Role of 14-3-3ζ in Platelet Glycoprotein Ibα-von Willebrand Factor Interaction-Induced Signaling
Abstract
:1. Introduction
2. Results
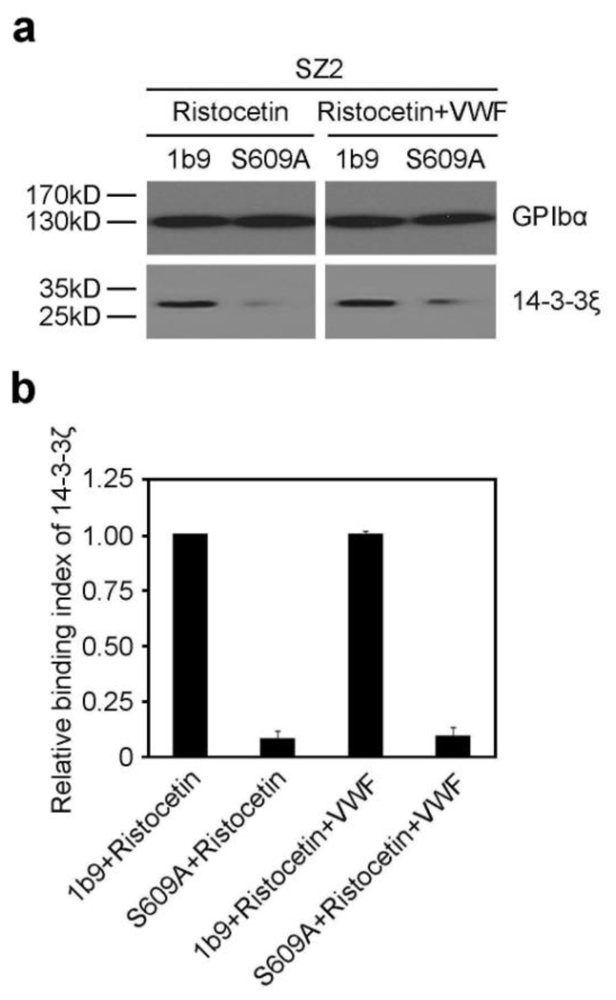
2.1. The S609A Mutation Disrupts the Association of 14-3-3ζ with GPIbα before and after VWF Binding to GPIbα
2.2. The S609A Mutation Inhibits the VWF-GPIb-IX Interaction-Induced Activation of Src Family Kinases
2.3. The S609A Mutation Inhibits the VWF-GPIb-IX Interaction-Induced Activation of PKC
2.4. Disruption of 14-3-3ζ Association with GPIbα Impairs Elevation of Intracellular Ca2+ Levels
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Lines Expressing Recombinant GPIb-IX and Mutants
4.3. Flow Cytometric Analysis of VWF Binding to GPIb-IX-Expressing Cells
4.4. Coimmunoprecipitation and Western Blotting
4.5. Measurement of Intracellular Ca2+ Levels
5. Conclusions
Supplementary Material
ijms-13-05364-s001.pdfAcknowledgements
- Declarations of InterestThe authors declare that they have no conflict of interest.
References
- Lopez, J.A. The platelet glycoprotein Ib-IX complex. Blood Coagul. Fibrinolysis 1994, 5, 97–119. [Google Scholar]
- Du, X. Signaling and regulation of the glycoprotein Ib-IX-V complex. Curr. Opin. Hematol 2007, 14, 262–269. [Google Scholar]
- Andrews, R.K.; Berndt, M.C. Platelet adhesion: A game of catch and release. J. Clin. Invest 2008, 118, 3009–3011. [Google Scholar]
- Kroll, M.H.; Harris, T.S.; Moake, J.L.; Handin, R.I.; Schafer, A.I. Von Willebrand factor binding to platelet GPIb initiates signals for platelet activation. J. Clin. Invest 1991, 88, 1568–1573. [Google Scholar]
- Kasirer-Friede, A.; Cozzi, M.R.; Mazzucato, M.; De Marco, L.; Ruggeri, Z.M.; Shattil, S.J. Signaling through GP Ib-IX-V activates αIIbβ3 independently of other receptors. Blood 2004, 103, 3403–3411. [Google Scholar]
- Feng, S.; Resendiz, J.C.; Lu, X.; Kroll, M.H. Filamin A binding to the cytoplasmic tail of glycoprotein Ibα regulates von Willebrand factor-induced platelet activation. Blood 2003, 102, 2122–2129. [Google Scholar]
- Williamson, D.; Pikovski, I.; Cranmer, S.L.; Mangin, P.; Mistry, N.; Domagala, T.; Chehab, S.; Lanza, F.; Salem, H.H.; Jackson, S.P. Interaction between platelet glycoprotein Ibα and filamin-1 is essential for glycoprotein Ib/IX receptor anchorage at high shear. J. Biol. Chem 2002, 277, 2151–2159. [Google Scholar]
- Englund, G.D.; Bodnar, R.J.; Li, Z.; Ruggeri, Z.M.; Du, X. Regulationn of von Willebrand factor binding to the platelet glycoprotein Ib-IX by a membrane skeleton-dependent inside-out signal. J. Biol. Chem 2001, 276, 16952–16959. [Google Scholar]
- Okita, J.R.; Pidard, D.; Newman, P.J.; Montgomery, R.R.; Kunicki, T.J. On the association of glycoprotein Ib and actin-binding protein in human platelets. J. Cell Biol 1985, 100, 317–321. [Google Scholar]
- Andrew, R.K.; Munday, A.D.; Mitchell, C.A.; Berndt, M.C. Interaction of calmodulin with the cytoplasmic domain of the platelet membrane glycoprotein Ib-IX-V complex. Blood 2001, 98, 681–687. [Google Scholar]
- Mu, F.T.; Andrews, R.K.; Arthur, J.F.; Munday, A.D.; Cranmer, S.L.; Jackson, S.P.; Stomski, F.C.; Lopez, A.F.; Berndt, M.C. A functional 14-3-3ζ-independent association of PI3-kinase with glycoprotein Ibα, the major ligand-binding subunit of the platelet glycoprotein Ib-IX-B complex. Blood 2008, 111, 4580–4587. [Google Scholar]
- Dai, K.; Bodnar, R.; Berndt, M.C.; Du, X. A critical role for 14-3-3ζ protein in regulating the VWF binding function of platelet glycoprotein Ib-IX and its therapeutic implications. Blood 2005, 106, 1975–1981. [Google Scholar]
- Bodnar, R.J.; Xi, X.; Li, Z.; Berndt, M.C.; Du, X. Regulation of glycoprotein Ib-IX-von Willebrand factor interaction by cAMP-dependent protein kinase-mediated phosphorylation at Ser 166 of glycoprotein Ibβ. J. Biol. Chem 2002, 277, 47080–47087. [Google Scholar]
- Yuan, Y.; Zhang, W.; Yan, R.; Liao, Y.; Zhao, L.; Ruan, C.; Du, X.; Dai, K. Identification of a novel 14-3-3ζ binding site within the cytoplasmic domain of platelet glycoprotein Ibα that plays a key role in regulating the von Willebrand factor binding function of glycoprotein Ib-IX. Circ. Res 2009, 105, 1177–1185. [Google Scholar]
- Gu, M.; Xi, X.; Englund, G.D.; Berndt, M.C.; Du, X. Analysis of the roles of 14-3-3 in the platelet glycoprotein Ib-IX-mediated activation of integrin αIIbβ3 using a reconstituted mammalian cell expression model. J. Cell Biol 1999, 147, 1085–1096. [Google Scholar]
- Bialkowska, K.; Zaffran, Y.; Meyer, S.C.; Fox, J.E. 14-3-3ζ mediates integrin-induced activation of Cdc42 and Rac. J. Biol. Chem 2003, 278, 33342–33350. [Google Scholar]
- Zaffran, Y.; Meyer, S.C.; Negrescu, E.; Reddy, K.B.; Fox, J.E. Signaling across the platelet adhesion receptor glycoprotein Ib-IX induces αIIbβ3 activation both in platelets and a transfected chinese hamster ovary cell system. J. Biol. Chem 2000, 275, 16779–16787. [Google Scholar]
- Wu, Y.; Asazuma, N.; Satoh, K.; Yakafuta, Y.; Berndt, M.C.; Ozaki, Y. Interaction between von Willebrand factor and glycoprotein Ib activates Src kinase in human platelets: Role of phosphoinositide 3-kinase. Blood 2003, 101, 3469–3476. [Google Scholar]
- Jackson, S.P.; Schoenwaelder, S.M.; Yuan, Y.; Rabinowitz, I.; Salem, H.H.; Mitchell, C.A. Adhesion receptor activation of phosphatidylinositol 3-kinase. Von Willebrand factor stimulates the cytoskeletal association and activation of phosphatidylinositol 3-kinase and pp60c-src in human platelets. J. Biol. Chem 1994, 269, 27093–27099. [Google Scholar]
- Mazzucato, M.; Pradella, P.; Cozzi, M.R.; de Marco, L.; Ruggeri, Z.M. Sequential cytoplasmic calcium signals in a 2-stage platelet activation process induced by the glycoprotein Ibα mechanoreceptor. Blood 2002, 100, 2793–2800. [Google Scholar]
- Li, S.; Wang, Z.; Liao, Y.; Zhang, W.; Shi, Q.; Yan, R.; Ruan, C.; Dai, K. The glycoprotein Ibα-von Willebrand factor interaction induces platelet apoptosis. J. Thromb. Haemost 2010, 8, 341–350. [Google Scholar]
- Mu, F.T.; Cranmer, S.L.; Andrews, R.K.; Berndt, M.C. Functional association of phosphoinositide-3-kinase with platelet glycoprotein Ibα, the major ligand-binding subunit of the glycoprotein Ib-IX-V complex. J. Thromb. Haemost 2010, 8, 324–330. [Google Scholar]
- Ruan, C.G.; Xi, X.D.; Gu, J.M. Studies on monoclonal antibodies to human von Willebrand factor. Chung Hua Nei KoTsa Chih 1986, 25. [Google Scholar]
- Ruan, C.G.; Du, X.P.; Xi, X.D.; Castaldi, P.A.; Berndt, M.C. A murine antiglycoprotein Ib complex monoclonal antibody, SZ 2, inhibits platelet aggregation induced by both ristocetin and collagen. Blood 1987, 69, 570–577. [Google Scholar]
- Li, S.; Shi, Q.; Liu, G.; Zhang, W.; Wang, Z.; Wang, Y.; Dai, K. Mechanism of platelet functional changes and effects of anti-platelet agents on in vivo hemostasis under different gravity conditions. J. Appl. Physiol 2010, 108, 1241–1249. [Google Scholar]



© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, W.; Zhao, L.; Liu, J.; Du, J.; Yan, R.; Dai, K. Role of 14-3-3ζ in Platelet Glycoprotein Ibα-von Willebrand Factor Interaction-Induced Signaling. Int. J. Mol. Sci. 2012, 13, 5364-5374. https://doi.org/10.3390/ijms13055364
Zhang W, Zhao L, Liu J, Du J, Yan R, Dai K. Role of 14-3-3ζ in Platelet Glycoprotein Ibα-von Willebrand Factor Interaction-Induced Signaling. International Journal of Molecular Sciences. 2012; 13(5):5364-5374. https://doi.org/10.3390/ijms13055364
Chicago/Turabian StyleZhang, Weilin, Lili Zhao, Jun Liu, Juan Du, Rong Yan, and Kesheng Dai. 2012. "Role of 14-3-3ζ in Platelet Glycoprotein Ibα-von Willebrand Factor Interaction-Induced Signaling" International Journal of Molecular Sciences 13, no. 5: 5364-5374. https://doi.org/10.3390/ijms13055364
APA StyleZhang, W., Zhao, L., Liu, J., Du, J., Yan, R., & Dai, K. (2012). Role of 14-3-3ζ in Platelet Glycoprotein Ibα-von Willebrand Factor Interaction-Induced Signaling. International Journal of Molecular Sciences, 13(5), 5364-5374. https://doi.org/10.3390/ijms13055364



