The Twenty-Year Story of a Plant-Based Vaccine Against Hepatitis B: Stagnation or Promising Prospects?
Abstract
:1. Premises of Plant-Based Vaccines
2. Production of HBV Antigens in Plant Systems
2.1. Small Hepatitis B Surface Antigen
2.2. Middle and Large Hepatitis B Surface Antigens
2.3. Hepatitis B Core Antigen
2.4. General Characterization of Plant-Produced HBV Antigens
3. Progress and Barriers of Oral Immunization
4. Plant-Produced HBV Antigens as Injection Vaccines
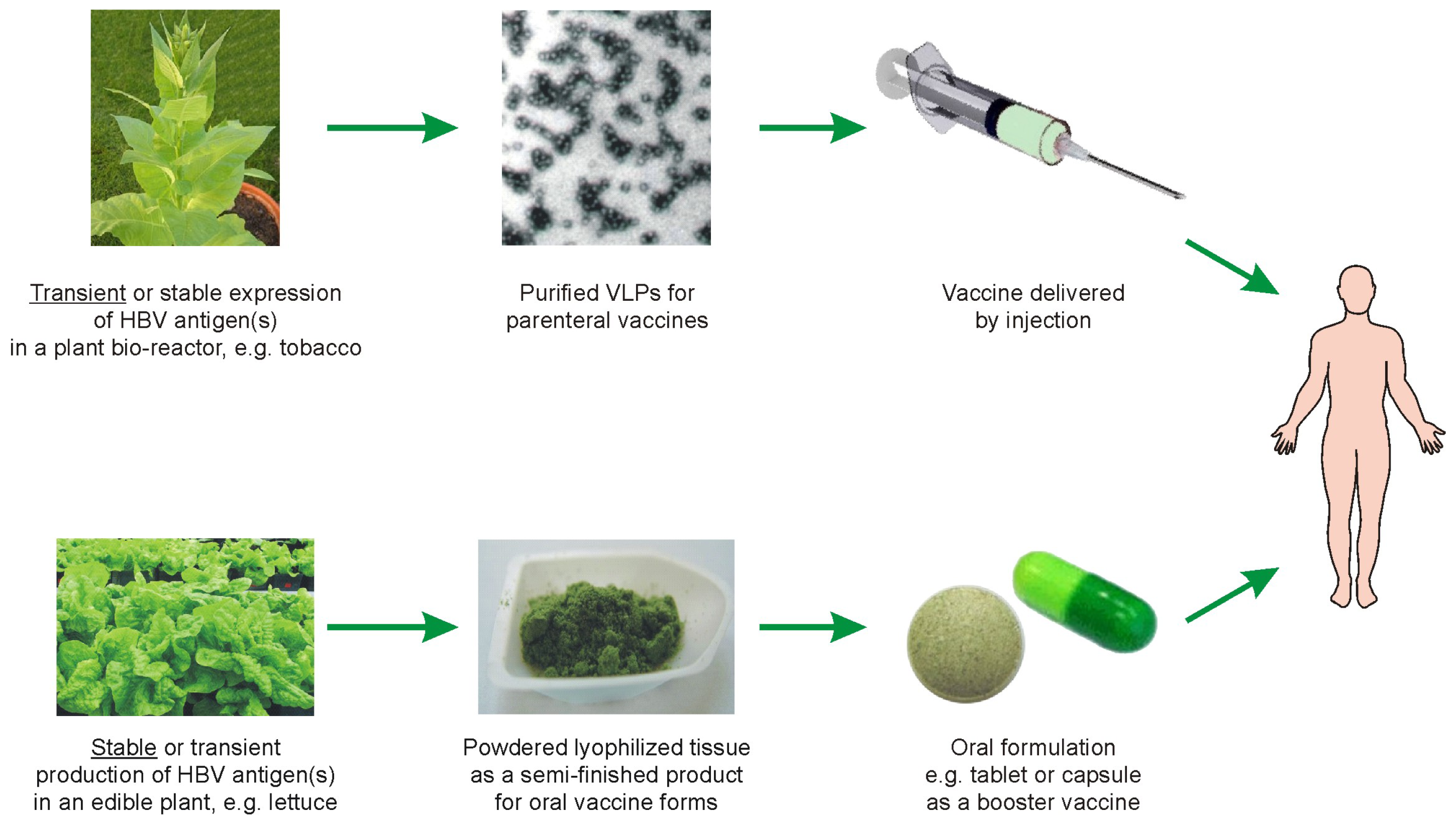
5. Possible Scenarios for Plant-Based Anti-HBV Vaccines
6. Conclusions
Abbreviations
| AlMV | Alfalfa Mosaic Virus |
| BeYDV | Bean Yellow Dwarf Virus |
| CaMV 35S promoter | promoter of 35S RNA of Cauliflower Mosaic Virus |
| CHO | cell line derived from Chinese hamster ovary |
| CLPs | Capsid-Like Particles |
| CPMV | Cowpea Mosaic Virus |
| CT | Cholera toxin |
| CTB | Cholera toxin subunit B |
| DW | dry weight |
| EMA | European Medicines Agency |
| ER | endoplasmic reticulum |
| FDA | U.S. Food and Drug Administration |
| FW | fresh weight |
| GALT | Gut-Associated Lymphoid Tissue |
| GMP | Good Manufacture Practice |
| HepB | Hepatitis B |
| HBV | Hepatitis B Virus |
| HBcAg | Hepatitis B core Antigen |
| HBsAg = HBs antigen(s) | (any) Hepatitis B surface Antigen(s) |
| rHBsAg | recombinant Hepatitis B surface Antigen |
| S-, M-, L-HBsAg | small, medium or large HBsAg |
| HCC | Hepatocellular carcinoma |
| LT | Heat-labile enterotoxin |
| MALT | Mucosa-Associated Lymphoid Tissue |
| mIU/mL | milli-International Unit/mL = unit of titer of anti-HBs antibodies |
| NALT | Nasal-Associated Lymphoid Tissue |
| NOS | nopaline synthase |
| PVX | Potato Virus X |
| S-IgA | secretory IgA |
| SVP | subviral particle |
| TEV | Tobacco Etch Virus |
| TSP | total soluble protein |
| 5′-UTR | 5′-untranslated region of mRNA |
| VLPs | Virus-Like Particles |
- Conflict of InterestThe authors declare no conflict of interest.
References
- Blumberg, B.S.; Alter, H.J.; Visnich, S. A “new” antigen in leukaemia sera. JAMA 1965, 191, 541–546. [Google Scholar]
- Krugman, S. The newly licensed hepatitis B vaccine. Characteristics and indications for use. JAMA 1982, 247, 2012–2015. [Google Scholar]
- Hilleman, M.R. Critical overview and outlook: Pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine 2003, 21, 4626–4649. [Google Scholar]
- McAleer, W.J.; Buynak, E.B.; Maigetter, R.Z. Human hepatitis B vaccine from recombinant yeast. Nature 1984, 307, 178–180. [Google Scholar]
- Young, M.D.; Rosenthal, M.H.; Dickson, B.; Du, W.; Maddrey, W.C. A multi-center controlled study of rapid hepatitis B vaccination using a novel triple antigen recombinant vaccine. Vaccine 2001, 19, 3437–3443. [Google Scholar]
- Singh, N.P.; Mandal, S.K.; Thakur, A.; Kapoor, D.; Anuradha, S.; Prakash, A.; Kohli, R.; Agarwal, S.K. Efficacy of GM-CSF as an adjuvant to hepatitis B vaccination in patients with chronic renal failure—Results of a prospective, randomized trial. Ren. Fail 2003, 25, 255–266. [Google Scholar]
- Tassopoulos, N.C.; Koutelou, M.G.; Polychronaki, H.; Paraloglou-Ioannides, M.; Hadziyannis, S.J. Recombinant interferon-α therapy for acute hepatitis B: A randomized, double-blind, placebo-controlled trial. J. Viral Hepat 1997, 4, 387–394. [Google Scholar]
- Deres, K.; Schroder, C.H.; Paessens, A.; Goldmann, S.; Hacker, H.J.; Weber, O.; Kramer, T.; Niewohner, U.; Pleiss, U.; Stoltefuss, J.; et al. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science 2003, 299, 893–896. [Google Scholar]
- Bruss, V. Hepatitis B virus morphogenesis. World J. Gastroenterol 2007, 13, 65–73. [Google Scholar]
- Zuckerman, J.N.; Sabin, C.; Craig, F.M.; Williams, A.; Zuckerman, A.J. Immune response to a new hepatitis B vaccine in healthcare workers who had not responded to standard vaccine: Randomised double blind dose-response study. BMJ 1997, 314, 329–333. [Google Scholar]
- Madaliński, K.; Sylvan, S.P.; Hellstrom, U.; Mikołajewicz, J.; Zembrzuska-Sadkowska, E.; Piontek, E. Antibody responses to preS components after immunization of children with low doses of BioHepB. Vaccine 2002, 20, 92–97. [Google Scholar]
- Rendi-Wagner, P.; Shouval, D.; Genton, B.; Lurie, Y.; Rümke, H.; Boland, G.; Cerny, A.; Heim, M.; Bach, D.; Schroeder, M.; et al. Comparative immunogenecity of a PreS/S hepatitis B vaccine in non- and low responders to conventional vaccine. Vaccine 2006, 24, 2781–2789. [Google Scholar]
- Yamada, T.; Iwabuki, H.; Kanno, T.; Tanaka, H.; Tomoji, K.; Fukuda, H.; Kondo, A.; Seno, M.; Tanizawa, K.; Kuroda, S. Physiochemical and immunological characterization of hepatitis B virus envelope particles exclusively consisting of the entire L (pre-S1 + pre-S2 + S) protein. Vaccine 2001, 19, 3154–3163. [Google Scholar]
- Han, X.; Ye, L.-B.; Li, B.; Bo, G.; Cai, W.; Hong, Z.; She, Y.-L.; Li, Y.; Kong, L.-B.; Wu, Z.-H. Expression, purification and characterization of Hepatitis B virus entire envelope large protein in Pichia pastoris. Protein Expres. Purif 2006, 49, 168–175. [Google Scholar]
- Brocke, P.; Schaefer, S.; Melber, K.; Jenzelewski, V.; Müller, F.; Dahlems, U.; Bartelsen, O.; Park, K.-N.; Janowicz, Z.A.; Gellissen, G. Recombinant Hepatitis B Vaccines: Disease Characterization and Vaccine Production. In Production of Recombinant Proteins. Novel Microbial and Eukaryotic Systems; Gellissen, G., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 319–359. [Google Scholar]
- Shouval, D.; Ilan, Y.; Adler, R.; Deepen, R.; Panet, A.; Even-Chen, Z.; Gorecki, M.; Gerlich, W.H. Improved immunogenicity in mice of a mammalian cell-derived recombinant hepatitis B vaccine containing pre-S1 and pre-S2 antigens as compared with conventional yeast-derived vaccines. Vaccine 1994, 12, 1453–1459. [Google Scholar]
- Couillin, I.; Pol, S.; Mancini, M.; Driss, F.; Brechot, C.; Tiollais, P.; Michel, M.-L. Specific vaccine therapy in chronic hepatitis B: Induction of T cell proliferative responses specific for envelope antigens. J. Infect. Dis 1999, 180, 15–26. [Google Scholar]
- Böcher, W.O.; Dekel, B.; Schwerin, W.; Geissler, M.; Hoffmann, S.; Rohwer, A.; Arditti, F.; Cooper, A.; Bernhard, H.; Berrebi, A.; et al. Induction of strong hepatitis B virus (HBV) specific T helper cell and cytotoxic T lymphocyte responses by therapeutic vaccination in the trimera mouse model of chronic HBV infection. Eur. J. Immunol 2001, 31, 2071–2079. [Google Scholar]
- Chen, X.; Li, M.; Le, X.; Ma, W.; Zhou, B. Recombinant hepatitis B core antigen carrying preS1 epitopes induce immune response against chronic HBV infection. Vaccine 2004, 22, 439–446. [Google Scholar]
- Langridge, W.H. Edible vaccines. Sci. Am 2000, 283, 66–71. [Google Scholar]
- Streatfield, S.J. Mucosal immunization using recombinant plant-based oral vaccines. Methods 2006, 38, 150–157. [Google Scholar]
- Peng, H.J.; Turner, M.W.; Strobel, S. The kinetics of oral hyposensitization to a protein antigen are determined by immune status and the timing, dose and frequency of antigen administration. Immunology 1989, 67, 425–430. [Google Scholar]
- Koprowski, H.; Jervis, G.A.; Norton, T.W. Immune responses in human volunteers upon oral administration of a rodent-adapted strain of poliomyelitis virus. Am. J. Hyg 1952, 55, 108–126. [Google Scholar]
- Matzinger, P. Tolerance, danger and extended family. Annu. Rev. Immunol 1994, 12, 991–1045. [Google Scholar]
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev 2003, 3, 331–341. [Google Scholar]
- Mason, H.S.; Lam, D.M.-K.; Arntzen, C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 1992, 89, 11745–11749. [Google Scholar]
- Thanavala, Y.; Yang, Y.-F.; Lyons, P.; Mason, H.S.; Arntzen, C.J. Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc. Natl. Acad. Sci. USA 1995, 92, 3358–3361. [Google Scholar]
- Kapusta, J.; Modelska, A.; Figlerowicz, M.; Pniewski, T.; Letellier, M.; Lisowa, O.; Yusibov, V.; Koprowski, H.; Plucienniczak, A.; Legocki, A.B. A plant-derived edible vaccine against hepatitis B virus. FASEB J 1999, 13, 1796–1799. [Google Scholar]
- Imani, J.; Berting, A.; Nitsche, S.; Schaefer, S.; Gerlich, W.H.; Neumann, K.-H. The integration of a major hepatitis B virus gene into cell-cycle synchronized carrot cell suspension cultures and its expression in regenerated carrot plants. Plant Cell Tiss. Org. Cult 2002, 71, 157–164. [Google Scholar]
- Pniewski, T.; Kapusta, J.; Bociąg, P.; Wojciechowicz, J.; Kostrzak, A.; Gdula, M.; Fedorowicz-Strońska, O.; Wójcik, P.; Otta, H.; Samardakiewicz, S.; et al. Low-dose oral immunization with lyophilized tissue of herbicide-resistant lettuce expressing hepatitis B surface antigen for prototype plant-derived vaccine tablet formulation. J. Appl. Genet 2011, 52, 125–136. [Google Scholar]
- Pniewski, T.; Kapusta, J.; Bociąg, P.; Kostrzak, A.; Fedorowicz-Strońska, O.; Czyż, M.; Gdula, M.; Krajewski, P.; Wolko, B.; Płucienniczak, A. Plant expression, lyophilisation and storage of HBV medium and large surface antigens for a prototype oral vaccine formulation. Plant Cell Rep 2012, 31, 585–595. [Google Scholar]
- Hayden, C.A.; Streatfield, S.J.; Lamphear, B.J.; Fake, G.M.; Keener, T.K.; Walker, J.H.; Clements, J.D.; Turner, D.D.; Tizard, I.R.; Howard, J.A. Bioencapsulation of the hepatitis B surface antigen and its use as an effective oral immunogen. Vaccine 2012, 30, 2937–2942. [Google Scholar]
- Hayden, C.A.; Egelkrout, E.M.; Moscoso, A.M.; Enrique, C.; Keener, T.K.; Jimenez-Flores, R.; Wong, J.C.; Howard, J.A. Production of highly concentrated, heat-stable hepatitis B surface antigen in maize. Plant Biotechnol. J 2012, 10, 979–984. [Google Scholar]
- Lam, D.M.-K.; Arntzen, C.J. Vaccines produced and administered through edible plants. U.S. Patent 5,484,719, 16 January 1996. [Google Scholar]
- Rukavtsova, E.B.; Zolova, O.E.; Buryanova, N.Ya.; Borisova, V.N.; Bykov, V.A.; Buryanov, Ya.I. Analysis of transgenic tobacco plants carrying the gene for the surface antigen of the Hepatitis B virus. Russ. J. Genet. 2003, 39, 41–45. [Google Scholar]
- Sunil Kumar, G.B.; Srinivas, L.; Ganapathi, T.R.; Bapat, V.A. Hepatitis B surface expression in transgenic tobacco (Nicotiana tabacum) plants using four different expression cassettes. Plant Cell Tiss. Org. Cult 2006, 84, 315–323. [Google Scholar]
- Ehsani, P.; Khabiri, A.; Domansky, N.N. Polypeptides of hepatitis B surface antigen produced in transgenic potato. Gene 1997, 190, 107–111. [Google Scholar]
- Shulga, N.Ya.; Rukavtsova, E.B.; Krymsky, M.A.; Borisova, V.N.; Melnikov, V.A.; Bykov, V.A.; Buryanov, Ya.I. Expression and Characterization of Hepatitis B Surface Antigen in Transgenic Potato Plants. Biochemistry 2004, 69, 1158–1164. [Google Scholar]
- Dogan, B.; Mason, H.S.; Richter, L.; Hunter, J.B.; Shuler, M.L. Process options in hepatitis B surface antigen extraction from transgenic potato. Biotechnol. Prog 2000, 16, 435–441. [Google Scholar]
- Richter, L.J.; Thanavala, Y.; Arntzen, C.J.; Mason, H.S. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat. Biotechnol 2000, 18, 1167–1171. [Google Scholar]
- Kong, Q.; Richter, L.; Yang, Y.F.; Arntzen, C.J.; Mason, H.S.; Thanavala, Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. USA 2001, 98, 11539–11544. [Google Scholar]
- Mason, H.S.; Thanavala, Y.; Arntzen, C.J.; Richter, E. Expression of immunogenic hepatitis B surface antigen in transgenic plants. U.S. Patent 6,551,820, 22 April 2003. [Google Scholar]
- Thanavala, Y.; Mahoney, M.; Pal, S.; Scott, A.; Richter, L.; Natarajan, N.; Goodwin, P.; Arntzen, C.J.; Mason, H.S. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. USA 2005, 102, 3378–3382. [Google Scholar]
- Joung, Y.H.; Youm, J.-W.; Jeon, J.H.; Lee, B.C.; Ryu, C.J.; Hong, H.J.; Kim, H.C.; Joung, H.; Kim, H.S. Expression of the hepatitis B surface S and preS2 antigens in tubers of Solanum tuberosum. Plant Cell Rep 2004, 22, 925–930. [Google Scholar]
- Zhao, C.H.; Wang, R.; Zhao, C.S.; Wang, G.L.; Tian, P. Expression of human HBV surface antigen gene with and without preS in transgenic tomato. Nongye Shengwu Jishu Xubao 2000, 8, 85–88. [Google Scholar]
- Gao, Y.; Ma, Y.; Li, M.; Cheng, T.; Li, S.-W.; Zhang, J.; Xia, N.-S. Oral immunization of animals with transgenic cherry tomatillo expressing HBsAg. World J. Gastroenterol 2003, 9, 996–1002. [Google Scholar]
- Srinivas, L.; Sunil Kumar, G.B.; Ganapathi, T.R.; Revathi, C.J.; Bapat, V.A. Transient and stable expression of hepatitis B surface antigen in tomato (Lycopersicon esculentum L.). Plant Biotechnol. Rep 2008, 2, 1–6. [Google Scholar]
- Sunil Kumar, G.B.; Ganapathi, T.R.; Revathi, C.J.; Prasad, K.S.N.; Bapat, V.A. Expression of hepatitis B surface antigen in transgenic banana plants and NT-1 cell line of tobacco. BARC News Lett 2003, 237, 85–96. [Google Scholar]
- Sunil Kumar, G.B.; Ganapathi, T.R.; Revathi, C.J.; Prasad, K.S.N.; Bapat, V.A. Expression of hepatitis B surface antigen in tobacco cell suspension cultures. Prot. Exp. Purif 2003, 32, 10–17. [Google Scholar]
- Sunil Kumar, G.B.; Ganapathi, T.R.; Srinivas, L.; Revathi, C.J.; Bapat, V.A. Secretion of hepatitis B surface antigen in transformed tobacco cell suspension cultures. Biotechnol. Lett 2005, 27, 927–932. [Google Scholar]
- Smith, M.L.; Mason, H.S.; Shuler, M.L. Hepatitis B surface antigen (HBsAg) expression in plant cell culture: Kinetics of antigen accumulation in batch culture and its intracellular form. Biotechnol. Bioeng 2002, 80, 812–822. [Google Scholar]
- Sojikul, P.; Buehner, N.; Mason, H.S. A plant signal peptide-hepatitis B surface antigen fusion protein with enhanced stability and immunogenicity expressed in plant cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2209–2214. [Google Scholar]
- Sunil Kumar, G.B.; Ganapathi, T.R.; Srinivas, L.; Revathi, C.J.; Bapat, V.A. Expression of hepatitis B surface antigen in potato hairy roots. Plant Sci 2006, 170, 918–925. [Google Scholar]
- Ganapathi, T.R.; Sunil Kumar, G.B.; Srinivas, L.; Revathi, C.J.; Bapat, V.A. Analysis of the limitations of hepatitis B surface antigen expression in soybean cell suspension cultures. Plant Cell Rep. 2007, 1575–1584. [Google Scholar]
- Peng, J.; Song, Q.; Chengkui, T. Expression of hepatitis B surface antigen gene (HBsAg) in Laminaria japonica (Laminariales, Phaeophyta). Chin. Sci. Bull 2002, 17, 1438–1440. [Google Scholar]
- Sunil Kumar, G.B.; Ganapathi, T.R.; Revathi, C.J.; Srinivas, L.; Bapat, V.A. Expression of hepatitis B surface antigen in transgenic banana plants. Planta 2005, 222, 484–493. [Google Scholar]
- Chen, H.Y.; Zhang, J.; Gao, Y.; Du, H.L.; Ma, Y.; Zheng, W.Z.; Xia, N.S. Transforming HBsAg into peanut and detection of its immunogenecity. Lett. Biotechnol 2002, 4, 245–250. [Google Scholar]
- Pniewski, T.; Kapusta, J.; Płucienniczak, A. Agrobacterium tumefaciens-mediated transformation of yellow lupin to generate callus tissue producing surface antigen of HBV in a long-term culture. J. Appl. Genet 2006, 47, 309–318. [Google Scholar]
- Kapusta, J.; Modelska, A.; Figlerowicz, M.; Pniewski, T.; Lisowa, O.; Koprowski, H.; Płucienniczak, A.; Legocki, A.B. Plant-based edible vaccine against HBV. Immunol. Lett 2000, 73, 269. [Google Scholar]
- Kapusta, J.; Modelska, A.; Pniewski, T.; Figlerowicz, M.; Jankowski, K.; Lisowa, O.; Plucienniczak, A.; Koprowski, H.; Legocki, A.B. Oral immunization of human with transgenic lettuce expressing hepatitis B surface antigen. Adv. Exp. Med. Biol 2001, 495, 299–303. [Google Scholar]
- Huang, Z.; Mason, H.S. Conformational analysis of hepatitis B surface antigen fusions in an Agrobacterium-mediated transient expression system. Plant Biotechnol. J 2004, 2, 241–249. [Google Scholar]
- Huang, Z.; Elkin, G.; Maloney, B.J.; Buehner, N.; Arntzen, C.J.; Thanavala, Y.; Mason, H.S. Virus-like particles expression and assembly in plants: Hepatitis B and Norwalk viruses. Vaccine 2005, 23, 1851–1858. [Google Scholar]
- Gleba, Y.; Klimyuk, V.; Marillonnet, S. Magnifection—A new platform for expressing recombinant vaccines in plants. Vaccine 2005, 23, 2042–2048. [Google Scholar]
- Huang, Z.; LePore, K.; Elkin, G.; Thanavala, Y.; Mason, H.S. High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol. J 2008, 6, 202–209. [Google Scholar]
- Salyaev, R.K.; Rekoslavskaya, N.I.; Stolbikov, A.S.; Hammond, R.W.; Shchelkunov, S.N. Synthesis of Hepatitis B Virus surface antigen in tomato plants transgenic for the preS2-S gene. Doklady Biochem. Biophys 2007, 416, 290–293. [Google Scholar]
- Salyaev, R.K.; Stolbikov, A.S.; Rekoslavskaya, N.I.; Shchelkunov, S.N.; Pozdnyakov, S.G.; Chepinoga, A.V.; Hammond, R.V. Obtaining tomato plants transgenic for the preS2-S-HDEL gene, which synthesize the major hepatitis B surface antigen. Doklady Biochem. Biophys 2010, 433, 187–190. [Google Scholar]
- Lou, X.-M.; Yao, Q.-H.; Zhang, Z.; Peng, R.-H.; Xiong, A.-S.; Wang, H.-K. Expression of the human Hepatitis B Virus large surface antigen gene in transgenic tomato plants. Clin. Vaccine Immunol 2007, 14, 464–469. [Google Scholar]
- Imamura, T.; Araki, M.; Miyanohara, A.; Nakao, J.; Yonemura, H.; Ohtomo, N.; Matsubara, K. Expression of hepatitis B virus middle and large surface antigen genes in Saccharomyces cerevisiae. J. Virol 1987, 61, 3543–3549. [Google Scholar]
- Chi, S.W.; Kim, D.H.; Kim, J.S.; Lee, M.K.; Han, K.H. Solution conformation of an immunodominant epitope in the hepatitis B virus preS2 surface antigen. Antivir. Res 2006, 72, 207–215. [Google Scholar]
- Chi, S.W.; Kim, D.H.; Lee, S.H.; Chang, I.; Han, K.H. Pre-structured motifs in natively unstructured preS1 surface antigen of hepatitis B virus. Protein Sci 2007, 16, 2108–2117. [Google Scholar]
- Qian, B.; Shen, H.; Liang, W.; Guo, X.; Zhang, C.; Wang, Y.; Li, G.; Wu, A.; Cao, K.; Zhang, D. Immunogenecity of recombinant hepatitis B virus surface antigen fused with preS1 epitopes expressed in rice seeds. Transgenic Res 2008, 17, 621–631. [Google Scholar]
- Bruss, V. Envelopment of the hepatitis B virus nucleocapsid. Virus Res 2004, 106, 199–209. [Google Scholar]
- Kratz, P.; Böttcher, B.; Nassal, M. Native display of complete foreign domains on the surface of hepatitis B virus capsids. Proc. Natl. Acad. Sci. USA 1999, 96, 1915–1920. [Google Scholar]
- Mihailova, M.; Boos, M.; Petrovskis, I.; Ose, V.; Skrastina, D.; Fiedler, M.; Sominskaya, I.; Ross, S.; Pumpens, P.; Roggendorf, M.; Viazov, S. Recombinant virus-like particles as a carrier of B- and T-cell epitopes of hepatitis C virus (HCV). Vaccine 2006, 24, 4369–4377. [Google Scholar]
- Tsuda, S.; Yoshioka, K.; Tanaka, T.; Iwata, A.; Yoshikawa, A.; Watanabe, Y.; Okada, Y. Application of the human Hepatitis B Virus core antigen from transgenic tobacco plants for serological diagnosis. Vox Sang 1998, 74, 148–155. [Google Scholar]
- Huang, Z.; Santi, L.; LePore, K.; Kilbourne, J.; Arntzen, C.J.; Mason, H.S. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine 2006, 24, 2506–2513. [Google Scholar]
- Mechtcheriakova, I.A.; Eldarov, M.A.; Nicholson, L.; Shanks, M.; Skryabin, K.G.; Lomonossoff, G.P. The use of viral vectors to produce hepatitis B virus core particles in plants. J. Virol. Methods 2006, 131, 10–15. [Google Scholar]
- Sainsbury, F.; Lomonossoff, G.P. Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol 2008, 148, 1212–1218. [Google Scholar]
- Huang, Z.; Chen, Q.; Hjelm, B.; Arntzen, C.; Mason, H. A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol. Bioeng 2009, 103, 706–714. [Google Scholar]
- Youm, J.-W.; Won, Y.-S.; Jeon, J.H.; Ryu, C.J.; Choi, Y.-K.; Kim, H.-C.; Kim, B.-D.; Joung, H.; Kim, H.S. Oral immunogenicity of potato-derived HBsAg middle protein in BALB/c mice. Vaccine 2007, 25, 577–584. [Google Scholar]
- Streatfield, S. Oral hepatitis B vaccine candidates produced and delivered in plant material. Immunol. Cell Biol 2005, 83, 257–262. [Google Scholar]
- Williamson, E.; Westrich, G.M.; Viney, J.L. Modulating dendritic cells to optimize mucosal immunization protocols. J. Immunol 1999, 163, 3668–3675. [Google Scholar]
- Isaka, M.; Yasuda, Y.; Mikozami, M.; Kozuka, S.; Taniguchi, T.; Matano, K.; Maeyama, J.; Mizuno, K.; Morokuma, K.; Goto, N.; et al. Mucosal immunization against hepatitis B virus by intranasal co-administration of recombinant hepatitis B surface antigen and recombinant cholera toxin B subunit as an adjuvant. Vaccine 2001, 19, 1460–1466. [Google Scholar]
- Brandtzaeg, P. Role of secretory antibodies in the defence against infections. Int. J. Med. Microbiol 2003, 293, 3–15. [Google Scholar]
- Kostrzak, A.; Cervantes Gonzales, M.; Guetard, D.; Nagaraju, D.B.; Wain-Hobson, S.; Tepfer, D.; Pniewski, T.; Sala, M. Oral administration of low doses of plant-based HBsAg induced antigen-specific IgAs and IgGs in mice, without increasing levels of regulatory T cells. Vaccine 2009, 27, 4798–4807. [Google Scholar]
- Pamer, E.G. Immune responses to commensal and environmental microbes. Nat. Immunol 2007, 8, 1173–1178. [Google Scholar]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med 2005, 11, S45–S53. [Google Scholar]
- Richman, L.K.; Chiller, J.M.; Brown, W.R.; Hanson, D.G.; Vaz, N.M. Enterically induced immunologic tolerance. I. Induction of suppressor T lymphocytes by intragastric administration of soluble proteins. J. Immunol 1978, 121, 2429–2434. [Google Scholar]
- Li, T.; Takeda, N.; Miyamura, T. Oral administration of hepatitis E virus-like particles induces a systemic and mucosal immune response in mice. Vaccine 2001, 19, 3476–3484. [Google Scholar]
- Kirk, D.D.; McIntosh, K.; Walmsley, A.M.; Peterson, R.K.D. Risk analysis for plant-made vaccines. Transgenic Res 2005, 14, 449–462. [Google Scholar]
- Mestecky, J.; Russell, M.W.; Elson, C.O. Perspectives on mucosal vaccines: Is mucosal tolerance a barrier? J. Immunol 2007, 179, 5633–5638. [Google Scholar]
- Wang, L.; Coppel, R.L. Oral vaccine delivery: Can it protect against non-mucosal pathogens? Expert Rev. Vaccines 2008, 7, 729–738. [Google Scholar]
- Swarbrick, E.T.; Stokes, C.R.; Soothill, J.F. Absorption of antigens after oral immunisation and the simultaneous induction of specific systemic tolerance. Gut 1979, 20, 121–125. [Google Scholar]
- Garside, P.; Mowat, A.M. Oral tolerance. Semin. Immunol 2001, 13, 177–185. [Google Scholar]
- Friedman, A.; Weiner, H.L. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc. Natl. Acad. Sci. USA 1994, 91, 6688–6692. [Google Scholar]
- Strobel, S. Immunity induced after a feed of antigen during early life: Oral tolerance v. sensitization. Proc. Nutr. Soc 2001, 60, 437–442. [Google Scholar]
- Taams, L.S.; van Rensen, A.J.; Poelen, M.C.; van Els, C.A.; Besseling, A.C.; Wagenaar, J.P.; van Eden, W.; Wauben, M.H. Anergic T cells actively suppress T cell responses via the antigen-presenting cell. Eur. J. Immunol 1998, 28, 2902–2912. [Google Scholar]
- Brandtzaeg, P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 2007, 25, 5467–5484. [Google Scholar]
- Daniell, H.; Singh, N.D.; Mason, H.; Streatfield, S.J. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci 2009, 14, 669–679. [Google Scholar]
- Sunil Kumar, G.B.; Ganapathi, T.R.; Bapat, V.A. Production of hepatitis B surface antigen in recombinant plant systems: An update. Biotechnol. Prog 2007, 23, 532–539. [Google Scholar]
- Shepard, C.W.; Simard, E.P.; Finelli, L.; Fiore, A.E.; Bell, B.P. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol. Rev 2006, 28, 112–125. [Google Scholar]
- Michel, M.-L.; Tiollais, P. Hepatitis B vaccines: Protective efficacy and therapeutic potential. Pathol. Biol 2010, 58, 288–295. [Google Scholar]
- Goldstein, S.T.; Fiore, A.E. Toward the global elimination of hepatitis B virus transmission. J. Pediatr 2001, 139, 343–345. [Google Scholar]
- Yuen, M.-F.; Lai, C.-L. Treatment of chronic hepatitis B: Evolution over two decades. J. Gastroenterol. Hepatol 2011, 26, S138–S143. [Google Scholar]
- WHO Media Centre. Hepatitis B. Available online: http://www.who.int/mediacentre/factsheets/fs204/en/ accessed on 1 July 2012.
- Kew, M.C. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol. Biol 2010, 58, 273–277. [Google Scholar]
- Romano, L.; Paladini, S.; van Damme, P.; Zanetti, A.R. The worldwide impact on the control and protection of viral hepatitis B. Dig. Liver Dis 2011, 43, S2–S7. [Google Scholar]
- Shchelkunov, S.N.; Shchelkunova, G.A. Plant-based vaccines against human hepatitis B virus. Expert Rev. Vaccines 2010, 9, 947–955. [Google Scholar]
- Guan, Z.-J.; Guo, B.; Huo, Y.-L.; Guan, Z.-P.; Wei, Y.-H. Overview of expression of hepatitis B surface antigen in transgenic plants. Vaccine 2010, 28, 7351–7362. [Google Scholar]
- Komarova, T.V.; Baschieri, S.; Donini, M.; Marusic, C.; Benvenuto, E.; Dorokhov, Y.L. Transient expression systems for plant-derived biopharmaceuticals. Expert Rev. Vaccines 2010, 9, 859–876. [Google Scholar]
- Tiwari, S.; Verma, P.C.; Singh, P.K.; Tuli, R. Plants as bioreactors for the production of vaccine antigens. Biotechnol. Adv. 2011, 27, 449–467. [Google Scholar]
- Çelik, E.; Çalık, P. Production of recombinant proteins by yeast cells. Biotechnol. Adv 2012, 30, 1108–1118. [Google Scholar]
- Bosch, D.; Schots, A. Plant glycans: Friend or foe in vaccine development? Exp. Rev. Vaccines 2010, 9, 835–842. [Google Scholar]
- Diminsky, D.; Moav, N.; Gorecki, M.; Barenholz, Y. Physical, chemical and immunological stability of CHO-derived hepatitis B surface antigen (HBsAg) particles. Vaccine 2000, 18, 3–17. [Google Scholar]
- Kapusta, J.; Pniewski, T.; Bociąg, P.; Wojciechowicz, J.; Płucienniczak, A. Nanogram doses of alum-adjuvanted HBs antigen induce humoral immune response in mice when orally administered. Arch. Immunol. Ther. Exp 2010, 58, 143–151. [Google Scholar]
- Borges, O.; Tavares, J.; de Sousa, A.; Borchard, G.; Junginger, H.E.; Cordeiro-da-Silva, A. Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles. Eur. J. Pharm. Sci 2007, 32, 278–290. [Google Scholar]
- Shukla, A.; Khatri, K.; Gupta, P.N.; Goyal, A.K.; Mehta, A.; Vyas, S.P. Oral immunization against hepatitis B using bile salt stabilized vesicles (bilosomes). J. Pharm. Pharm. Sci 2008, 11, 59–66. [Google Scholar]
- Pniewski, T. Is an oral plant-based vaccine against Hepatitis B Virus possible? Curr. Pharm. Biotechnol 2012, 13, 2692–2704. [Google Scholar]
- Wee, J.L.; Scheerlinck, J.P.; Snibson, K.J.; Edwards, S.; Pearse, M.; Quinn, C.; Sutton, P. Pulmonary delivery of ISCOMATRIX influenza vaccine induces both systemic and mucosal immunity with antigen dose sparing. Mucosal Immunol. 2008, 1, 489–496; erratum. Mucosal Immunol 2009, 2, 184. [Google Scholar]
- Skene, C.D.; Sutton, P. Saponin-adjuvanted particulate vaccines for clinical use. Methods 2006, 40, 53–59. [Google Scholar]
- Vajdy, M. Immunomodulatory properties of vitamins, flavonoids and plant oils and their potential as vaccine adjuvants and delivery systems. Expert Opin. Biol. Ther 2011, 11, 1501–1513. [Google Scholar]
- Wang, Y.; Wang, W.; Li, N.; Yu, Y.; Cao, X. Activation of antigen-presenting cells by immunostimulatory plant DNA: A natural resource for potential adjuvant. Vaccine 2002, 20, 2764–2771. [Google Scholar]
- Lai, H.; Chen, Q. Bioprocessing of plant-derived virus-like particles of Norwalk virus capsid protein under current Good Manufacture Practice regulations. Plant Cell Rep 2012, 31, 573–584. [Google Scholar]
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pniewski, T. The Twenty-Year Story of a Plant-Based Vaccine Against Hepatitis B: Stagnation or Promising Prospects? Int. J. Mol. Sci. 2013, 14, 1978-1998. https://doi.org/10.3390/ijms14011978
Pniewski T. The Twenty-Year Story of a Plant-Based Vaccine Against Hepatitis B: Stagnation or Promising Prospects? International Journal of Molecular Sciences. 2013; 14(1):1978-1998. https://doi.org/10.3390/ijms14011978
Chicago/Turabian StylePniewski, Tomasz. 2013. "The Twenty-Year Story of a Plant-Based Vaccine Against Hepatitis B: Stagnation or Promising Prospects?" International Journal of Molecular Sciences 14, no. 1: 1978-1998. https://doi.org/10.3390/ijms14011978



