Adsorption of Sodium Dodecyl Sulfate on Ge Substrate: The Effect of a Low-Polarity Solvent
Abstract
:1. Introduction
2. Results and Discussion
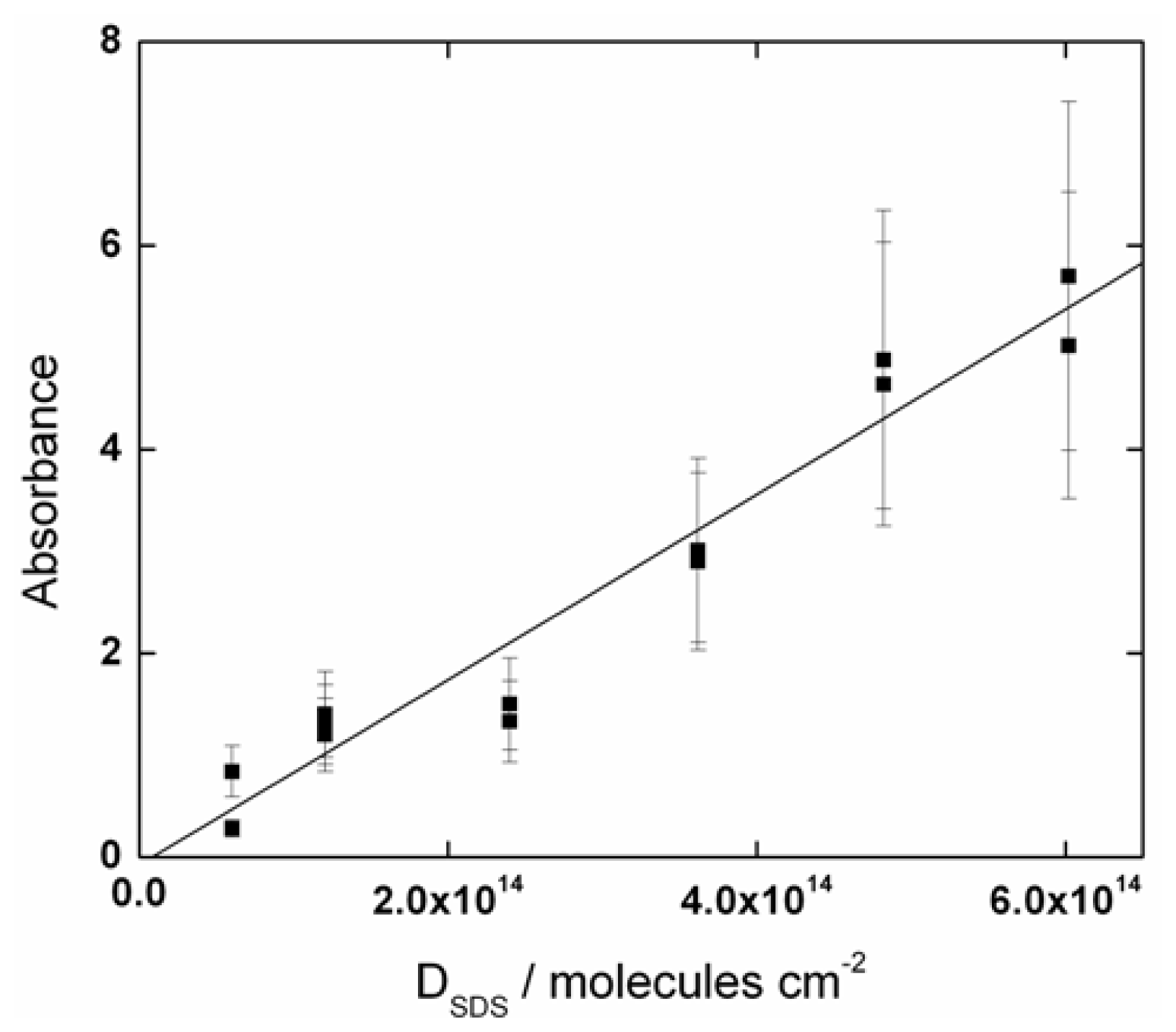
2.1. SDS Deposition and Adsorption Analysis
2.2. Atomic Force Microscopy Results
2.3. Infrared Spectroscopy of SDS Adsorbed on Germanium Substrate
2.4. Linear Dichroism Measurements
3. Experimental Section
4. Conclusions
Acknowledgments
References
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed; Wiley-Interscience: New York, NY, USA, 2004. [Google Scholar]
- Paria, S.; Khilar, K.C. A review on experimental studies of surfactant adsorption at the hydrophilic solid–water interface. Adv. Colloid Interface Sci 2004, 110, 75–95. [Google Scholar]
- Mobius, D.; Miller, R. Organized Monolayers and Assemblies: Structure, Processes and Function; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Holler, F.; Callis, J.B. Conformation of the hydrocarbon chains of sodium dodecyl sulfate molecules in micelles: An FTIR Study. J. Phys. Chem 1989, 93, 2053–2058. [Google Scholar]
- Sperline, R.P.; Song, Y.; Freiser, H. Fourier transform infrared attenuated total reflection spectroscopy linear dichroism study of sodium dodecyl sulfate adsorption at the Al2O3/Water interface using Al2O3-Coated optics. Langmuir 1992, 8, 2183–2191. [Google Scholar]
- Sperline, R.P. Infrared spectroscopic study of the crystalline phases of sodium dodecyl sulfate. Langmuir 1997, 13, 3715–3726. [Google Scholar]
- Sperline, R.P.; Song, Y.; Freiser, H. Temperature dependent structure of adsorbed sodium dodecyl sulfate at the Al2O3/water interface. Langmuir 1997, 13, 3727–3732. [Google Scholar]
- Manne, S.; Cleveland, J.P.; Gaub, H.E.; Stucky, G.D.; Hansma, P.K. Direct visualization of surfactant hemimicelles by force microscopy of the electrical double layer. Langmuir 1994, 10, 4409–4413. [Google Scholar]
- Patrick, H.N.; Warr, G.G.; Manne, S.; Aksay, I.A. Surface micellization patterns of quaternary ammonium surfactants on Mica. Langmuir 1999, 15, 1685–1692. [Google Scholar]
- Paruchuri, V.K.; Nalaskovski, J.; Shah, D.O.; Miller, J.D. The effect of cosurfactants on sodium dodecyl sulfate micellar structures at a graphite surface. Colloids Surf. A 2006, 272, 157–163. [Google Scholar]
- Prosser, A.J.; Franses, E.I. Infrared reflection absorption spectroscopy (IRRAS) of aqueous nonsurfactant salts, ionic surfactants, and mixed ionic surfactants. Langmuir 2002, 18, 9234–9242. [Google Scholar]
- Conboy, J.C.; Messmer, M.C.; Richmond, G.L. Investigation of surfactant conformation and order at the liquid-liquid interface by total internal reflection sum-frequency vibrational spectroscopy. J. Phys. Chem 1996, 100, 7617–7622. [Google Scholar]
- Li, H.; Tripp, C.P. Use of infrared bands of the surfactant headgroup to identify mixed surfactant structures adsorbed on Titania. J. Phys. Chem. B 2004, 108, 18318–18326. [Google Scholar]
- Kawai, T.; Kamio, H.; Kondo, T.; Kon-No, K. Effects of concentration and temperature on SDS monolayers at the air-solution interface studied by infrared external reflection spectroscopy. J. Phys. Chem. B 2005, 109, 4497–4500. [Google Scholar]
- Roberts, G. Langmuir-Blodgett Films; Plenum Press: New York, NY, USA, 1990. [Google Scholar]
- Kimura, F.; Umemura, J.; Takenaka, T. FTIR-ATR studies on langmuir-blodgett films of stearic acid with 1–9 monolayers. Langmuir 1986, 2, 96–101. [Google Scholar]
- Wen, B.M.; Liu, C.Y.; Liu, Y. Sovolthermal synthesis of ultralong single-crystalline TiO2 nanowire. New J. Chem 2005, 29, 969–971. [Google Scholar]
- Su, C.Y.; Lin, H.C.; Yang, T.K.; Lin, C.K. The effect of vacuum annealing on the structure and surface chemistry of iron nanoparticles. J. Nanopart. Res 2010, 12, 1755–1765. [Google Scholar]
- Gong, J.; Zu, X.; Li, Y.; Mu, W.; Deng, Y. Janus particles with tunable coverage of zinc oxide nanowires. J. Mater. Chem 2011, 21, 2067–2069. [Google Scholar]
- Liu, Z.; Hu, Z.; Xie, Q.; Yang, B.; Wu, J.; Qian, Y. Surfactant-assisted growth of uniform nanorods of crystalline tellurium. J. Mater. Chem 2003, 13, 159–162. [Google Scholar]
- Sun, D.; Riley, A.E.; Cadby, A.J.; Richman, E.K.; Korlann, S.D.; Tolbert, S.H. Hexagonal nanopourous germanium through surfactant-driven self-assembly of Zintl clusters. Nature 2006, 441, 1126–1130. [Google Scholar]
- Collic, M.; Fuerstenau, D.W. Influence of the dielectric constant of the media on oxide stability in surfactant solutions. Langmuir 1997, 13, 6644–6649. [Google Scholar]
- Kandel, D.; Kaxiras, E. The surfactant effect in semiconductor thin-film growth. Solid State Phys 1999, 54, 219–262. [Google Scholar]
- Bakkers, E.P.; van Dam, J.A.; De Franceschi, S.; Kouwenhoven, L.P.; Kaiser, M.; Verheijen, M.; Wondergem, H.; van der Sluis, P. Epitaxial growth of InP nanowires on germanium. Nat. Mater 2004, 3, 769–773. [Google Scholar]
- Bosi, M.; Anttolini, G. Germanium: Epitaxy and its applications. Prog. Cryst. Growth Charact 2010, 56, 146–174. [Google Scholar]
- Loscutoff, P.W.; Bent, S.F. Reactivity of the germanium surface: Chemical passivation and functionalization. Ann. Rev. Phys. Chem 2006, 57, 467–495. [Google Scholar]
- Buriak, J.M. Organometallic Chemistry on Silicon and Germanium Surfaces. Chem. Rev 2002, 102, 1271–1308. [Google Scholar]
- Pei, L.Z.; Cai, Z.Y. A Review on Germanium Nanowires. Recent Pat Nanotechnol 2012, 6, 44–59. [Google Scholar]
- Tang, J.; Wang, C.Y.; Xiu, F.; Zhou, Y.; Chen, L.J.; Wang, K.L. Formation and device application of Ge nanowire heterostructures via rapid thermal annealing. Adv. Mater. Sci. Eng 2011, 2011, 1–16. [Google Scholar]
- Xing, R.; Rankin, S.E. Three-stage multilayer formation kinetics during adsorption of an anionic fluorinated surfactant onto germanium. 1. Concentration Effect. J. Phys. Chem. B 2006, 110, 295–304. [Google Scholar]
- Biswas, S.C.; Chattoraj, D.K. Kinetics adsorption of cationic surfactants at silica-water interface. J. Colloid Interface Sci 1994, 205, 12–20. [Google Scholar]
- Ahn, D.J.; Franses, E.I. Determination of molecular orientations in Langmuir-Blodgett films by polarized Fourier transform IR attenuated total reflection and transmission spectroscopy. Thin Solid Films 1994, 244, 971–976. [Google Scholar]
- Hidalgo, A.A.; Pimentel, A.S.; Tabak, M.; Oliveira, O.N. Thermodynamic and infrared analyses of the interaction of chlorpromazine with phospholipids monolayer. J. Phys. Chem. B 2006, 110, 19637–19646. [Google Scholar]
- Esumi, K.; Kobayashi, T.; Meguro, K. Characterization of surfactants layer on alumina and water-dioxane mixtures. Colloids Surf 1991, 54, 189–196. [Google Scholar]
- Zhang, Z.; Kitamori, T.; Sawada, T.; Tsuyumoto, I. Effect of organic phase on dynamic and collective behavior of surfactants at liquid/liquid interfaces by a time-resolved quasi-elastic laser-scattering method. Anal. Sci 2000, 16, 1199–1202. [Google Scholar]
- Schmitt, F.J.; Muller, M. Conformation and orientation analysis of modified polyglutamates in thin films by ATR infrared spectroscopy. Thin Solid Films 1997, 310, 138–147. [Google Scholar]
- Martin, I.; Goormaghtigh, E.; Ruysschaert, J.M. Attenuated total reflection IR spectroscopy as a tool to investigate the orientation and tertiary structure changes in fusion proteins. Biochim. Biophys. Acta 2003, 1614, 97–103. [Google Scholar]
- Snyder, R.G.; Hsu, S.L.; Krimm, S. Vibrational spectp in the C-H stretching region and the structure of the polymethylene chain. Spectrochim. Acta A 1978, 34, 395–406. [Google Scholar]
- Snyder, R.G. Vibrational study of chain conformation of liquid n-paraffins and molten polyethylene. J. Chem. Phys 1967, 47, 1316–1360. [Google Scholar]
- Snyder, R.G. Vibrational spectra of crystalline n-paraffins: 2. intermolecular effects. J. Mol. Spectrosc 1964, 7, 116–144. [Google Scholar]
- Wong, T.K.; Wong, N.B.; Tanner, P.A. Fourier transform IR study of the phase transitions and molecular order in the hexadecyltrimethylammonium sulfate/water system. J. Colloid Interface Sci 1997, 186, 325–331. [Google Scholar]
- Weers, J.G.; Scheuring, D.R. Structure/performance relationships in monoalkyl/diakyl cationic surfactant mixtures. J. Colloid Interface Sci 1991, 145, 563–580. [Google Scholar]
- Scheuing, D.R.; Weers, J.G. A Fourier transform infrared spectroscopic study of dodecyltrimethylammonium chloride/sodium dodecyl sulfate surfactant mixtures. Langmuir 1990, 6, 665–671. [Google Scholar]
- Vaia, R.A.; Teukolsky, R.K.; Giannelis, E.P. Interlayer structure and molecular environment of alkylammonium layered silicates. Chem. Mater 1994, 6, 1017–1022. [Google Scholar]
- Venkataraman, N.V.; Vasudevan, S. Interdigitation of an intercalated surfactant bilayer. J. Phys. Chem. B 2001, 105, 7639–7650. [Google Scholar]
- Prosser, A.J.; Franses, E.I. Adsorption and surface tension of ionic surfactants at the air–water interface: review and evaluation of equilibrium models. Colloids Surf. A 2001, 178, 1–40. [Google Scholar]
- Gaikar, V.G.; Padalkar, K.V.; Aswal, V.K. Characterization of mixed micelles of structural isomers of sodium butyl benzene sulfonate and sodium dodecyl sulfate by SANS, FTIR spectroscopy and NMR spectroscopy. J. Mol. Liq 2008, 138, 155–167. [Google Scholar]
- Padalkar, K.V.; Gaikar, V.G.; Aswal, V.K. Characterization of mixed micelles of sodium cumene sulfonate with sodium dodecyl sulfate and cetyl-trimethylammonium bromide by SANS, FTIR spectroscopy and NMR spectroscopy. J. Mol. Liq 2009, 144, 40–49. [Google Scholar]
- Kawai, T.; Umemura, J.; Takenaka, T.; Kodoma, M.; Seki, S. Fourier transform infrared study on the phase transitions of an octadecyltrimethylammonium chloride-water system. J. Colloid Interface Sci 1985, 103, 56–61. [Google Scholar]
- Dobson, K.D.; Roddick–Lanzilotta, A.D.; McQuillan, A.J. An in situ infrared spectroscopic investigation of adsorption of sodium dodecyl sulfate and of cetyltrimethylammonium bromide surfactants to TiO2, ZrO2, Al2O3, and Ta2O4 Particle Films from Aqueous Solutions. Vib. Spectrosc 2000, 24, 287–295. [Google Scholar]
- Viana, R.B.; da Silva, A.B.F; Pimentel, A.S. Infrared Spectroscopy of Anionic, Cationic, and Zwitterionic Surfactants. Adv. Phys. Chem 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Harrick, N.J. Internal Reflection Spectroscopy; Harrick Scientific: New York, NY, USA, 1987. [Google Scholar]
- Neivandt, D.J.; Gee, M.L.; Hair, M.L.; Tripp, C.P. Polarized Infrared Attenuated Total Reflection for the in Situ Determination of the Orientation of Surfactant Adsorbed at the Solid/Solution Interface. J. Phys. Chem. B 1998, 102, 5107–5114. [Google Scholar]
- Haller, G.L.; Rice, R.W. Study of adsorption on single crystals by internal reflectance spectroscopy. J. Phys. Chem 1970, 74, 4386–4393. [Google Scholar]
- Poirier, J.S.; Tripp, C.P.; Neivandt, D.J. Templated surfactant readsorption on polyelectrolyte-induced depleted surfactant surfaces. Langmuir 2005, 21, 2876–2880. [Google Scholar]
- Singh, P.K.; Adler, J.J.; Rabinovitch, Y.I.; Moudgil, B.M. Investigation of self-assembled surfactant structures at the solid-liquid interface using FT-IR/ATR. Langmuir 2001, 17, 468–473. [Google Scholar]
- Muller, M.; Kessler, B.; Lunkwitz, K. Induced orientation of α-helical polypeptides in polyelectrolyte multilayers. J. Phys. Chem. B 2003, 107, 8189–8197. [Google Scholar]
- Marsh, D. Quantitation of secondary structure in ATR infrared spectroscopy. Biophys. J 1999, 77, 2630–2637. [Google Scholar]
- Ahn, D.J.; Franses, E.I. Orientations of chain axes and transition moments in Langmuir-Blodgett monolayer determined by polarized FTIR-ATR spectroscopy. J. Phys. Chem 1992, 96, 9952–9959. [Google Scholar]
- Ahn, D.J.; Franses, E.I. Determination of molecular orientations in Langmuir-Blodgett films by polarized Fourier transform IR attenuated total reflection and transmission spectroscopy. Thin Solid Films 1994, 244, 971–976. [Google Scholar]
- Lobo, R.F.M.; Pereira-da-Silva, M.A.; Raposo, M.; Faria, R.M.; Oliveira, O.N. In situ thickness measurements of ultra-thin multilayer polymer films by atomic force microscopy. Nanotechnology 1999, 10, 389–393. [Google Scholar]
- Ivanov, D.; Dubreuil, N.; Raussens, V.; Ruysschaert, J.M.; Goormaghtigh, E. Evaluation of the ordering of membranes in multilayer stacks built on an ATR-FTIR germanium crystal with atomic force microscopy: The case of the H+,K+-ATPase-containing gastric tubulovesicle membranes. Biophys. J 2004, 87, 1307–1315. [Google Scholar]


| CH2 | CH3 | ||
|---|---|---|---|
| νa (α = 0) | νs (α = 0) | νa (α = 90) | |
| A⊥ | 1.591 | 0.724 | 0.251 |
| A// | 2.703 | 1.272 | 0.324 |
| LD | 0.589 | 0.569 | 0.775 |
| γ (α = 0°) | 48.1 | 47.0 | - |
| S (α = 0°) | 0.169 | 0.198 | - |
| γ (α = 90°) | - | - | 47.7 |
| S (α = 90°) | - | - | 0.179 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Viana, R.B.; Da Silva, A.B.F.; Pimentel, A.S. Adsorption of Sodium Dodecyl Sulfate on Ge Substrate: The Effect of a Low-Polarity Solvent. Int. J. Mol. Sci. 2012, 13, 7980-7993. https://doi.org/10.3390/ijms13077980
Viana RB, Da Silva ABF, Pimentel AS. Adsorption of Sodium Dodecyl Sulfate on Ge Substrate: The Effect of a Low-Polarity Solvent. International Journal of Molecular Sciences. 2012; 13(7):7980-7993. https://doi.org/10.3390/ijms13077980
Chicago/Turabian StyleViana, Rommel B., Albérico B. F. Da Silva, and André S. Pimentel. 2012. "Adsorption of Sodium Dodecyl Sulfate on Ge Substrate: The Effect of a Low-Polarity Solvent" International Journal of Molecular Sciences 13, no. 7: 7980-7993. https://doi.org/10.3390/ijms13077980




