The Ascorbate-glutathione-α-tocopherol Triad in Abiotic Stress Response
Abstract
:1. Introduction
2. ROS Formation in Abiotic Stress
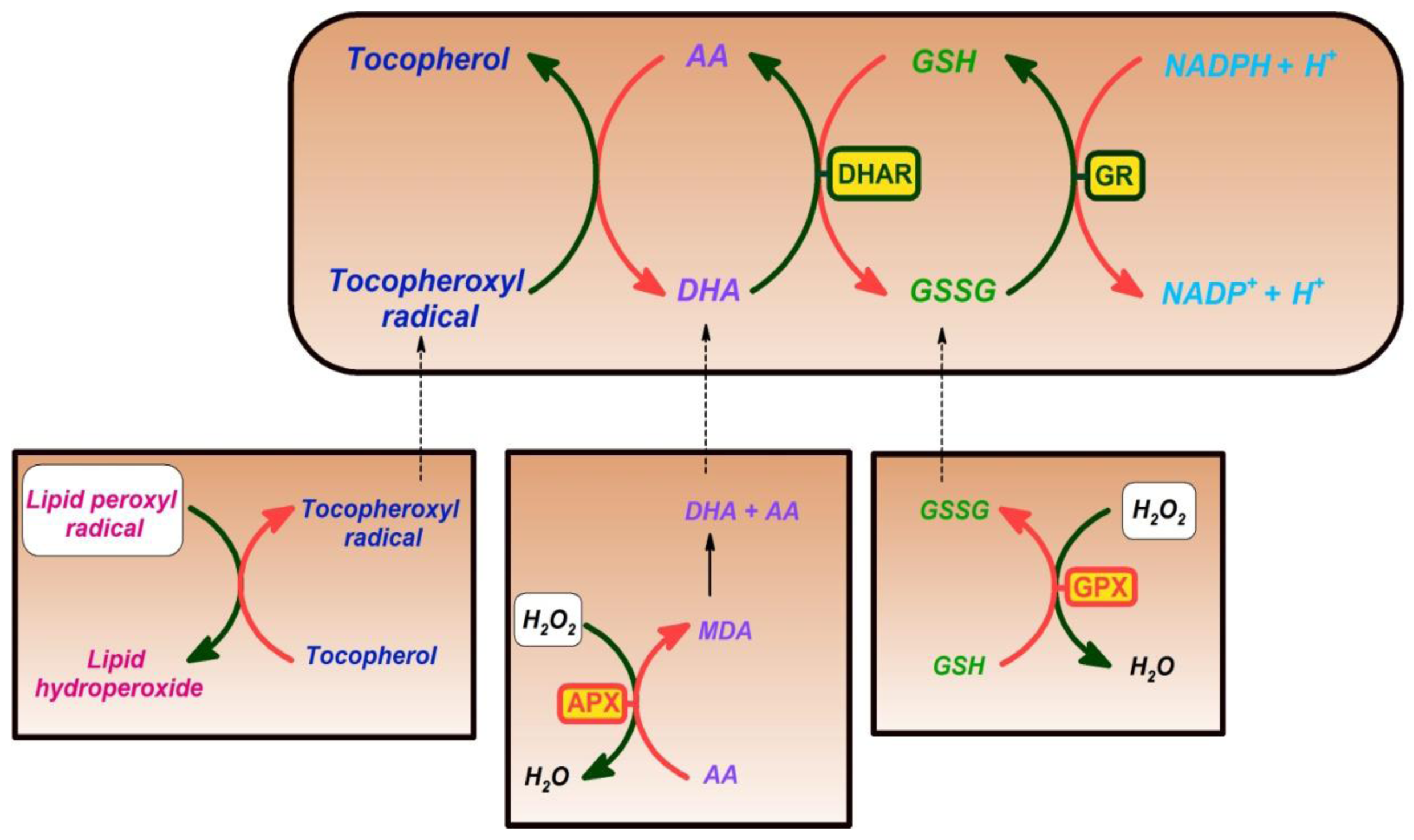
3. Ascorbic Acid
4. Glutathione
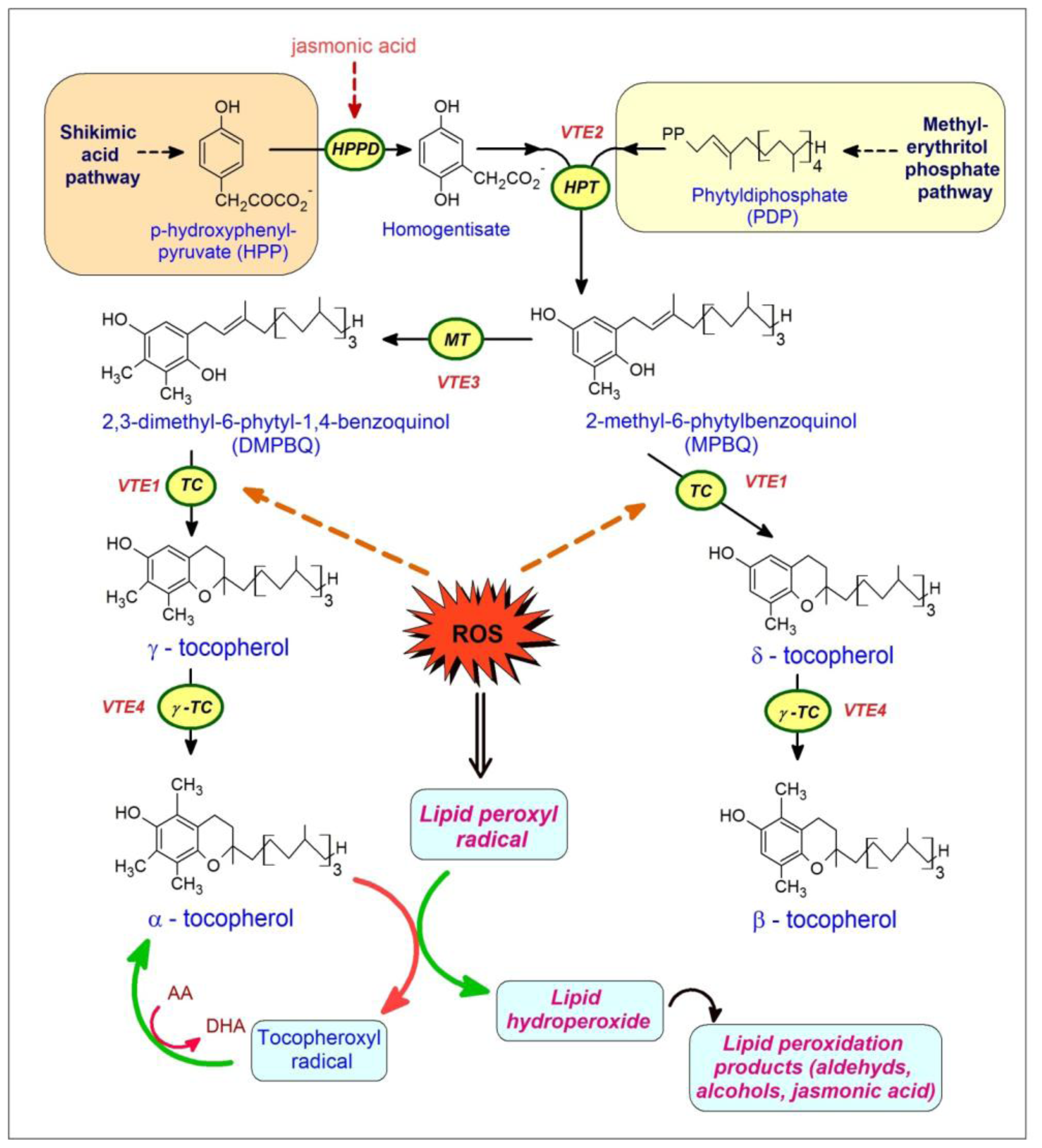
5. Vitamin E
6. Conclusions—Or Lessons to the Human Being from the Plant Cell
Acknowledgments
References
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 2002, 7, 405–410. [Google Scholar]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 2010, 33, 453–467. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem 2010, 48, 909–930. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol 2011, 155, 2–18. [Google Scholar]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol 2000, 3, 229–235. [Google Scholar]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Biochem. Mol. Biol 2000, 35, 291–314. [Google Scholar]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol 2010, 30, 161–175. [Google Scholar]
- Meyer, A.J. The integration of glutathione homeostasis and redox signaling. Plant Physiol 2008, 165, 1390–403. [Google Scholar]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci 2008, 13, 178–182. [Google Scholar]
- Nishiyama, Y.; Yamamoto, H.; Allakhverdiev, I.S.; Suleyman, I.; Masami, I.; Yokota, A.; Murata, N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 2001, 20, 5587–5594. [Google Scholar]
- Allakhverdiev, S.I.; Murata, N. Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of Photosystem II in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 2004, 1657, 23–32. [Google Scholar]
- Chow, W.S.; Lee, H.Y.; He, J.; Hendrickson, L.; Hong, Y.N.; Matsubara, S. Photoinactivation of Photosystem II in leaves. Photosynth. Res 2005, 84, 35–41. [Google Scholar]
- Feller, U.; Crafts-Brandner, S.J.; Salvucci, M.E. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol 1998, 116, 539–546. [Google Scholar]
- Eckardt, N.A.; Portis, A.R., Jr. Heat denaturation profiles of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and Rubisco activase and the inability of Rubisco activase to restore activity of heat-denatured Rubisco. Plant Physiol. 1997, 113, 243–248. [Google Scholar]
- Brooks, A.; Farquhar, G.D. Effect of temperature on the CO2/O2 specificity of ribulose-1,5- bisphosphate carboxylase oxygenase and the rate of respiration in the light—estimates from gas—exchange measurements on spinach. Planta 1985, 165, 397–406. [Google Scholar]
- Wise, R.R. Chilling-enhanced photooxidation—the production, action and study of reactive oxygen species produced during chilling in the light. Photosynth. Res 1995, 45, 79–97. [Google Scholar]
- Sanda, S.; Yoshida, K.; Kuwano, M.; Kawamura, T.; Munekage, Y.N.; Akashi, K.; Yokota, A. Responses of the photosynthetic electron transport system to excess light energy caused by water deficit in wild watermelon. Physiol. Plant 2011, 142, 247–264. [Google Scholar]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestier, C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J 2002, 32, 539–548. [Google Scholar]
- Jaspers, P.; Kangasjarvi, J. Reactive oxygen species in abiotic stress signaling. Physiol. Plant 2010, 138, 405–413. [Google Scholar]
- Puntarulo, S.; Sanchez, R.A.; Boveris, A. Hydrogen peroxide metabolism in soybean embryonic axes at the onset of germination. Plant Physiol 1988, 86, 626–630. [Google Scholar]
- Moller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant. Mol. Biol 2001, 52, 561–591. [Google Scholar]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol 2006, 141, 357–366. [Google Scholar]
- Blokhina, O.; Fagerstedt, K.V. Reactive oxygen species and nitric oxide in plant mitochondria: Origin and redundant regulatory systems. Physiol. Plant 2010, 138, 447–462. [Google Scholar]
- Prasad, T.K.; Anderson, M.D.; Stewart, C.R. Acclimation, hydrogen-peroxide, and abscisic-acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Physiol 1994, 105, 619–627. [Google Scholar]
- Prasad, T.K.; Anderson, M.D.; Martin, B.A.; Stewart, C.R. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen-peroxide. Plant Cell 1994, 6, 65–74. [Google Scholar]
- Purvis, A.C.; Shewfelt, R.L.; Gegogeine, J.W. Superoxide production by mitochondria isolated from green bell pepper fruit. Physiol. Plant 1995, 94, 743–749. [Google Scholar]
- Hernandez, J.A.; Corpas, F.J.; Gomez, M.; Delrio, L.A.; Sevilla, F. Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol. Plant 1993, 89, 103–110. [Google Scholar]
- Mittova, V.; Tal, M.; Volokita, M.; Guy, M. Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 2003, 26, 845–856. [Google Scholar]
- Schwarzlander, M.; Fricker, M.D.; Sweetlove, L.J. Monitoring the in vivo redox state of plant mitochondria: Effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim. Biophys. Acta 2009, 1787, 468–475. [Google Scholar]
- Juszczuk, I.M.; Wagner, A.M.; Rychter, A.M. Regulation of alternative oxidase activity during phosphate deficiency in bean roots (Phaseolus vulgaris). Physiol Plant 2001, 113, 185–192. [Google Scholar]
- Parsons, H.L.; Yip, J.Y.; Vanlerberghe, G.C. Increased respiratory restriction during phosphate-limited growth in transgenic tobacco cells lacking alternative oxidase. Plant Physiol 1999, 121, 1309–1320. [Google Scholar]
- Malusa, E.; Laurenti, E.; Juszczuk, I.; Ferrari, R.P.; Rychter, A.M. Free radical production in roots of Phaseolus vulgaris subjected to phosphate deficiency stress. Plant Physiol. Biochem 2002, 40, 963–967. [Google Scholar]
- Kromer, S. Respiration during photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol 1995, 46, 45–70. [Google Scholar]
- Del Río, L.A.; Sandalio, L.M.; Corpas, F.J.; Palma, J.M.; Barroso, J.B. Reactive oxygen species and reactive nitrogen species in peroxisomes: Production, scavenging, and role in cell signaling. Plant Physiol 2006, 141, 330–335. [Google Scholar]
- Sandalio, L.M.; Fernandez, V.M.; Ruperez, F.L.; del Río, L.A. Superoxide free radicals are produced in glyoxysomes. Plant Physiol 1988, 87, 1–4. [Google Scholar]
- López-Huertas, E.; Corpas, F.J.; Sandalio, L.M.; del Río, L.A. Characterization of membranepolypeptides from pea leaf peroxisomes involved in superoxide radical generation. Biochem. J 1999, 337, 531–536. [Google Scholar]
- Del Río, L.A.; Corpas, F.J.; Sandalio, L.M.; Palma, J.M.; Gómez, M.; Barroso, J.B. Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J. Exp. Bot. 2002, 53, 1255–1272. [Google Scholar]
- Nila, A.G.; Sandalio, L.M.; López, M.G.; Gómez, M.; del Río, L.A.; Gómez-Lim, M.A. Expression of a peroxisome proliferator-activated receptor gene (xPPARa) from Xenopus laevis in tobacco (Nicotiana tabacum) plants. Planta 2006, 224, 569–581. [Google Scholar]
- Halliwell, B.; Foyer, C.H. Ascorbic acid, metal ions and the superoxide radical. Biochem. J 1976, 155, 697–700. [Google Scholar]
- Takahashi, M.; Asada, K. Superoxide production in aprotic interior of chloroplast thylakoids. Arch. Biochem. Biophys 1988, 267, 714–722. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol 2011, 155, 12–18. [Google Scholar]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant. Biol 2000, 3, 229–235. [Google Scholar]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Biochem. Mol. Biol 2000, 35, 291–314. [Google Scholar]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar]
- Dowdle, J.; Ishikawa, T.; Gatzek, S.; Rolinski, S.; Smirnoff, N. Two genes in Arabidopsis thaliana encoding GDPp-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 2007, 52, 673–689. [Google Scholar]
- Laing, W.A.; Wright, M.A.; Cooney, J.; Bulley, S.M. The missing step of the l-galactose pathway of ascorbate biosynthesis in plants, an l-galactose guanyltransferase, increases leaf ascorbate content. Proc. Nat. Acad. Sci. USA 2007, 104, 9534–9539. [Google Scholar]
- Linster, C.L.; Gomez, T.A.; Christensen, K.C.; Adler, L.N.; Young, B.D.; Brenner, C.; Clarke, S.G. Arabidopsis vtc2 encodes a GDP-l-galactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway to ascorbic acid in plants. J. Biol. Chem 2007, 282, 18879–18885. [Google Scholar]
- Agius, F.; Gonzalez-Lamothe, R.; Caballero, J.L.; Munoz-Blanco, J.; Botella, M.A.; Valpuesta, V. Engineering increased vitamin C levels in plants by overexpression of a d-galacturonic acid reductase. Nat. Biotechnol 2003, 21, 177–181. [Google Scholar]
- Loewus, F.A. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochem 1999, 52, 193–210. [Google Scholar]
- Wolucka, B.A.; Van Montagu, M. Gdp-mannose 3′,5′-epimerase forms GDP-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem 2003, 278, 47483–47490. [Google Scholar]
- Wolucka, B.A.; Van Montagu, M. The VTC2 cycle and the de novo biosynthesis pathways for vitamin C in plants: An opinion. Phytochemistry 2007, 68, 2602–2613. [Google Scholar]
- Lorence, A.; Chevone, B.I.; Mendes, P.; Nessler, C.L. Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol 2004, 134, 1200–1205. [Google Scholar]
- Conklin, P.L.; Norris, S.R.; Wheeler, G.L.; Williams, E.H.; Smirnoff, N.; Last, R.L. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 4198–4203. [Google Scholar]
- Reuhs, B.L.; Glenn, J.; Stephens, S.B.; Kim, J.S.; Christie, D.B.; Glushka, J.G.; Zablackis, E.; Albersheim, P.; Darvill, A.G.; O’Neill, M.A. l-galactose replaces l-fucose in the pectic polysaccharide rhamnogalacturonan II synthesized by the l-fucose-deficient mur1 Arabidopsis mutant. Planta 2004, 219, 147–157. [Google Scholar]
- Dutilleul, C.; Garmier, M.; Noctor, G.; Mathieu, C.; Chetrit, P.; Foyer, C.H.; de Paepe, R. Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell 2003, 15, 1212–1226. [Google Scholar]
- Bartoli, C.G.; Yu, J.; Gomez, F.; Fernandez, L.; McIntosh, L.; Foyer, C.H. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J. Exp. Bot 2006, 57, 1621–1631. [Google Scholar]
- Yabuta, Y.; Mieda, T.; Rapolu, M.; Nakamura, A.; Motoki, T.; Maruta, T.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot 2007, 58, 2661–2671. [Google Scholar]
- Bartoli, C.G.; Tambussi, E.A.; Diego, F.; Foyer, C.H. Control of ascorbic acid synthesis and accumulation and glutathione by the incident light red/far red ratio in Phaseolus vulgaris leaves. FEBS Lett 2009, 583, 118–122. [Google Scholar]
- Zechmann, B. Subcellular distribution of ascorbate in plants. Plant Signal. Behav 2011, 6, 360–363. [Google Scholar]
- Conklin, P.L.; Williams, E.H.; Last, R.L. Environmental stress sensitivity of an ascorbic acid deficient Arabidopsis mutant. Proc. Natl. Acad. Sci. USA 1996, 93, 9970–9974. [Google Scholar]
- Gao, Q.; Zhang, L. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J. Plant Physiol 2008, 165, 138–148. [Google Scholar]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot 2005, 56, 3041–3049. [Google Scholar]
- Zsigmond, L.; Tomasskovics, B.; Deak, V.; Rigo, G.; Szabados, L.; Banhegyi, G.; Szarka, A. Enhanced activity of galactono-1,4-lactone dehydrogenase and ascorbate-glutathione cycle in mitochondria from complex III deficient Arabidopsis. Plant Physiol. Biochem 2011, 49, 809–815. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol 1998, 49, 249–279. [Google Scholar]
- Szarka, A.; Horemans, N.; Banhegyi, G.; Asard, H. Facilitated glucose and dehydroascorbate transport in plant mitochondria. Arch. Biochem. Biophys 2004, 428, 73–80. [Google Scholar]
- Szarka, A.; Horemans, N.; Kovacs, Z.; Grof, P.; Mayer, M.; Banhegyi, G. Dehydroascorbate reduction in plant mitochondria is coupled to the respiratory electron transfer chain. Physiol. Plantatrum 2007, 129, 225–232. [Google Scholar]
- Chen, Z.; Young, T.E.; Ling, J.; Chang, S.C.; Gallie, D.R. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. USA 2003, 100, 3525–3530. [Google Scholar]
- Wang, Z.; Xiao, Y.; Chen, W.; Tang, K.; Zhang, L. Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J. Integr. Plant Biol 2010, 52, 400–409. [Google Scholar]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Morishima, I.; Shibahara, T.; Inanaga, S.; Tanaka, K. Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiol. Plantarum 2006, 127, 57–65. [Google Scholar]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Shibahara, T.; Inanaga, S.; Tanaka, K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 2007, 225, 1255–1264. [Google Scholar]
- Li, F.; Wu, Q.Y.; Sun, Y.L.; Wang, L.Y.; Yang, X.H.; Meng, Q.W. Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol. Plantarum 2010, 139, 421–434. [Google Scholar]
- Eltelib, H.A.; Badejo, A.A.; Fujikawa, Y.; Esaka, M. Gene expression of monodehydroascorbate reductase and dehydroascorbate reductase during fruit ripening and in response to environmental stresses in acerola (Malpighia glabra). J. Plant Physiol 2011, 168, 619–627. [Google Scholar]
- Wells, W.W.; Xu, D.P. Dehydroascorbate reduction. J. Bioenerg. Biomembr 1994, 26, 369–377. [Google Scholar]
- Foyer, C.H.; Halliwell, B. Purification and properties of dehydroascorbate reductase from spinach leaves. Phytochemistry 1977, 16, 1347–1350. [Google Scholar]
- Hossain, M.A.; Asada, K. Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol 1984, 25, 85–92. [Google Scholar]
- Mhamdi, A.; Hager, J.; Chaouch, S.; Queval, G.; Han, Y.; Taconnat, L.; Saindrenan, P.; Gouia, H.; Issakidis-Bourguet, E.; Renou, J.P.; et al. Arabidopsis glutathione reductase1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. J. Plant Physiol 2010, 153, 1144–1160. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ 2011, 2, 454–484. [Google Scholar]
- Bashandy, T.; Guilleminot, J.; Vernoux, T.; Caparros-Ruiz, D.; Ljung, K.; Meyer, Y.; Reichheld, J.P. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 2010, 22, 376–391. [Google Scholar]
- Noctor, G.; Gomez, L.; Vanacker, H.; Foyer, C.H. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J. Exp. Bot 2002, 53, 1283–1304. [Google Scholar]
- Wachter, A.; Wolf, S.; Steininger, H.; Bogs, J.; Rausch, T. Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: Implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J 2005, 41, 15–30. [Google Scholar]
- Maughan, S.C.; Pasternak, M.; Cairns, N.; Kiddle, G.; Brach, T.; Jarvis, R.; Haas, F.; Nieuwland, J.; Lim, B.; Muller, C.; et al. Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2331–2336. [Google Scholar] [Green Version]
- Zechmann, B.; Tomasic, A.; Horvat, L.; Fulgosi, H. Subcellular distribution of glutathione and cysteine in Cyanobacteria. Protoplasma 2010, 246, 65–72. [Google Scholar]
- Zechmann, B.; Muller, M.; Zellnig, G. Intracellular adaptations of glutathione content in Cucurbita pepo l. Induced by treatment with reduced glutathione and buthionine sulfoximine. Protoplasma 2006, 227, 197–209. [Google Scholar]
- Zechmann, B.; Mauch, F.; Sticher, L.; Muller, M. Subcellular immunocytochemical analysis detects the highest concentrations of glutathione in mitochondria and not in plastids. J. Exp. Bot 2008, 59, 4017–4027. [Google Scholar]
- Queval, G.; Jaillard, D.; Zechmann, B.; Noctor, G. Increased intracellular ho availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ 2011, 34, 21–32. [Google Scholar]
- Gromes, R.; Hothorn, M.; Lenherr, E.D.; Rybin, V.; Scheffzek, K.; Rausch, T. The redox switch of gamma-glutamylcysteine ligase via a reversible monomer-dimer transition is a mechanism unique to plants. Plant J 2008, 54, 1063–1075. [Google Scholar]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol 2002, 53, 159–182. [Google Scholar]
- Grill, E.; Loffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar]
- Clemens, S.; Kim, E.J.; Neumann, D.; Schroeder, J.I. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J 1999, 18, 3325–3333. [Google Scholar]
- Vatamaniuk, O.K.; Mari, S.; Lu, Y.P.; Rea, P.A. Atpcs1, a phytochelatin synthase from Arabidopsis: Isolation and in vitro reconstitution. Proc. Natl. Acad. Sci. USA 1999, 96, 7110–7115. [Google Scholar]
- Ha, S.B.; Smith, A.P.; Howden, R.; Dietrich, W.M.; Bugg, S.; O’Connell, M.J.; Goldsbrough, P.B.; Cobbett, C.S. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 1999, 11, 1153–1164. [Google Scholar]
- Vatamaniuk, O.K.; Mari, S.; Lang, A.; Chalasani, S.; Demkiv, L.O.; Rea, P.A. Phytochelatin synthase, a dipeptidyltransferase that undergoes multisite acylation with gamma-glutamylcysteine during catalysis: Stoichiometric and site-directed mutagenic analysis of Arabidopsis thaliana PCS1-catalyzed phytochelatin synthesis. J. Biol. Chem 2004, 279, 22449–22460. [Google Scholar]
- Clemens, S. Evolution and function of phytochelatin synthases. J. Plant Physiol 2006, 163, 319–332. [Google Scholar]
- Queval, G.; Thominet, D.; Vanacker, H.; Miginiac-Maslow, M.; Gakiere, B.; Noctor, G. H2O2-activated up-regulation of glutathione in Arabidopsis involves induction of genes encoding enzymes involved in cysteine synthesis in the chloroplast. Mol. Plant 2009, 2, 344–356. [Google Scholar]
- Foyer, C.H.; Souriau, N.; Perret, S.; Lelandais, M.; Kunert, K.J.; Pruvost, C.; Jouanin, L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. J. Plant Physiol 1995, 109, 1047–1057. [Google Scholar]
- Strohm, M.; Jouanin, L.; Kunert, K.J.; Pruvost, C.; Polle, A.; Foyer, C.H.; Rennenberg, H. Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula × Populus alba) overexpressing glutathione synthetase. Plant J 1995, 7, 141–145. [Google Scholar]
- Noctor, G.; Strohm, M.; Jouanin, L.; Kunert, K.J.; Foyer, C.H.; Rennenberg, H. Synthesis of glutathione in leaves of transgenic poplar overexpressing gamma-glutamylcysteine synthetase. Plant Physiol 1996, 112, 1071–1078. [Google Scholar]
- Arisi, A.C.M.; Noctor, G.; Foyer, C.H.; Jouanin, L. Modification of thiol contents in poplars (Populus tremula × P. alba) overexpressing enzymes involved in glutathione synthesis. Planta 1997, 203, 362–372. [Google Scholar]
- Creissen, G.; Fermin, J.; Fryer, M.; Kular, B.; Leyland, N.; Reynolds, H.; Pastori, G.; Wellburn, F.; Baker, N.; Wellburn, A.; et al. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco paradoxically causes increased oxidative stress. Plant Cell 2000, 12, 301–301. [Google Scholar]
- Liedschulte, V.; Wachter, A.; An, Z.G.; Rausch, T. Exploiting plants for glutathione (GSH) production: Uncoupling GSH synthesis from cellular controls results in unprecedented GSH accumulation. Plant Biotech. J 2010, 8, 807–820. [Google Scholar]
- Zhu, Y.L.; Pilon-Smits, E.A.H.; Tarun, A.S.; Weber, S.U.; Jouanin, L.; Terry, N. Cadmium tolerance and accumulation in indian mustard is enhanced by overexpressing gamma-glutamylcysteine synthetase. J. Plant Physiol 1999, 121, 1169–1177. [Google Scholar]
- Gullner, G.; Komives, T.; Rennenberg, H. Enhanced tolerance of transgenic poplar plants overexpressing gamma-glutamylcysteine synthetase towards chloroacetanilide herbicides. J. Exp. Bot 2001, 52, 971–979. [Google Scholar]
- Ivanova, L.A.; Ronzhina, D.A.; Ivanov, L.A.; Stroukova, L.V.; Peuke, A.D.; Rennenberg, H. Over-expression of gsh1 in the cytosol affects the photosynthetic apparatus and improves the performance of transgenic poplars on heavy metal-contaminated soil. Plant Biol 2011, 13, 649–659. [Google Scholar]
- Wagner, U.; Edwards, R.; Dixon, D.P.; Mauch, F. Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol. Biol 2002, 49, 515–532. [Google Scholar]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; van Montagu, M.; Inze, D.; van Camp, W. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 1997, 16, 4806–4816. [Google Scholar]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar]
- Sappl, P.G.; Carroll, A.J.; Clifton, R.; Lister, R.; Whelan, J.; Harvey Millar, A.; Singh, K.B. The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. Plant J 2009, 58, 53–68. [Google Scholar]
- Esterbauer, H.; Grill, D. Seasonal variation of glutathione and glutathione reductase in needles of Picea abies. J. Plant Physiol 1978, 61, 119–121. [Google Scholar]
- Tausz, M.; Sircelj, H.; Grill, D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot 2004, 55, 1955–1962. [Google Scholar]
- Foyer, C.; Lelandais, M.; Galap, C.; Kunert, K.J. Effects of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. J. Plant Physiol 1991, 97, 863–872. [Google Scholar]
- Aono, M.; Kubo, A.; Saji, H.; Tanaka, K.; Kondo, N. Enhanced tolerance to photooxidative stress of transgenic Nicotiana-tabacum with high chloroplastic glutathione-reductase activity. Plant Cell Physiol 1993, 34, 129–135. [Google Scholar]
- Broadbent, P.; Creissen, G.P.; Kular, B.; Wellburn, A.R.; Mullineaux, P.M. Oxidative stress responses in transgenic tobacco containing altered levels of glutathione-reductase activity. J. Plant 1995, 8, 247–255. [Google Scholar]
- Kornyeyev, D.; Logan, B.A.; Allen, R.D.; Holaday, A.S. Field-grown cotton plants with elevated activity of chloroplastic glutathione reductase exhibit no significant alteration of diurnal or seasonal patterns of excitation energy partitioning and CO2 fixation. Field Crops Res 2005, 94, 165–175. [Google Scholar]
- Ding, S.; Lu, Q.; Zhang, Y.; Yang, Z.; Wen, X.; Zhang, L.; Lu, C. Enhanced sensitivity to oxidative stress in transgenic tobacco plants with decreased glutathione reductase activity leads to a decrease in ascorbate pool and ascorbate redox state. Plant Mol. Biol 2009, 69, 577–592. [Google Scholar]
- Le Martret, B.; Poage, M.; Shiel, K.; Nugent, G.D.; Dix, P.J. Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotech. J 2011, 9, 661–673. [Google Scholar]
- Munne-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci 2002, 21, 31–57. [Google Scholar]
- Grusak, M.A.; DellaPenna, D. Improving the nutrient composition of plants to enhance human nutrition and health1. Rev. Plant Physiol. Plant Mol. Biol 1999, 50, 133–161. [Google Scholar]
- Soll, J.; Schultz, G.; Joyard, J.; Douce, R.; Block, M.A. Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch. Biochem. Biophys 1985, 238, 290–299. [Google Scholar]
- Arango, Y.; Heise, K.P. Tocopherol synthesis from homogentisate in Capsicum anuum l. (yellow pepper) chromoplast membranes: Evidence for tocopherol cyclase. Biochem. J 1998, 336, 531–533. [Google Scholar]
- Lichtenthaler, H.K.; Prenzel, U.; Douce, R.; Joyard, J. Localization of prenylquinones in the envelope of spinach chloroplasts. Biochim. Biophys. Acta 1981, 641, 99–105. [Google Scholar]
- Grumbach, K.H. On the role of carotenoids in photosynthesis. J. Physical. Chem 1983, 364, 1133–1134. [Google Scholar]
- Fryer, M.J. The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ 1992, 15, 381–392. [Google Scholar]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci 1998, 3, 147–151. [Google Scholar]
- Wise, R.R.; Naylor, A.W. Chilling-enhanced photooxidation: Evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. J. Plant Physiol 1987, 83, 278–282. [Google Scholar]
- Norris, S.R.; Shen, X.; DellaPenna, D. Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. J. Plant Physiol 1998, 117, 1317–1323. [Google Scholar]
- Collakova, E.; DellaPenna, D. Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. Pcc 6803 and Arabidopsis. J. Plant Physiol 2001, 127, 1113–1124. [Google Scholar]
- Savidge, B.; Weiss, J.D.; Wong, Y.H.; Lassner, M.W.; Mitsky, T.A.; Shewmaker, C.K.; Post-Beittenmiller, D.; Valentin, H.E. Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. Pcc 6803 and Arabidopsis. J. Plant Physiol 2002, 129, 321–332. [Google Scholar]
- Cheng, Z.; Sattler, S.; Maeda, H.; Sakuragi, Y.; Bryant, D.A.; DellaPenna, D. Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in Cyanobacteria and photosynthetic eukaryotes. Plant Cell 2003, 15, 2343–2356. [Google Scholar]
- Van Eenennaam, A.L.; Lincoln, K.; Durrett, T.P.; Valentin, H.E.; Shewmaker, C.K.; Thorne, G.M.; Jiang, J.; Baszis, S.R.; Levering, C.K.; Aasen, E.D.; et al. Engineering vitamin E content: From Arabidopsis mutant to soy oil. Plant Cell 2003, 15, 3007–3019. [Google Scholar]
- Porfirova, S.; Bergmuller, E.; Tropf, S.; Lemke, R.; Dormann, P. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 12495–12500. [Google Scholar]
- Sattler, S.E.; Cahoon, E.B.; Coughlan, S.J.; DellaPenna, D. Characterization of tocopherol cyclases from higher plants and Cyanobacteria. Evolutionary implications for tocopherol synthesis and function. J. Plant Physiol 2003, 132, 2184–2195. [Google Scholar]
- Tsegaye, Y.; Shintani, D.K.; DellaPenna, D. Overexpression of the enzyme p-hydroxyphenolpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiol. Biochem 2002, 40, 913–920. [Google Scholar]
- Collakova, E.; DellaPenna, D. Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. J. Plant Physiol 2003, 131, 632–642. [Google Scholar]
- Kanwischer, M.; Porfirova, S.; Bergmuller, E.; Dormann, P. Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress. J. Plant Physiol 2005, 137, 713–723. [Google Scholar]
- Shintani, D.; DellaPenna, D. Elevating the vitamin E content of plants through metabolic engineering. Science 1998, 282, 2098–2100. [Google Scholar]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar]
- Fahrenholtz, S.R.; Doleiden, F.H.; Trozzolo, A.M.; Lamola, A.A. On the quenching of singlet oxygen by α-tocopherol. Photochem. Photobiol 1974, 20, 505–509. [Google Scholar]
- Munne-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol 2005, 162, 743–748. [Google Scholar]
- Tanaka, M.; Murai, M.; Tokunaga, H.; Kimura, T.; Okada, S. Tocopherol succinate reference standard (control 881) of National Institute of Hygienic Sciences. Eisei Shikenjo Hokoku 1990, (108), 156–158. [Google Scholar]
- Moran, J.F.; Becana, M.; Iturbeormaetxe, I.; Frechilla, S.; Klucas, R.V.; Apariciotejo, P. Drought induces oxidative stress in pea-plants. Planta 1994, 194, 346–352. [Google Scholar]
- Price, A.H.; Atherton, N.M.; Hendry, G.A. Plants under drought-stress generate activated oxygen. Free Radic. Res. Commun 1989, 8, 61–66. [Google Scholar]
- Bartoli, C.G.; Simontacchi, M.; Tambussi, E.; Beltrano, J.; Montaldi, E.; Puntarulo, S. Drought and watering-dependent oxidative stress: Effect on antioxidant content in Triticum aestivum L. leaves. J. Exp. Bot 1999, 50, 375–383. [Google Scholar]
- Munne-Bosch, S.; Schwarz, K.; Alegre, L. Enhanced formation of alpha-tocopherol and highly oxidized abietane diterpenes in water-stressed rosemary plants. J. Plant Physiol 1999, 121, 1047–1052. [Google Scholar]
- Munne-Bosch, S.; Alegre, L. Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. J. Plant Physiol 2001, 125, 1094–1102. [Google Scholar]
- Garcia-Plazaola, J.I.; Becerril, J.M. Effects of drought on photoprotective mechanisms in European beech (Fagus sylvatica L.) seedlings from different provenances. Trees Strut. Funct 2000, 14, 485–490. [Google Scholar]
- Boo, Y.C.; Jung, J. Water deficit-induced oxidative stress and antioxidative defenses in rice plants. J. Plant Physiol 1999, 155, 255–261. [Google Scholar]
- Falk, J.; Krauss, N.; Dahnhardt, D.; Krupinska, K. The senescence associated gene of barley encoding 4-hydroxyphenylpyruvate dioxygenase is expressed during oxidative stress. J. Plant Physiol 2002, 159, 1245–1253. [Google Scholar]
- Sandorf, I.; Hollander-Czytko, H. Jasmonate is involved in the induction of tyrosine aminotransferase and tocopherol biosynthesis in Arabidopsis thaliana. Planta 2002, 216, 173–179. [Google Scholar]
- Munne-Bosch, S.; Penuelas, J. Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 2003, 217, 758–766. [Google Scholar]
- Liu, X.; Hua, X.; Guo, J.; Qi, D.; Wang, L.; Liu, Z.; Jin, Z.; Chen, S.; Liu, G. Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis thaliana. Biotechnol. Lett 2008, 30, 1275–1280. [Google Scholar]
- Collin, V.C.; Eymery, F.; Genty, B.; Rey, P.; Havaux, M. Vitamin E is essential for the tolerance of Arabidopsis thaliana to metal-induced oxidative stress. Plant Cell Environ 2008, 31, 244–257. [Google Scholar]
- Munne-Bosch, S.; Alegre, L. Interplay between ascorbic acid and lipophilic antioxidant defences in chloroplasts of water-stressed Arabidopsis plants. FEBS Lett 2002, 524, 145–148. [Google Scholar]
- Liebler, D.C.; Kling, D.S.; Reed, D.J. Antioxidant protection of phospholipid bilayers by alpha-tocopherol. Control of α-tocopherol status and lipid peroxidation by ascorbic acid and glutathione. J. Biol. Chem 1986, 261, 12114–12119. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The Ascorbate-glutathione-α-tocopherol Triad in Abiotic Stress Response. Int. J. Mol. Sci. 2012, 13, 4458-4483. https://doi.org/10.3390/ijms13044458
Szarka A, Tomasskovics B, Bánhegyi G. The Ascorbate-glutathione-α-tocopherol Triad in Abiotic Stress Response. International Journal of Molecular Sciences. 2012; 13(4):4458-4483. https://doi.org/10.3390/ijms13044458
Chicago/Turabian StyleSzarka, András, Bálint Tomasskovics, and Gábor Bánhegyi. 2012. "The Ascorbate-glutathione-α-tocopherol Triad in Abiotic Stress Response" International Journal of Molecular Sciences 13, no. 4: 4458-4483. https://doi.org/10.3390/ijms13044458
APA StyleSzarka, A., Tomasskovics, B., & Bánhegyi, G. (2012). The Ascorbate-glutathione-α-tocopherol Triad in Abiotic Stress Response. International Journal of Molecular Sciences, 13(4), 4458-4483. https://doi.org/10.3390/ijms13044458




