2002, 102, 2357–2368. [Google Scholar]
- Banks, E.; Ueba, Y.; Okamoto, Y. Lanthanide fluorescence as a probe of ionomer structure. Ann. NY. Acad. Sci 1981, 366, 356–372. [Google Scholar]
- Barja, B.C.; Remorino, A.; Aramendía, P.F. Luminescence quenching of Eu(III) carboxylates by Cu(II) in a composite polymer xerogel film. Photochem. Photobiol 2006, 82, 43–49. [Google Scholar]
- Wu, M.; Lakowicz, J.R.; Geddes, C.D. Enhanced lanthanide luminescence using silver nanostructures: Opportunities for a new class of probes with exceptional spectral characteristics. J. Fluoresc 2005, 15, 53–59. [Google Scholar]
- Bowen, L.M.; Muller, G.; Riehl, J.P.; Dupureur, C.M. lanthanide spectroscopic studies of the dinuclear and Mg(II)-dependent PvuII restriction endonuclease. Biochemistry 2004, 43, 15286–15295. [Google Scholar]
- Strohriegl, P.; Grazulevicius, J.V. Charge-transporting molecular glasses. Adv. Mater 2002, 14, 1439–1452. [Google Scholar]
- Chen, A.C.A.; Culligan, S.W.; Geng, Y.; Chen, S.H.; Klubek, K.P.; Vaeth, K.M.; Tang, C.W. Organic polarized light-emitting diodes via Förster energy transfer using monodisperse conjugated oligomers. Adv. Mater 2004, 16, 783–738. [Google Scholar]
- Kline, R.J.; McGehee, M.D. Morphology and charge transport in conjugated polymers. J. Macromol. Sci. Part C 2006, 46, 27–45. [Google Scholar]
- Rivaton, A.; Mailhot, B.; Derderian, G.; Bussiere, P.O.; Gardette, J.L. Investigation of the photophysical processes and photochemical reactions involved in PVK Films irradiated at λ > 300 nm. Macromolecules 2003, 36, 5815–5824. [Google Scholar]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar]
- Liang, H.; Wu, W.; Wang, Y.; Li, Z. Fluorescence study of Eu chelates in solution and polymer matrix. Spectrochim. Acta Part A 2005, 61, 2687–2690. [Google Scholar]
- Guan, M.; Bian, M.; Li, Z.Q.; Xin, F.Y.; Huang, C.H. Bright red light-emitting electroluminescence devices based on a functionalized europium complex. New J. Chem 2003, 27, 1731–1734. [Google Scholar]
- Lam, M.K.; Kwok, K.L.; Tse, S.C.; So, S.K.; Yuan, J.B.; Leung, L.M.; Gong, M.L. Heterojunction OLEDs fabricated by Eu ternary complexes with conducting secondary ligands. Opt. Mater 2006, 28, 709–713. [Google Scholar]
- Li, H.; Inoue, S.; Machida, K.; Adachi, G. Preparation and luminescence properties of organically modified silicate composite phosphors doped with a europium (III) β-diketonate complex. Chem. Mater 1999, 11, 3171–3176. [Google Scholar]
- Wada, A.; Watanabe, M.; Yamanoi, Y.; Nishihara, H. Modification of the luminescence spectra of chloro(tetrapyridylcyclotetramine) europium complexes by fine tuning of the Eu-Cl distance with outer-sphere counterions in the solid state, in a polymer matrix and in solution. Chem. Commun 2008, 1671–1673. [Google Scholar]
- Wen, X.; Li, M.; Wang, Y.; Zhang, J.; Fu, L.; Hao, R.; Ma, Y.; Ai, X. Colloidal nanoparticles of a europium complex with enhanced luminescent properties. Langmuir 2008, 24, 6932–6936. [Google Scholar]
- Féau, C.; Klein, E.; Kerth, P.; Lebeau, L. Synthesis of a coumarin-based europium complex for bioanalyte labeling. Bioorg. Med. Chem. Lett 2007, 17, 1499–1503. [Google Scholar]
- Alonso, I.; Carretero, J.C.; Ruano, J.L.G. Benzyl methyl (S)-2-(p-tolylsulfinyl)maleate: An efficient dienophile for the enantioselective synthesis of cyclohexadienes. J. Org. Chem 1993, 58, 3231–3232. [Google Scholar]
- Lee, J.F.; Chen, Y.C.; Lin, J.T.; Wu, C.C.; Chen, C.Y.; Dai, C.A.; Chao, C.Y.; Chen, H.L.; Liau, W.B. Blue light-emitting and electron-transporting materials based on dialkyl-functionlized anthracene imidazophenanthrolines. Tetrahedron 2011, 67, 1696–1702. [Google Scholar]
- Su, W.F.; Lee, J.F. Bismuth titanate nanoparticle dispersed polyacrylate smart material. Polym. Prepr 2002, 43, 1219–1220. [Google Scholar]
- Su, W.F.; Lee, J.F.; Chen, M.Y.; Ho, R.M. Bismuth titanate nanoparticles dispersed polyacrylates. J. Mater. Res 2004, 19, 2343–2348. [Google Scholar]
- Patel, D.G.; Graham, K.R.; Reynolds, J.R. A Diels–Alder crosslinkable host polymer for improved PLED performance: the impact on solution processed doped device and multilayer device performance. J. Mater. Chem 2012, 22, 3004–3014. [Google Scholar]
- Feng, L.; Hu, J.; Liu, Z.; Zhao, F.; Liu, G. Preparation and properties of optically active poly(N-methacryloyl l-leucine methyl ester). Polymer 2007, 48, 3616–3623. [Google Scholar]
- Mondal, J.A.; Ramakrishna, G.; Singh, A.K.; Ghosh, H.N.; Mariappan, M.; Maiya, B.G.; Mukherjee, T.; Palit, D.K. Ultrafast intramolecular electronic energy-transfer dynamics in a bichromophoric molecule. J. Phys. Chem. A 2004, 108, 7843–7852. [Google Scholar]
- Maciel, G.S.; Kim, K.S.; Chung, S.J.; Swiatkiewicz, J.; He, G.S.; Prasad, P.N. Linear and nonlinear optical properties of an erbium two-photon dye salt. J. Phys. Chem. B 2001, 105, 3155–3157. [Google Scholar]
- Zhang, L.; Li, B.; Zhang, L.; Chen, P.; Liu, S. Synthesis, characterization, and luminescent properties of europium complexes with fluorine functionalized phenanthroline. J. Electrochem. Soc 2009, 156, H202–H207. [Google Scholar]
- Yang, C.; Fu, L.M.; Wang, Y.; Zhang, J.P.; Wong, W.T.; Ai, X.C.; Qiao, Y.F.; Zou, B.S.; Gui, L.L. A Highly luminescent europium complex showing visible-light-sensitized red emission: Direct observation of the singlet pathway. Angew. Chem. Int. Ed 2004, 43, 5010–5013. [Google Scholar]
- Bondarev, S.L.; Knyukshto, V.N.; Stepuro, V.I.; Stupak, A.P.; Turbana, A.A. Fluorescence and electronic structure of the laser dye DCM in solutions and in polymethylmethacrylate. J. Appl. Spectrosc 2004, 71, 194–201. [Google Scholar]
- Espinosa, J.F.; Fernández, M.J.; Grant, K.B.; Gude, L.; Rodrigo, M.M.; Lorente, A. Synthesis, DNA intercalation and europium(III)-triggered DNA photocleavage by a bis-proflavine succinamide conjugate. Tetrahedron Lett 2004, 45, 4017–4020. [Google Scholar]
- Montgomery, C.P.; Murray, B.S.; New, E.J.; Pal, R.; Parker, D. Cell-penetrating metal complex optical probes: Targeted and responsive systems based on lanthanide luminescence. Acc. Chem. Res 2009, 42, 925–937. [Google Scholar]
- Decker, C. Kinetic Study and new applications of UV radiation curing. Macromol. Rapid Commun 2002, 23, 1067–1093. [Google Scholar]
- Yamada, M.; Nakamura, Y.; Hasegawa, T.; Itoh, A.; Kuroda, S.; Shimao, I. Oxidative chlorination of 1,10-Phenanthroline and its derivatives by phosphorus pentachloride in phosphoryl chloride. Bull. Chem. Soc. Jpn 1992, 65, 2007–2009. [Google Scholar]
- Kessler, M.A. Determination of copper at ng mL−1-levels based on quenching of the europium chelate luminescence. Anal. Chim. Acta 1998, 364, 125–129. [Google Scholar]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev 2009, 109, 4283–4374. [Google Scholar]
- Steck, E.A.; Day, A.R. Reactions of phenanthraquinone and retenequinone with aldehydes and ammonium acetate in acetic acid solution. J. Am. Chem. Soc 1943, 65, 452–456. [Google Scholar]








| Compound | λabsa (nm) | Band gap b (eV) | PL λmax sol/film c | Φ d,e sol/film | LUMO/HOMO f (eV) | Tm/Tg g (°C) |
|---|---|---|---|---|---|---|
| Cl-MIP | 288 | 3.4 | 394 | 0.58/0.32 | −2.40/−5.80 | 255/N.D |
| Eu(DBM)3Cl-MIP | 294, 352 | 3.0 | 611 | 0.38/0.63 | −2.98/−5.98 | 234/137 |
| PBMA | 257, 263 | 4.8 | 274 | N.D h | N.D | N.D/47 |
| Compound | τ1 (a1) | τ2 (a2) | Avg. lifetime, μs |
|---|---|---|---|
| Eu(DBM)3Cl-MIP | 565 (0.625) | 565 (0.300) | 565 |
| Eu(DBM)3Cl-MIP/PBMA | 695 (0.648) | 142 (0.276) | 651 |
| * Eu(DBM)3Cl-MIP/PBMA | 505 (0.822) | 1251 (0.143) | 730 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, J.-F.; Chen, H.-L.; Lee, G.-S.; Tseng, S.-C.; Lin, M.-H.; Liau, W.-B. Photosensized Controlling Benzyl Methacrylate-Based Matrix Enhanced Eu3+ Narrow-Band Emission for Fluorescence Applications. Int. J. Mol. Sci. 2012, 13, 3718-3737. https://doi.org/10.3390/ijms13033718
Lee J-F, Chen H-L, Lee G-S, Tseng S-C, Lin M-H, Liau W-B. Photosensized Controlling Benzyl Methacrylate-Based Matrix Enhanced Eu3+ Narrow-Band Emission for Fluorescence Applications. International Journal of Molecular Sciences. 2012; 13(3):3718-3737. https://doi.org/10.3390/ijms13033718
Chicago/Turabian StyleLee, Jiann-Fong, Hsuen-Li Chen, Geneh-Siang Lee, Shao-Chin Tseng, Mei-Hsiang Lin, and Wen-Bin Liau. 2012. "Photosensized Controlling Benzyl Methacrylate-Based Matrix Enhanced Eu3+ Narrow-Band Emission for Fluorescence Applications" International Journal of Molecular Sciences 13, no. 3: 3718-3737. https://doi.org/10.3390/ijms13033718




