Pharmacokinetics and Tissue Distribution Study of Praeruptorin D from Radix Peucedani in Rats by High-Performance Liquid Chromatography (HPLC)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Plasma and Tissue Samples
2.2. Method Validation
2.2.1. Specificity
2.2.2. Linearity of Calibration Curve and Lower Limit of Quantification
2.2.3. Precision and Accuracy
2.2.4. Recovery and Stability
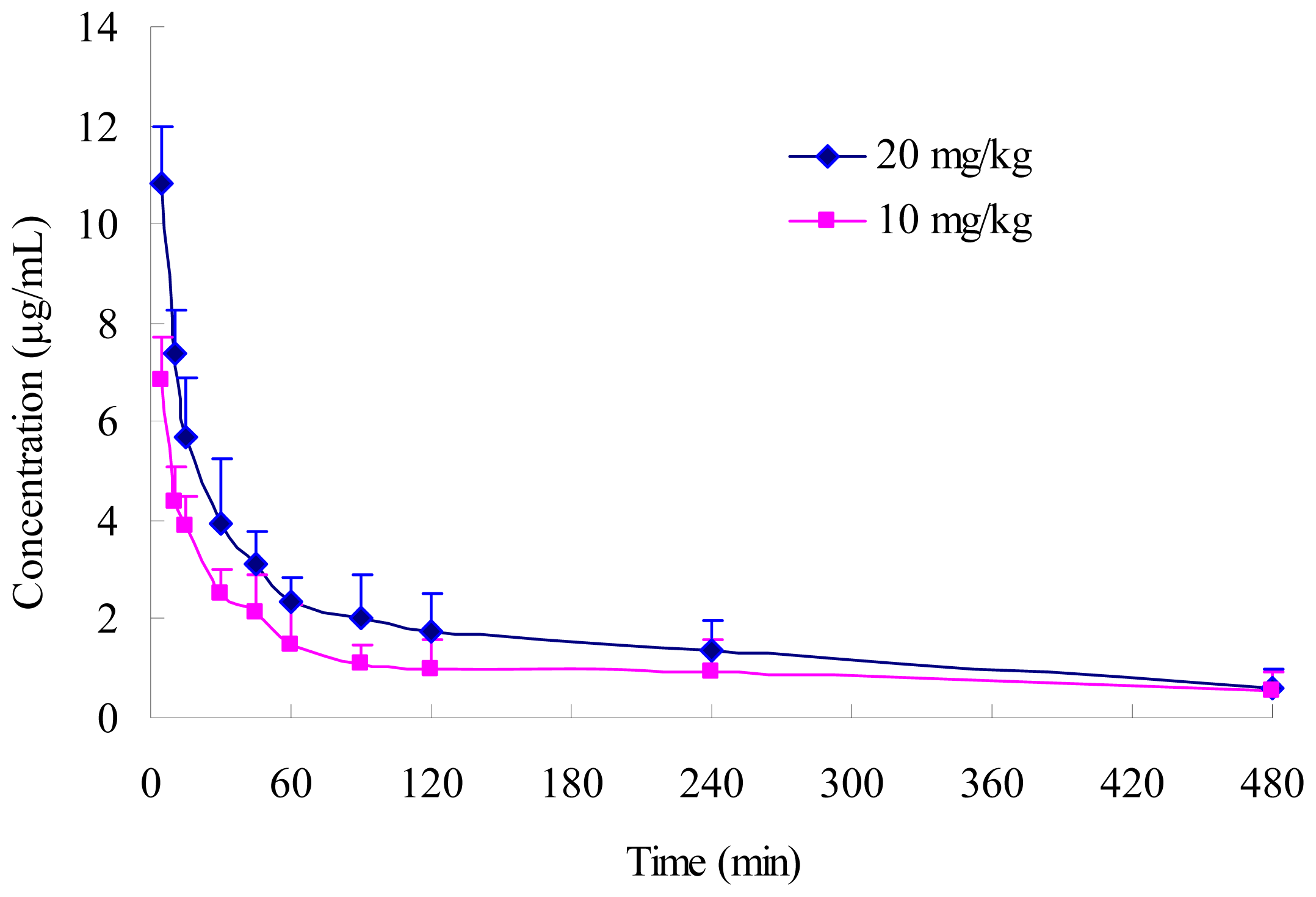
2.3. Pharmacokinetics of PD in Rats
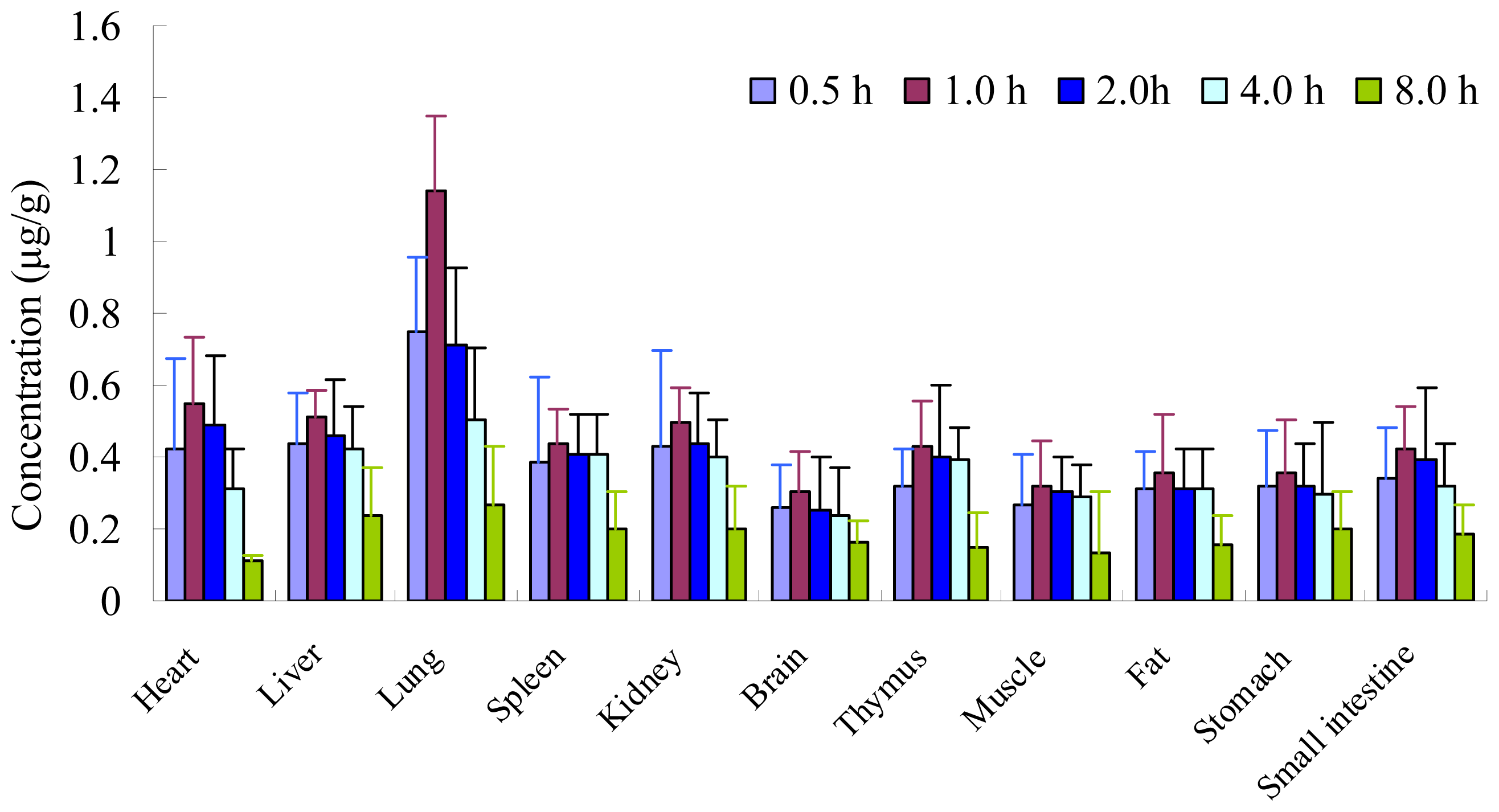
2.4. Tissue Distribution Study
3. Experimental Section
3.1. Materials and Reagents
3.2. Animals
3.3. Instrumentation and Chromatographic Conditions
3.4. Calibration Standards and Quality Control (QC) Samples Preparation
3.5. General Procedure of Sample Preparation for PD Analysis in Plasma and Tissue Samples
3.6. Method Validation
3.6.1. Specificity
3.6.2. Calibration Curves and Lower Limit of Quantification
3.6.3. Precision and Accuracy
3.6.4. Extraction Recovery and Stability
3.7. In Vivo Pharmacokinetic Study
3.8. Tissue Distribution Study
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
References
- The State Pharmacopoeia Commission of P.R. China, Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2010; Volume 1, p. 248.
- Chang, H.M.; But, P.P.H.; Yao, S.C.; Wang, L.L.; Yeung, S.C.S. Pharmacology and Applications of Chinese Materia Medica; World Scientific Publisher: Singapore, 2001; Volume 2, p. 905. [Google Scholar]
- Chen, Z.X.; Huang, B.S.; She, Q.L.; Zeng, G.F. The chemical constituents of Bai-Hua-Qian-Hu, the root of Peucedanum praeruptorum Dunn. (Umbelliferae)—Four new coumarins. Acta Pharm. Sin 1979, 14, 486–496. [Google Scholar]
- Takata, M.; Shibata, S.; Okuyama, T. Structures of angular pyranocoumarins of Bai-Hua Qian-Hu, the root of Peucedanum praeruptorum. Planta Med 1990, 56, 307–311. [Google Scholar]
- Chang, H.T.; Okada, Y.; Ma, T.J.; Okuyama, T.; Tu, P.F. Two new coumarin glycosides from Peucedanum praeruptorum. J. Asian Nat. Prod. Res 2008, 10, 577–581. [Google Scholar]
- Ishii, H.; Okada, Y.; Baba, M.; Okuyama, T. Studies of coumarins from the Chinese drug Qianhu, XXVII: Structure of a new simple coum arin glycoside from Bai-Hua Qianhu, Peucedanum praeruptorum. Chem. Pharm. Bull 2008, 56, 1349–1351. [Google Scholar]
- Fong, W.F.; Zhang, J.X.; Wu, J.Y.; Tse, K.W.; Wang, C.; Cheung, H.Y.; Yang, M.S. Pyranocoumarin(±)-4′-O-acetyl-3′-O-angeloyl-cis-khellactone induces mitochondrial-dependent apoptosis in HL-60 cells. Planta Med 2004, 70, 489–495. [Google Scholar]
- Liang, T.G.; Yue, W.Y.; Li, Q.S. Chemopreventive effects of Peucedanum praeruptorum Dunn and its major constituents on SGC7901 gastric cancer cells. Molecules 2010, 15, 8060–8071. [Google Scholar]
- Mizuno, A.; Okada, Y.; Nishino, H.; Okuyama, T. Anti-cancer activity of coumarins from Peucedanum praeruptum on second stage cancer. J. Tradit. Med 1994, 11, 220–224. [Google Scholar]
- Zhao, N.C.; Jin, W.B.; Zhang, X.H.; Guan, F.L.; Sun, Y.B.; Adachi, H.; Okuyama, T. Relaxant effects of pyranocoumarin compounds isolated from a Chinese medical plant, Bai-Hua Qian-Hu, on isolated rabbit tracheas and pulmonary arteries. Biol. Pharm. Bull 1999, 22, 984–987. [Google Scholar]
- Shen, X.L.; Chen, G.Y.; Zhu, G.Y.; Fong, W.F. (±)-3′-O,4′-O-dicynnamoyl-cis-khellactone, a derivative of (±)-praeruptorin A, resverses P-glycoprotein mediated multidrug resistance in cancer cells. Bioorg. Med. Chem 2006, 14, 7138–7145. [Google Scholar]
- Wu, J.Y.; Fong, W.F.; Zhang, J.X.; Leung, C.H.; Kwong, H.L.; Yang, M.S.; Li, D.; Cheung, H.Y. Reversal of multidrug resistance in cancer cells by pyranocoumarins isolated from Radix Peucedani. Eur. J. Pharmacol 2003, 473, 9–17. [Google Scholar]
- Li, J.M.; Chang, T.H.; Sun, X.D.; Hao, L.Y.; Wang, Y.P.; Yu, Y.F.; Zhang, K.Y. Effect of dl-praeruptorin A on calcium current in ventricular cells of guinea pig. Acta Pharmacol. Sin 1994, 15, 525–527. [Google Scholar]
- Feng, W.Y.; Li, J.M.; Zhang, K.Y. Effect of Pd Ia on elctrophysiological properties of guinea pig ventricular cell (In Chinese). Chin. Pharm. J 1998, 33, 660–662. [Google Scholar]
- Zhang, Z.; Liu, Y.Y.; Su, M.Q.; Liang, X.F.; Wang, W.F.; Zhu, X. Pharmacokinetics, tissue distribution and excretion study of dl-praeruptorin A of Peucedanum praeruptorum in rats by liquid chromatography tandem mass spectrometry. Phytomedicine 2011, 18, 527–532. [Google Scholar]
- Ruan, H.; Zhang, Z.; Zhu, X. Liquid chromatography tandem mass spectrometry pharmacokinetic study of DL-praeruptorin A in rat plasma. Biomed. Chromatogr 2010, 24, 1193–1198. [Google Scholar]
- Jing, W.H.; Song, Y.L.; Yan, R.; Bi, H.C.; Li, P.T.; Wang, Y.T. Transport and metabolism of (±)-praeruptorin A in Caco-2 cell monolayers. Xenobiotica 2011, 41, 71–81. [Google Scholar]
- Ruan, H.; Zhang, Z.; Liang, X.F.; Fu, Y.; Su, M.Q.; Liu, Q.L.; Wang, X.M.; Zhu, X. Metabolism of dl-praeruptorin A in rat liver microsomes using HPLC-electrospray ionization tandem mass spectrometry. Arch. Pharm. Res 2011, 34, 1311–1321. [Google Scholar]
- Song, Y.L.; Jing, W.H.; Zhao, H.Y.; Yan, R.; Li, P.T.; Wang, Y.T. Stereoselective metabolism of (±)-praeruptorin A, a calcium channel blocker from Peucedani Radix, in pooled liver microsomes of rats and humans. Xenobiotica 2012, 42, 231–237. [Google Scholar]
- Nishino, H.; Okuyama, T.; Takata, M.; Shibata, S.; Tokuda, H.; Takayasu, J.; Hasegawa, T.; Nishino, A.; Ueyama, H.; Iwashima, A. Studies on the anti-tumor-promoting activity of naturally occurring substances. IV. Pd-II [(+)anomalin, (+)praeruptorin B], a seselin-type coumarin, inhibits the promotion of skin tumor formation by 12-O-tetradecanoylphorbol-13-acetate in 7,12-dimethylbenz[a]anthracene-initiated mice. Carcinogenesis 1990, 11, 1557–1561. [Google Scholar]
- Yu, P.J.; Jin, H.; Zhang, J.Y.; Wang, G.F.; Li, J.R.; Zhu, Z.G.; Tian, Y.X.; Wu, S.Y.; Xu, W.; Zhang, J.J.; Wu, S.G. Pyranocoumarins isolated from Peucedanum praeruptorum Dunn suppress lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-κB and STAT3 activation. Inflammation 2012, 35, 967–977. [Google Scholar]
- Wu, W.; Chen, J.; Liu, S.; Yu, N.; Fang, C.; Zhang, Q. Simultaneous determination of three coumarin constituents in roots of Peucedanum praeruptorum by RP-HPLC (In Chinese). Chin. J. Chin. Mater. Med 2009, 34, 1121–1123. [Google Scholar]
- Song, Y.L.; Yan, R.; Jing, W.H.; Zhao, H.Y.; Wang, Y.T. Characterization of metabolism of (+)-praeruptorin B and (+)-praeruptorin E in human and rat liver microsomes by liquid chromatography coupled with ion trap mass spectrometry and time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom 2011, 25, 719–730. [Google Scholar]
- Zhang, W.D.; Zhang, C.; Liu, R.H.; Li, H.L.; Zhang, J.T.; Mao, C.; Moran, S.; Chen, C.L. Preclinical pharmacokinetics and tissue distribution of a natural cardioprotective agent astragaloside IV in rats and dogs. Life Sci 2006, 79, 808–815. [Google Scholar]
- Chen, C.L.; Tai, H.L.; Zhu, D.M.; Uckun, F.M. Pharmacokinetic features and metabolism of calphostin C, a naturally occurring perylenequinone with antileukemic activity. Pharm. Res 1999, 16, 1003–1009. [Google Scholar]
- Chen, C.L.; Rawwas, J.; Sorrell, A.; Eddy, L.; Uckun, F.M. Bioavailability and pharmacokinetic features of etoposide in childhood acute lymphoblastic leukemia patients. Leuk. Lymphoma 2001, 42, 317–327. [Google Scholar]
- Chen, C.L.; Sangiah, S.; Bourne, D.W.; Roder, J.D.; Chen, H.; Alavi, F.K.; Clarke, C.R.; Garrison, G.L.; Berlin, K.D.; Couch, K.M.; et al. Metabolism and metabolite pharmacokinetics of BRB-I-28, a class Ib antiarrhythmic agent. Eur. J. Drug Metab. Pharmacokinet 1995, 20, 151–161. [Google Scholar]


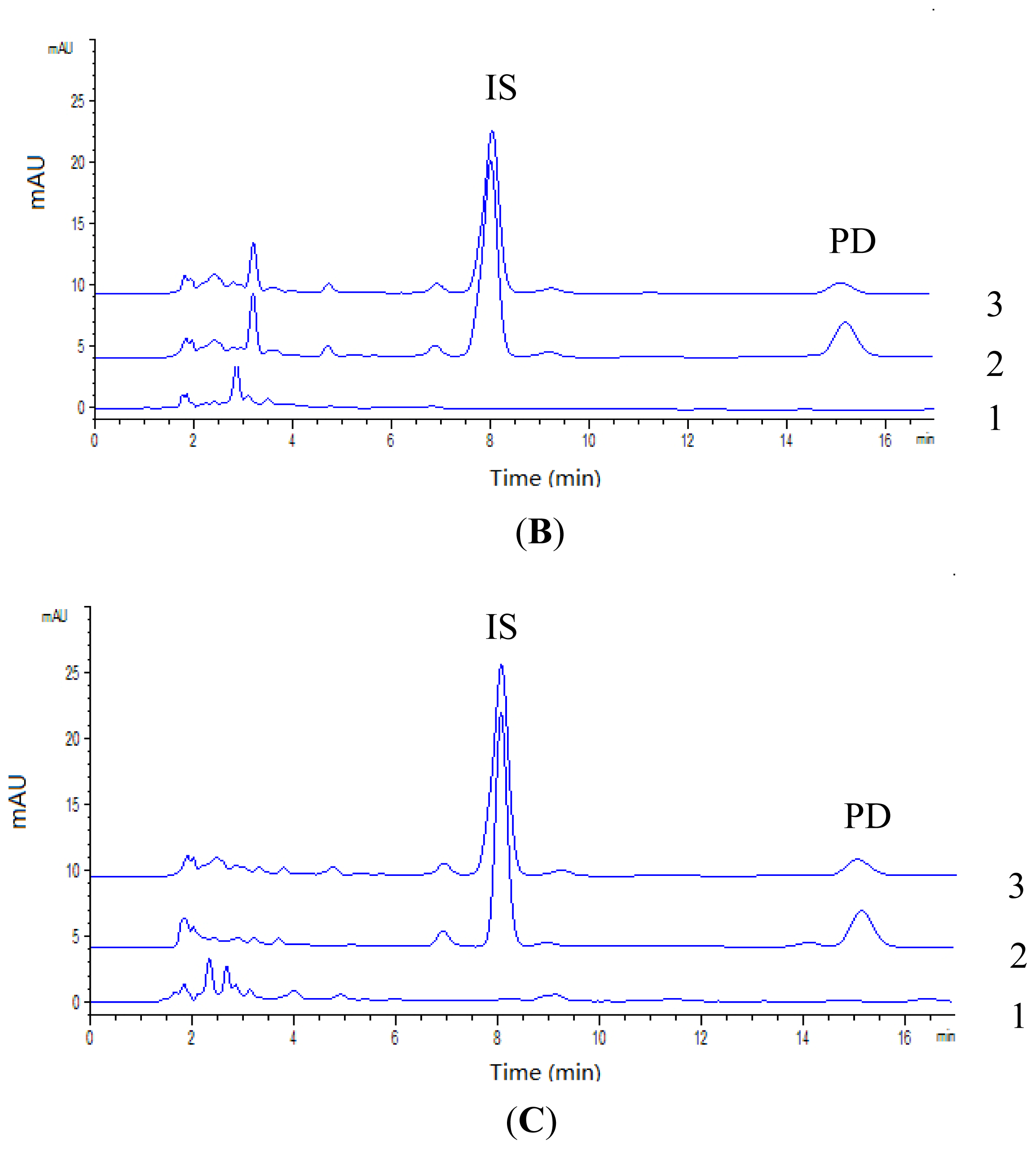
| Biosamples | Calibration curves | R2 | Linear range (μg/mL) |
|---|---|---|---|
| Plasma | Y = 0.0876X + 0.0016 | 0.9991 | 0.0512–51.2 |
| Lung | Y = 0.1074X + 0.0054 | 0.9999 | 0.0512–51.2 |
| Liver | Y = 0.1426X + 0.0812 | 0.9984 | 0.0512–51.2 |
| Heart | Y = 0.1314X + 0.0094 | 0.9989 | 0.0512–51.2 |
| Spleen | Y = 0.1381X + 0.0017 | 0.9993 | 0.0512–51.2 |
| Stomach | Y = 0.1318X + 0.0142 | 0.9981 | 0.0512–51.2 |
| Small intestine | Y = 0.1315X + 0.0072 | 0.9973 | 0.0512–51.2 |
| Brain | Y = 0.1417X + 0.0114 | 0.9988 | 0.0512–51.2 |
| Thymus | Y = 0.1381X + 0.0020 | 0.9994 | 0.0512–51.2 |
| Muscle | Y = 0.1493X + 0.0147 | 0.9982 | 0.0512–51.2 |
| Fat | Y = 0.1517X + 0.0039 | 0.9979 | 0.0512–51.2 |
| Kidney | Y = 0.1359X + 0.0018 | 0.9991 | 0.0512–51.2 |
| Biosamples | Concentration (μg/mL) | Intra-day | Inter-day | Recovery | ||
|---|---|---|---|---|---|---|
| Precision (RSD%) | Accuracy (RE%) | Precision (RSD%) | Accuracy (RE%) | Mean ± SD (%) | ||
| Plasma | 0.0512 | 8.80 | 9.79 | 11.25 | −10.56 | 84.00 ± 5.88 |
| 3.2 | 5.43 | −8.94 | 6.20 | 8.01 | 83.12 ± 4.06 | |
| 51.2 | 2.91 | −3.62 | 4.07 | 5.98 | 88.63 ± 6.75 | |
| Liver | 0.0512 | 7.29 | −8.51 | 9.62 | −11.03 | 86.56 ± 5.31 |
| 3.2 | 8.47 | −8.48 | 9.97 | −5.44 | 84.51 ± 5.64 | |
| 51.2 | 5.56 | 6.33 | 5.56 | 3.07 | 83.63 ± 6.93 | |
| Lung | 0.0512 | 6.90 | 9.14 | 5.94 | 10.19 | 84.35 ± 5.20 |
| 3.2 | 6.62 | −5.75 | 8.90 | 7.85 | 85.12 ± 4.17 | |
| 51.2 | 5.88 | 6.87 | 10.52 | 7.19 | 82.07 ± 6.42 | |
| Biosamples | Concentration (μg/mL) | Accuracy (RE%) | ||
|---|---|---|---|---|
| Short-term stability | Freeze-thaw stability | Long term stability | ||
| Plasma | 0.0512 | −11.76 | 10.28 | 9.30 |
| 3.2 | −5.22 | −8.75 | −7.04 | |
| 51.2 | 4.43 | −7.11 | 8.89 | |
| Liver | 0.0512 | −8.05 | 5.29 | −8.57 |
| 3.2 | 7.57 | −6.45 | −10.81 | |
| 51.2 | 5.81 | 7.76 | 6.06 | |
| Lung | 0.0512 | −11.35 | 9.03 | −7.15 |
| 3.2 | −9.76 | 7.05 | 6.92 | |
| 51.2 | 8.13 | 5.02 | −4.25 | |
| Parameter | Unit | Dose | |
|---|---|---|---|
| 10 mg/kg | 20 mg/kg | ||
| t1/2α | h | 0.119 ± 0.036 | 0.130 ± 0.045 |
| t1/2β | h | 2.408 ± 0.409 | 2.640 ± 0.612 |
| AUC0-∞ | mg·h/L | 18.145 ± 6.265 | 40.790 ± 11.746 * |
| CLs | L/h/kg | 0.576 ± 0.193 | 0.513 ± 0.185 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liang, T.; Yue, W.; Du, X.; Ren, L.; Li, Q. Pharmacokinetics and Tissue Distribution Study of Praeruptorin D from Radix Peucedani in Rats by High-Performance Liquid Chromatography (HPLC). Int. J. Mol. Sci. 2012, 13, 9129-9141. https://doi.org/10.3390/ijms13079129
Liang T, Yue W, Du X, Ren L, Li Q. Pharmacokinetics and Tissue Distribution Study of Praeruptorin D from Radix Peucedani in Rats by High-Performance Liquid Chromatography (HPLC). International Journal of Molecular Sciences. 2012; 13(7):9129-9141. https://doi.org/10.3390/ijms13079129
Chicago/Turabian StyleLiang, Taigang, Wenyan Yue, Xue Du, Luhui Ren, and Qingshan Li. 2012. "Pharmacokinetics and Tissue Distribution Study of Praeruptorin D from Radix Peucedani in Rats by High-Performance Liquid Chromatography (HPLC)" International Journal of Molecular Sciences 13, no. 7: 9129-9141. https://doi.org/10.3390/ijms13079129




