Synthesis and Electrochemical Proprieties of Novel Unsymmetrical Bis-Tetrathiafulvalenes and Electrical Conductivity of Their Charge Transfer Complexes with Tetracyanoquinodimethane (TCNQ)
Abstract
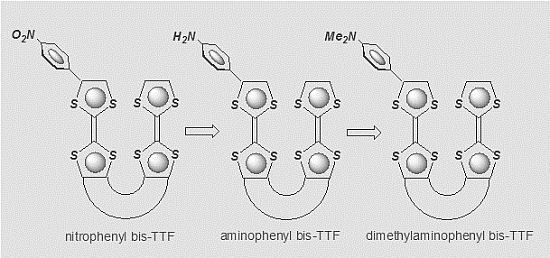
:1. Introduction
2. Results and Discussion
2.1. Electrochemical Studies
2.2. Preparation and Electrical Conductivity of Charge Transfer Complexes
3. Experimental Section
3.1. General
3.2. 2-Methylthio-4-(p-nitrophenyl)-1,3-dithiolium Trifluoromethane Sulfonate 2
3.3. 2-Methylthio-4-(p-nitrophenyl)-1,3-dithiole 3
3.4. 4-(p-Nitrophenyl)-1,3-dithiolium Tetrafluoroborate 4
3.5. 2,3-Bis(2-cyanoethylthio)-6-p-nitrophenyl Tetrathiafulvalene 9
3.5.1. Method 1
3.5.2. Method 2
3.6. Synthesis of Mono-TTFs 10a and 10b
3.7. Synthesis of Bis-TTFs 11a and 11b
3.8. Synthesis of Bis-TTFs 12a and 12b
3.9. Synthesis of Bis-TTFs 13a and 13b
3.10. Synthesis of Bis-TTFs 14a and 14b
4. Conclusions
Acknowledgments
References
- Ferraris, J.; Cowan, D.O.; Walatka, V.; Perlstein, J.H. Electron transfer in a new highly conducting donor-acceptor complex. J. Am. Chem. Soc 1973, 95, 948–949. [Google Scholar]
- Segura, J.L.; Martin, N. New concepts in tetrathiafulvalene chemistry. Angew. Chem. Int. Ed 2001, 40, 1372–1409. [Google Scholar]
- Fabre, J.M. Dimensionality and electrical properties in organic synthetic metals—Current results through selected recent examples. J. Solid State Chem 2002, 168, 367–383. [Google Scholar]
- Clemente-León, M.; Coronado, E.; Galan-Mascaros, J.R.; Gime-nez-Saiz, C.; Gomez-Garcia, C.J.; Fabre, J.M.; Mousdis, G.A.; Papavassiliou, G.C. Hybrid molecular materials based upon organic π-electron donors and inorganic metal complexes. conducting salts of bis(ethylenediseleno)tetrathiafulvalene (BEST) with the octahedral anions hexacyanoferrate(III) and nitroprusside. J. Solid State Chem 2002, 168, 616–625. [Google Scholar]
- Devic, T.; Evain, M.; Moelo, Y.; Canadell, E.; Auban-Senzier, P.; Fourmigué, M.; Batail, P. Single crystalline commensurate metallic assemblages of π-slabs and CdI2-type layers: Synthesis and properties of β-(EDT-TTF-I2)2[Pb5/61/6I2]3 and β-(EDT-TTF-I2)2[Pb2/3+xAg1/3-2xxI2]3, x = 0.05. J. Am. Chem. Soc 2003, 125, 3295–3301. [Google Scholar]
- Suzuki, W.; Fujiwara, E.; Kobayashi, A.; Fujishiro, Y.; Nishibori, E.; Takata, M.; Sakata, M.; Fujiwara, H.; Kobayashi, H. Highly conducting crystals based on single-component gold complexes with extended-TTF dithiolate ligands. J. Am. Chem. Soc 2003, 125, 1486–1487. [Google Scholar]
- Nishikawa, H.; Machida, A.; Morimoto, T.; Kikuchi, K.; Kodama, T.; Ikemoto, I.; Yamada, J.-I.; Yoshino, H.; Murata, K. A new organic superconductor, (DODHT)2BF4·H2O. Chem. Commun 2003, 494–495. [Google Scholar]
- Yamada, J.I.; Toita, T.; Akutsu, H.; Nakatsuji, S.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K.; Choi, E.S.; Graf, D.; Brooks, J.S. A new organic superconductor, β-(BDA-TTP)2GaCl4[BDA-TTP = 2,5-(1,3-dithian-2-ylidene)-1,3,4,6-tetrathiapentalene]. Chem. Commun 2003, 2230–2231. [Google Scholar]
- Coronado, E.; Galan-Mascaros, J.R.; Gimenez-Saiz, C.; Gomez-Garcia, C.J.; Ruis-Perez, C. Hybrid organic/inorganic molecular materials formed by tetrathiafulvalene radicals and magnetic trimeric clusters of dimetallic oxalate-bridged complexes: The series (TTF)4{MII(H2O)2[MIII(ox)3]2}·nH2O (MII = Mn, Fe, Co, Ni, Cu and Zn; MIII = Cr and Fe; ox = C2O42−). Eur. J. Inorg. Chem 2003, 2003, 2290–2298. [Google Scholar]
- Miyazaki, A.; Okabe, K.; Enomoto, K.; Nishijo, J.; Enoki, T.; Setifi, F.; Golhen, S.; Ouahab, L.; Toita, T.; Yamada, J.I. π-d Interaction-based molecular magnets. Polyhedron 2003, 22, 2227–2234. [Google Scholar]
- Enoki, T.; Yamazaki, H.; Okabe, K.; Enomoto, K.; Kato, T.; Miyazaki, A.; Ogura, E.; Kuwqatani, Y.; Iyoda, M. Unconventional TTF-based molecular magnets. Synth. Met 2003, (133–134), 501–503. [Google Scholar]
- Otero, M.; Herranz, M.A.; Seoane, C.; Martin, N.; Garin, J.; Orduna, J.; Alcala, R.; Villacampa, B. Synthesis and properties of push-pull chromophores for second-order nonlinear optics derived from π-extended tetrathiafulvalenes (TTFs). Tetrahedron 2002, 58, 7463–7475. [Google Scholar]
- Farren, C.; Christensen, C.A.; Fitzgerald, S.; Bryce, M.R.; Beeby, A. Synthesis of novel phthalocyanine-tetrathiafulvalene hybrids; intramolecular fluorescence quenching related to molecular geometry. J. Org. Chem 2002, 67, 9130–9139. [Google Scholar]
- Bryce, M.R. Functionalised tetrathiafulvalenes: New applications as versatile π-electron systems in materials chemistry. J. Mater. Chem 2000, 10, 589–598. [Google Scholar]
- Asakawa, M.; Ashton, P.R.; Balzani, V.; Credi, A.; Hamers, C.; Mattersteig, G.; Montalti, M.; Shipway, A.N.; Spencer, N.; Stoddart, J.F.; et al. A chemically and electrochemically switchable [2] catenane incorporating a tetrathiafulvalene unit. Angew. Chem. Int. Engl 1998, 37, 333–337. [Google Scholar]
- Laukhina, E.; Vidal-Gancedo, J.; Laukhin, V.; Veciana, J.; Chuev, I.; Tkacheva, V.; Wurst, K.; Rovira, C. Multistability in a BEDT-TTF based molecular conductor. J. Am. Chem. Soc 2003, 125, 3948–3953. [Google Scholar]
- Nishikawa, H.; Machida, A.; Morimoto, T.; Kikuchi, K.; Kodama, T.; Ikemoto, I.; Yamada, J.I.; Yoshino, H.; Murata, K. A new organic superconductor, (DODHT)2BF4·H2O. Chem. Commun 2003, 494–495. [Google Scholar]
- Martin, N.; Sanchez, L.; Guldi, D.M. Stabilisation of charge-separated states via gain of aromaticity and planarity of the donor moiety in C60-based dyads. Chem. Commun 2000, 113–114. [Google Scholar]
- Roncali, J. Linearly extended π-donors: When tetrathiafulvalene meets conjugated oligomers and polymers. J. Mater. Chem 1997, 7, 2307–2321. [Google Scholar]
- Hansen, T.K.; Jorgensen, T.; Stein, P.C.; Becher, J. Crown ether derivatives of tetrathiafulvalene. J. Org. Chem 1992, 57, 6403–6409. [Google Scholar]
- Bengs, H.; Ebert, M.; Karthaus, O.; Kohne, B.; Praefcke, K.; Ringsdorf, H.; Wendorff, J.H.; Wustefeld, R. Induction and variation of discotic columnar phases through doping with electron acceptors. Adv. Mater 1990, 2, 141–144. [Google Scholar]
- Bryce, M.R.; Devonport, W.; Goldenberg, L.M.; Wang, C. Macromolecular tetrathiafulvalene chemistry. Chem. Commun 1998, 9, 945–952. [Google Scholar]
- Abbaz, T.; Gouasmia, A.K.; Fujiwara, H.; Hiraoka, T.; Sugimoto, T.; Taillefer, M.; Fabre, J.M. New TTF and bis-TTF containing thiophene units: Electrical properties of the resulting salts. Synth. Met 2007, 157, 508–516. [Google Scholar]
- Subias, G.; Abbaz, T.; Fabre, J.M.; Fraxedas, J. Characterization of the anion-ordering transition in (TMTTF)2ReO4 by x-ray absorption and photoemission spectroscopies. Phys. Rev. B 2007, 76, 085103–085107. [Google Scholar]
- Lamère, J.F.; Malfant, I.; Sournia-Saquet, A.; Lacroix, P.G.; Fabre, J.M.; Kaboub, L.; Abbaz, T.; Gouasmia, A.K.; Asselberghs, I.; Clays, K. Quadratic nonlinear optical response in partially charged donor-substituted tetrathiafulvalene: From a computational investigation to a rational synthetic feasibility. Chem. Mater 2007, 19, 805–815. [Google Scholar]
- Clausen, R.P.; Becher, J. The 1,2,3-thiadiazole route to new vinylogue tetrathiafulvalenes. Tetrahedron 1996, 52, 3171–3188. [Google Scholar]
- Binet, L.; Fabre, J.M.; Montginoul, C.; Simonsen, K.B.; Becker, J. Reparation and chemistry of new unsymmetrically substituted tetrachalcogenofulvalenes bearing CN(CH2)2X and HO(CH2)2X groups (X = S or Se). J. Chem. Soc. Perkin Trans 1996, 1, 783–788. [Google Scholar]
- Campaigne, E.; Hamilton, R.D.; Jacobsen, N.W. Dithiolium derivatives. II. Some new 1,3-dithiolium perchlorates. J. Org. Chem 1964, 29, 1708–1710. [Google Scholar]
- Misaki, Y.; Nishikawa, H.; Kawakami, K.; Yamabe, T.; Mori, T.; Inokuchi, H.; Mori, H.; Tanaka, S. Ethylenedioxy substituted 2,5-bis(1′,3′-dithiol-2′-ylidene)-1,3,4,6-tetrathiapentalenes and their conducting salts. Chem. Lett 1993, 22, 2073–2076. [Google Scholar]
- Binet, L.; Fabre, J.M. Synthesis of new functionalized π-electron donors: Primary hydroxy and primary amino multisulfur tetrathiafulvalenes. Synthesis 1997, 10, 1179–1184. [Google Scholar]
- Akamatu, H.; Inokuchi, H.; Matsunaga, Y. Organic semiconductors with high conductivity. I. Complexes between polycyclic aromatic hydrocarbons and halogens. Bull. Chem. Soc. Jpn 1956, 29, 213–218. [Google Scholar]
- Acker, D.S.; Harder, R.J.; Hertler, W.R.; Mahler, W.; Melby, L.R.; Benson, R.E.; Mochel, W.E. 7,7,8,8-tetracyanoqunodimethane and its electrically conducting anion-radical derivatives. J. Am. Chem. Soc 1960, 82, 6408–6409. [Google Scholar]
- Wudl, F.; Smith, G.M.; Hufnagel, E. 1,3-Bis-dithiole chloride. J. Chem. Soc. D Chem. Commun 1970, 1453–1954. [Google Scholar]




| Donor | E1ox (mV) | E2ox (mV) | E3ox (mV) | ΔEox (mV) |
|---|---|---|---|---|
| 9 | 652 | 988 | - | 336 |
| 10a | 643 | 980 | - | 337 |
| 10b | 640 | 977 | - | 337 |
| 11a | 553 | 687 | 1018 | 465 |
| 11b | 550 | 684 | 1014 | 464 |
| 12a | 538 | 673 | 1004 | 466 |
| 12b | 536 | 670 | 1001 | 465 |
| 13a | 514 | 646 | 978 | 464 |
| 13b | 512 | 643 | 974 | 462 |
| 14a | 530 | 665 | 996 | 466 |
| 14b | 528 | 663 | 994 | 466 |
| Complex | σRT (S cm−1) | mp (°C) |
|---|---|---|
| 9-TCNQ | 1.30 × 10−2 | 265 |
| 10a-TCNQ | 3.45 × 10−1 | 215 |
| 10b-TCNQ | 8.60 × 10−1 | 209 |
| 11a-TCNQ | 4.36 × 10−3 | 231 |
| 11b-TCNQ | 6.82 × 10−3 | 226 |
| 12a-TCNQ | 0.27 × 10−3 | 274 |
| 12b-TCNQ | 2.85 × 10−3 | 268 |
| 13a-TCNQ | 4.35 × 10−4 | 234 |
| 13b-TCNQ | 7.20 × 10−4 | 228 |
| 14a-TCNQ | 0.54 × 10−6 | 247 |
| 14b-TCNQ | 1.30 × 10−6 | 239 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abbaz, T.; Bendjeddou, A.; Gouasmia, A.; Regainia, Z.; Villemin, D. Synthesis and Electrochemical Proprieties of Novel Unsymmetrical Bis-Tetrathiafulvalenes and Electrical Conductivity of Their Charge Transfer Complexes with Tetracyanoquinodimethane (TCNQ). Int. J. Mol. Sci. 2012, 13, 7872-7885. https://doi.org/10.3390/ijms13077872
Abbaz T, Bendjeddou A, Gouasmia A, Regainia Z, Villemin D. Synthesis and Electrochemical Proprieties of Novel Unsymmetrical Bis-Tetrathiafulvalenes and Electrical Conductivity of Their Charge Transfer Complexes with Tetracyanoquinodimethane (TCNQ). International Journal of Molecular Sciences. 2012; 13(7):7872-7885. https://doi.org/10.3390/ijms13077872
Chicago/Turabian StyleAbbaz, Tahar, Amel Bendjeddou, Abdelkrim Gouasmia, Zine Regainia, and Didier Villemin. 2012. "Synthesis and Electrochemical Proprieties of Novel Unsymmetrical Bis-Tetrathiafulvalenes and Electrical Conductivity of Their Charge Transfer Complexes with Tetracyanoquinodimethane (TCNQ)" International Journal of Molecular Sciences 13, no. 7: 7872-7885. https://doi.org/10.3390/ijms13077872