Fly Ash-based Geopolymer Lightweight Concrete Using Foaming Agent
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Fluorescence (XRF) Analysis
2.2. Compressive Strength, Density, Porosity and Water Absorption
2.3. X-ray Diffraction (XRD) Analysis
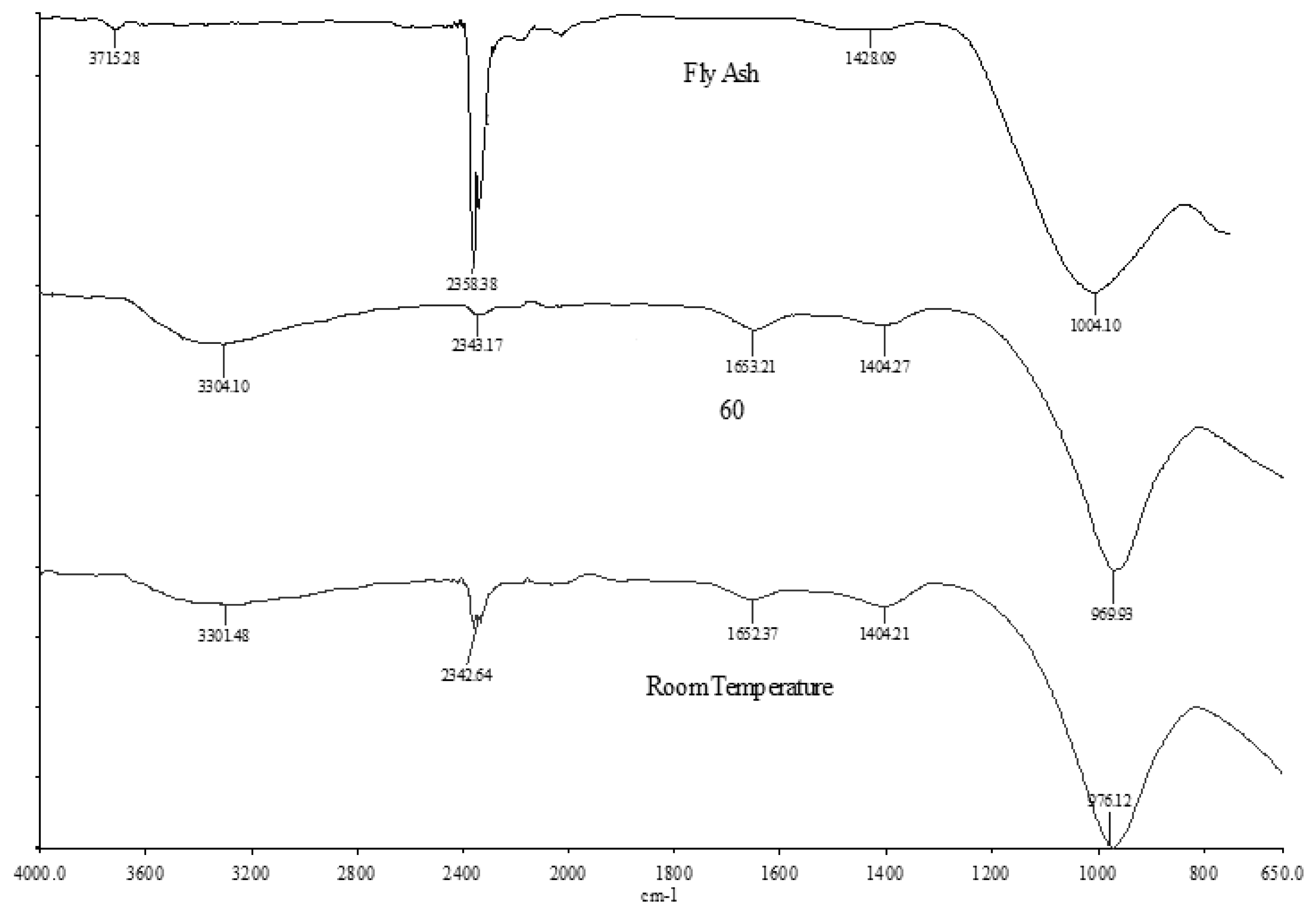
2.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.5. Microstructure Analysis
3. Experimental Section
3.1. Materials
3.2. Mix Design and Mixing Process
3.3. Testing
3.3.1. Compressive Strength
3.3.2. The Water Absorption
3.3.3. Porosity
3.3.4. X-ray Diffraction (XRD)
3.3.5. Scanning Electron Microscope (SEM)
3.3.6. Fourier Transform Infrared Spectroscopy (FTIR)
4. Conclusions
- The compressive strength of foamed geopolymer concrete LW2 with heat curing (60 °C) produced the maximum compressive strength on days 1, 7, and 28 (11.0, 17.6, and 18.2 MPa), respectively.
- The compressive strength of the LW2 samples was greater than the compressive strength of the LW1 samples. This was attributed to the fact that the porosity and water absorption of the LW2 samples, at 6.78% and 1.22% respectively, were lower than the porosity and water absorption of the LW1 samples, at 15.29% and 2.35%, respectively.
- Based on SEM observations, the LW2 samples had a denser matrix than the LW1 samples. This occurred because heat curing increased the rate of geopolymerization and hence, increased the strength. The LW1 samples had microcracks that resulted in increased water absorption and porosity, thus the strength was reduced.
Acknowledgments
References
- Narayanan, N.; Ramamurthy, K. Structure and properties of aerated concrete: A review. Cem. Concr. Compos 2000, 22, 321–329. [Google Scholar]
- Fahrizal, Z.; Mahyuddin, R. Performance and characteristic foamed concrete mix design with silica fume for housing development. Int. J. Acad. Res 2011, 3, 1198–1206. [Google Scholar]
- Brook, K.M.; Malhotra, V.M.; Croswell, S.F.; Atkinson, G. No-Fines Concrete; Cement and Concrete Association of Australia: North Sydney, Australia, 1999. [Google Scholar]
- National Ready Mixed Concrete Association (NRMCA), Structural Lightweight Concrete1; NRMCA: Silver Spring, MD, USA, 2003.
- Ramamurthy, K.; Kunhanandan Nambiar, E.K.; Indu Siva Ranjani, G. A classification of studies on properties of foam concrete. Cem. Concr. Compos 2009, 31, 388–396. [Google Scholar]
- Cement and Concrete Institute, Foamed Concrete; Cement and Concrete Institute: Midrand, South Africa, 2010.
- Kearsley, E.P.; Wainwright, P.J. The effect of high fly ash content on the compressive strength of foam concrete. Cem. Concr. Res 2001, 31, 105–112. [Google Scholar]
- De Rose, L.; Morris, J. The Influence of Mix Design on the Properties of Microcellular Concrete. In Specialist Techniques and Materials for Construction; Dhir, R.K., Handerson, N.A., Eds.; Thomas Telford: London, UK, 1999; pp. 185–197. [Google Scholar]
- Turner, M. Fast Set Foamed Concrete for Same Day Reinstatement of Openings in Highways. Proceedings of One Day Seminar on Foamed Concrete: Properties, Applications and Latest Technological Developments, Loughborough University: Leicestershire, UK, July 2001; pp. 12–18.
- Jones, M.R.; McCarthy, A. Behaviour and assessment of foamed concrete for construction application. In Use of Foamed Concrete in Construction; Dhir, R.K., Newlands, M.D., McCarthy, A., Eds.; Thomas Telford: London, UK, 2005; pp. 61–88. [Google Scholar]
- Jones, M.R.; McCarthy, A. Preliminary views on the potential of foamed concrete as a structural material. Mag. Concr. Res 2005, 57, 21–31. [Google Scholar]
- Jones, M.R.; McCarthy, A. Utilizing unprocessed low-lime coal ash in foamed concrete. Fuel 2005, 84, 1398–1409. [Google Scholar]
- Jones, M.R.; McCarthy, A. Heat of hydration in foamed concrete: Effect of mix constituent and plastic density. Cem. Concr. Res 2006, 36, 1032–1041. [Google Scholar]
- Papayianni, I.; Milud, I.A. Production of Foamed Concrete with High Calcium Fly Ash. In Use of Foamed Concrete in Construction; Dhir, R.K., Newlands, M.D., McCarthy, A., Eds.; Thomas Telford: London, UK, 2005; pp. 23–28. [Google Scholar]
- Pickford, C.; Crompton, S. Foam concrete in bridge construction. Concrete 1996, 14–15. [Google Scholar]
- Wee, T.H.; Babu, D.S.; Tamilselvan, T.; Lin, H.S. Air-void system of foamed concrete and its effect on mechanical properties. ACI Mater. J 2006, 103, 45–52. [Google Scholar]
- Kearsley, E.P. The Use of Foamed Concrete for Affordable Development in Third World Countries. In Appropriate Concrete Technology; Dhir, R.K., McCarthy, M.J., Eds.; E & FN Spon: London, UK, 1996; pp. 233–243. [Google Scholar]
- Byun, K.J.; Song, H.W.; Park, S.S. Development of structural lightweight foamed concrete using polymer foam agent. Int. Congr. Polym. Concr 1998, 1–9. [Google Scholar]
- Fujiwara, H.; Sawada, E.; Ishikawa, Y. Manufacturing of high strength aerated concrete containing silica fume. Proceedings of the Fifth International Conference on Fly Ash, Silica Fume, Slag and Natural Pozzolana in Concrete, Milwaukee, WI, USA, 4–9 June 1995; pp. 779–791.
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res 2007, 37, 1583–1589. [Google Scholar]
- Kumar, S.; Kumar, R.; Alex, T.C.; Bandopadhyay, A.; Mehrotra, S.P. Effect of mechanically activated fly ash on the properties of geopolymer cement. Proceedings of the 4th World Congress on Geopolymer, Saint-Quentin, France, 30 June–2 July 2005; pp. 113–116.
- Wallah, S.E. Creep Behaviour of Fly Ash-Based Geopolymer Concrete. Civ. Eng. Dimens 2010, 12, 73–78. [Google Scholar]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.J.; Rangan, B.V. On the development of fly ash based geopolymer concete. ACI Mater. J 2004, 101, 467–472. [Google Scholar]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristic of class c fly ash geopolymer. Cem. Concr. Compos 2010, 32, 142–147. [Google Scholar]
- Wallah, S.E.; Hardjito, D.; Sumajouw, D.M.J.; Rangan, B.V. Sulfate and acid resistance of fly ash-based geopolymer concrete. Proceedings of the Australian Structural Engineering Conference,, Newcastle, Australia, 11 October 2005.
- Song, X-J.; Marosszeky, M.; Brungs, M.; Chang, Z-T. Response of geopolymer concrete to sulphuric acid attack. Proceedings of World Congress Geopolymer, Saint-Quentin, France, 29 June–1 July 2005; pp. 157–160.
- Hardjito, D.; Rangan, B.V. Development and properties of low-calcium fly ash-based geopolymer concrete. In Research Report GC 1; Faculty of Engineering, Curtin University of Technology: Perth, Australia, 2005. [Google Scholar]
- Xu, H.; van Deventer, J.S.J.; Lukey, G.C. Effect of alkali metals on the preferential geopolymerization of stilbite/kaolinite mixtures. Ind. Eng. Chem. Res 2001, 40, 3749–3756. [Google Scholar]
- Abdullah, M.M.A.; Kamarudin, H.; Mohammed, H.; Khairul Nizar, I.; Rafiza, A.R.; Zarina, Y. The relationship of NaOH molarity, Na2SiO3/NaOH ratio, fly Ash/Alkaline activator ratio, and curing temperature to the strength of fly ash-based geopolymer. Adv. Mater. Res 2011, 328–330, 1475–1482. [Google Scholar]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloids Surf. A 2007, 301, 246–254. [Google Scholar]
- Swanepoel, J.C.; Strydom, C.A. Utilisation of fly Ash in a geopolymeric material. Appl. Geochem 2002, 17, 1143–1148. [Google Scholar]
- Fernandez-Jimenez, A.; Palomo, A. Composition and microstructure of alkali activated fly Ash binder: Effect of the activator. Cem. Concr. Res 2005, 35, 1984–1992. [Google Scholar]
- Lee, W.K.W.; van Deventer, J.S.J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A 2002, 211, 115–126. [Google Scholar]
- Lee, W.K.W.; van Deventer, J.S.J. Structural reorganization of class F Fly Ash in alkaline silicate solutions. Colloids Surf. A 2002, 211, 49–66. [Google Scholar]
- Fernandez-Jimenez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali activated fly ash structure. Microporous Mesoporous Mater 2005, 86, 207–214. [Google Scholar]
- Criado, M.; Palomo, A.; Fernandez-Jimenez, A. Alkali activation of fly ashes. Part 1. Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar]
- Palomo, A.; Blanco-Varela, M.T.; Granizo, M.L.; Puertas, F.; Vazquez, T.; Grutzeck, M.W. Chemical stability of cementitious materials based on metakaolin. Cem. Concr. Res 1999, 29, 997–1004. [Google Scholar]
- Fernandez-Jimenez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res 2005, 35, 1204–1209. [Google Scholar]
- Fernandez-Jimenez, A.; Palomo, A. Characterization of fly ashes, potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar]
- Bakri, A.M.M.A.; Kamarudin, H.; Bnhussain, M.; Khairul Nizar, I.; Rafiza, A.R.; Zarina, Y. Microstructure of different naoh molarity of fly ash-based green polymeric cement. J. Eng. Technol. Res 2011, 3, 44–49. [Google Scholar]
- Nehdi, M.; Djebbar, Y.; Khan, A. Neural network model for preformed-foam cellular concrete. ACI Mater. J 2001, 98, 402–409. [Google Scholar]





| Chemical Composition | Fly Ash | Samples Cured at Room Temperature (LW1) | Samples Cured at 60 °C (LW2) |
|---|---|---|---|
| SiO2 | 26.4 | 35.1 | 37.6 |
| Al2O3 | 9.3 | 11.8 | 12.8 |
| CaO | 21.6 | 19.6 | 18.7 |
| Fe2O3 | 30.1 | 23.3 | 21.6 |
| MnO | 0.3 | 0.2 | 0.2 |
| TiO2 | 3.1 | 2.3 | 2.10 |
| K2O | 2.6 | 2.7 | 2.7 |
| SO3 | 1.3 | 0.9 | 0.8 |
| Sample | Curing | Compressive Strength (Mpa) | Porosity (%) | Water Absorption (%) | Density (kg/m3) | ||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 28 | |||||
| LW1 | Room temp. | 3.3 | 13.5 | 18.1 | 15.29 | 2.35 | 1650 |
| LW2 | 60 °C | 11.0 | 17.6 | 18.2 | 6.78 | 1.22 | 1667 |
| Bonds | Fly Ash (cm−1) | LW1 (cm−1) | LW2 (cm−1) |
|---|---|---|---|
| Stretching vibration (OH, H-O-H) [24,33,37] | 3715–2358 | 3301–2333 | 3304–2343 |
| Bending vibration (H-O-H) [30] | - | 1652 | 1653 |
| Stretching vibration (O-C-O) [31,33,35] | 1437 | - | - |
| Asymmetric stretching (Si-O-Si & Al-O-Si) [31–36] | 1082 | 970 | 969 |
| Sample | Fly Ash: Activator | Sodium Silicate: NaOH (Activator) | Foam: Geopolymer Paste | Curing Temperature |
|---|---|---|---|---|
| LW1 | 2:1 | 2.5:1 | 2:1 | Room temperature |
| LW2 | 2:1 | 2.5:1 | 2:1 | 60°C |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abdullah, M.M.A.B.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Yahya, Z.; Abdul Razak, R. Fly Ash-based Geopolymer Lightweight Concrete Using Foaming Agent. Int. J. Mol. Sci. 2012, 13, 7186-7198. https://doi.org/10.3390/ijms13067186
Abdullah MMAB, Hussin K, Bnhussain M, Ismail KN, Yahya Z, Abdul Razak R. Fly Ash-based Geopolymer Lightweight Concrete Using Foaming Agent. International Journal of Molecular Sciences. 2012; 13(6):7186-7198. https://doi.org/10.3390/ijms13067186
Chicago/Turabian StyleAbdullah, Mohd Mustafa Al Bakri, Kamarudin Hussin, Mohamed Bnhussain, Khairul Nizar Ismail, Zarina Yahya, and Rafiza Abdul Razak. 2012. "Fly Ash-based Geopolymer Lightweight Concrete Using Foaming Agent" International Journal of Molecular Sciences 13, no. 6: 7186-7198. https://doi.org/10.3390/ijms13067186
APA StyleAbdullah, M. M. A. B., Hussin, K., Bnhussain, M., Ismail, K. N., Yahya, Z., & Abdul Razak, R. (2012). Fly Ash-based Geopolymer Lightweight Concrete Using Foaming Agent. International Journal of Molecular Sciences, 13(6), 7186-7198. https://doi.org/10.3390/ijms13067186





