Corneal Stromal Cell Growth on Gelatin/Chondroitin Sulfate Scaffolds Modified at Different NHS/EDC Molar Ratios
Abstract
:1. Introduction
2. Results and Discussion
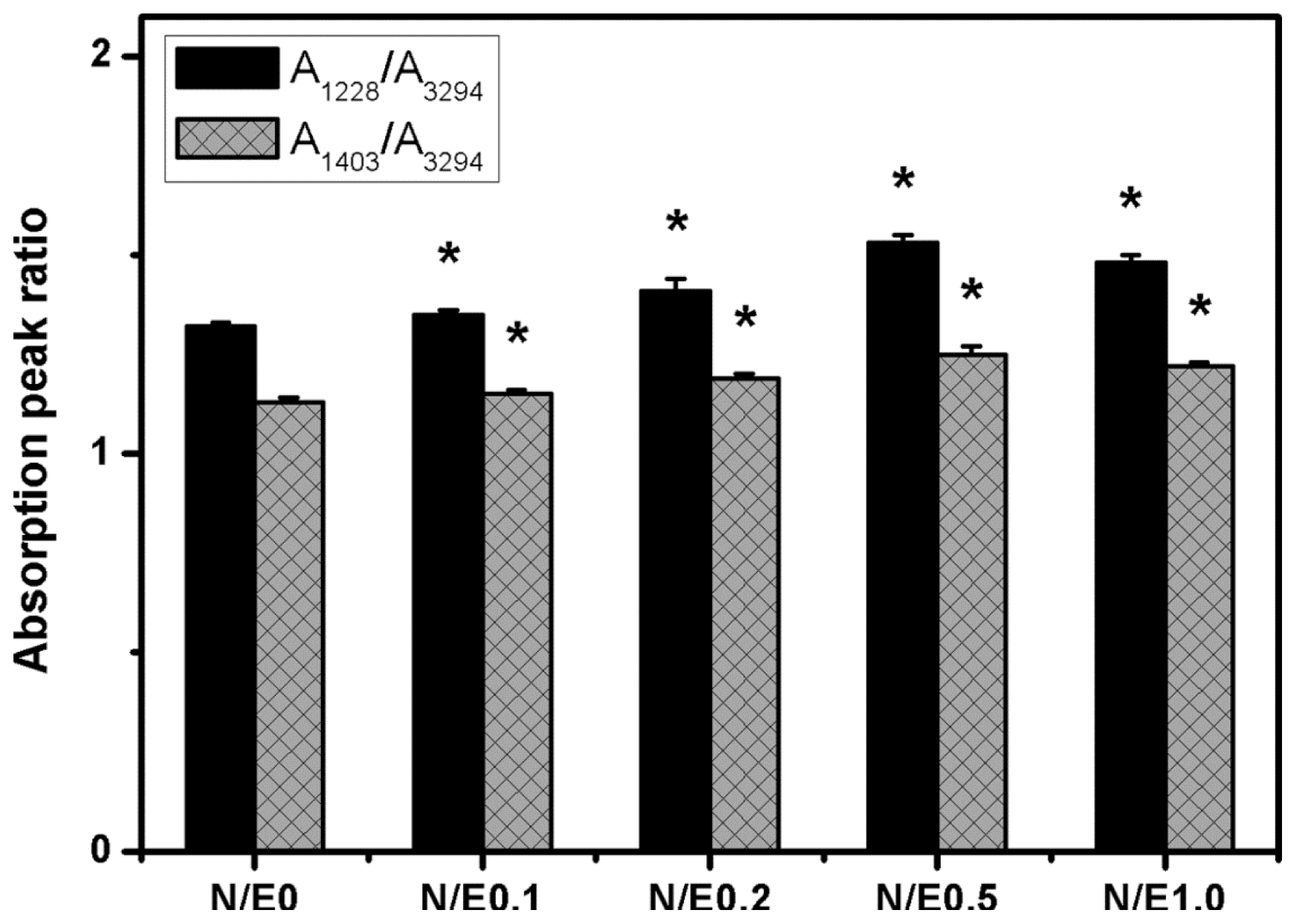
2.1. Fourier Transform Infrared (FTIR) Spectroscopy
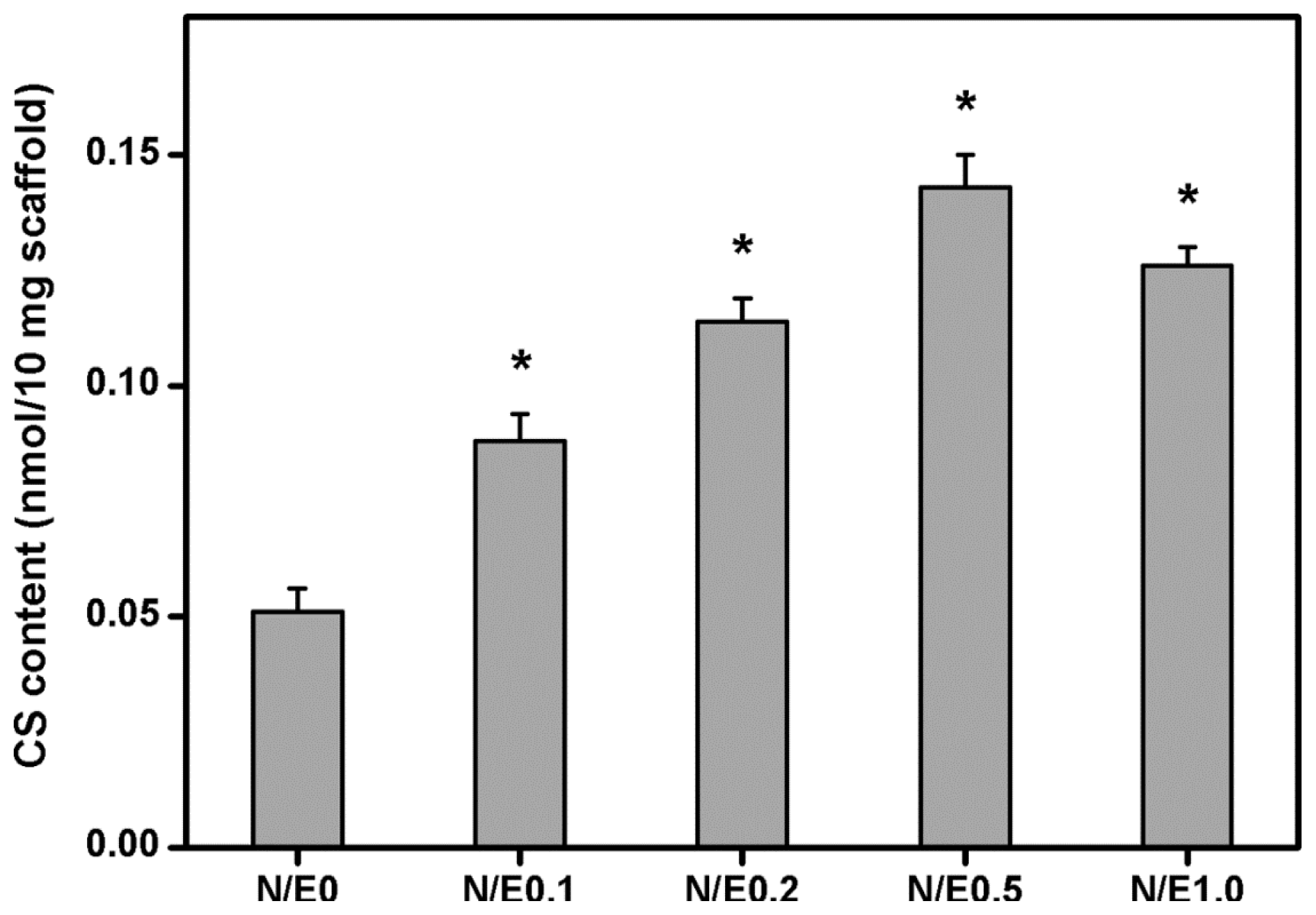
2.2. Dimethylmethylene Blue Assays
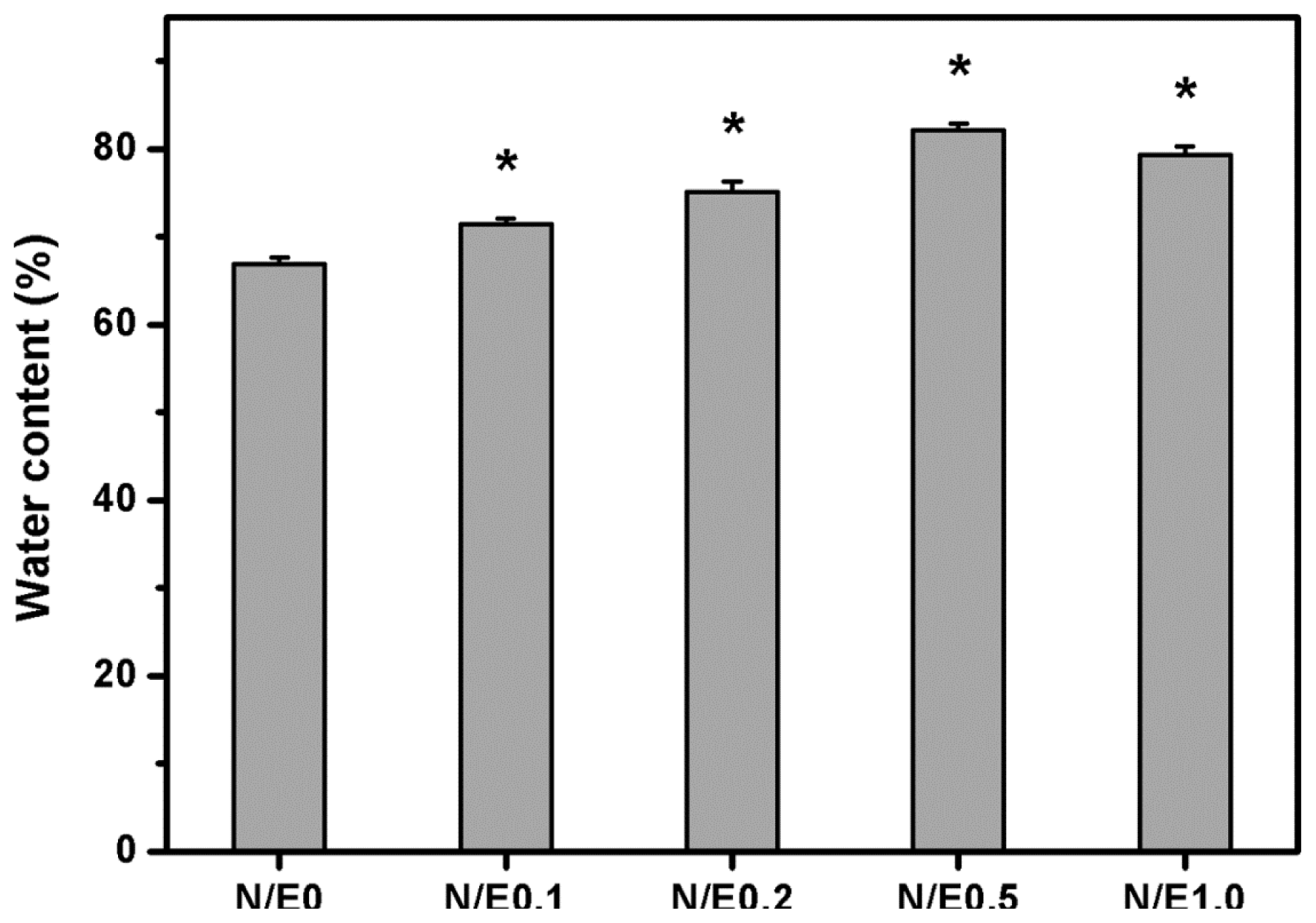
2.3. Water Content Measurements
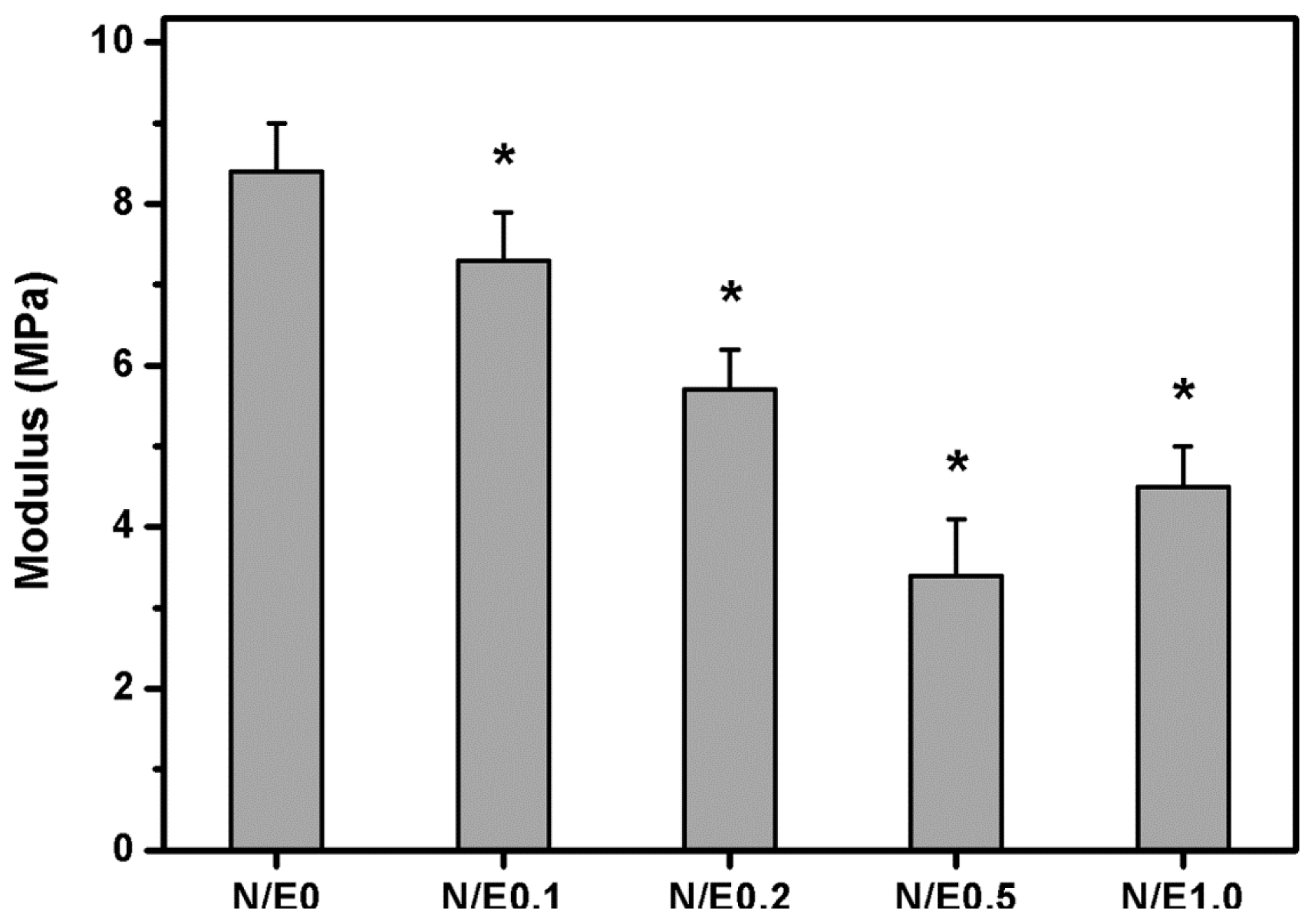
2.4. Mechanical Tests
2.5. Glucose Permeation Studies
2.6. In Vitro Biocompatibility Studies
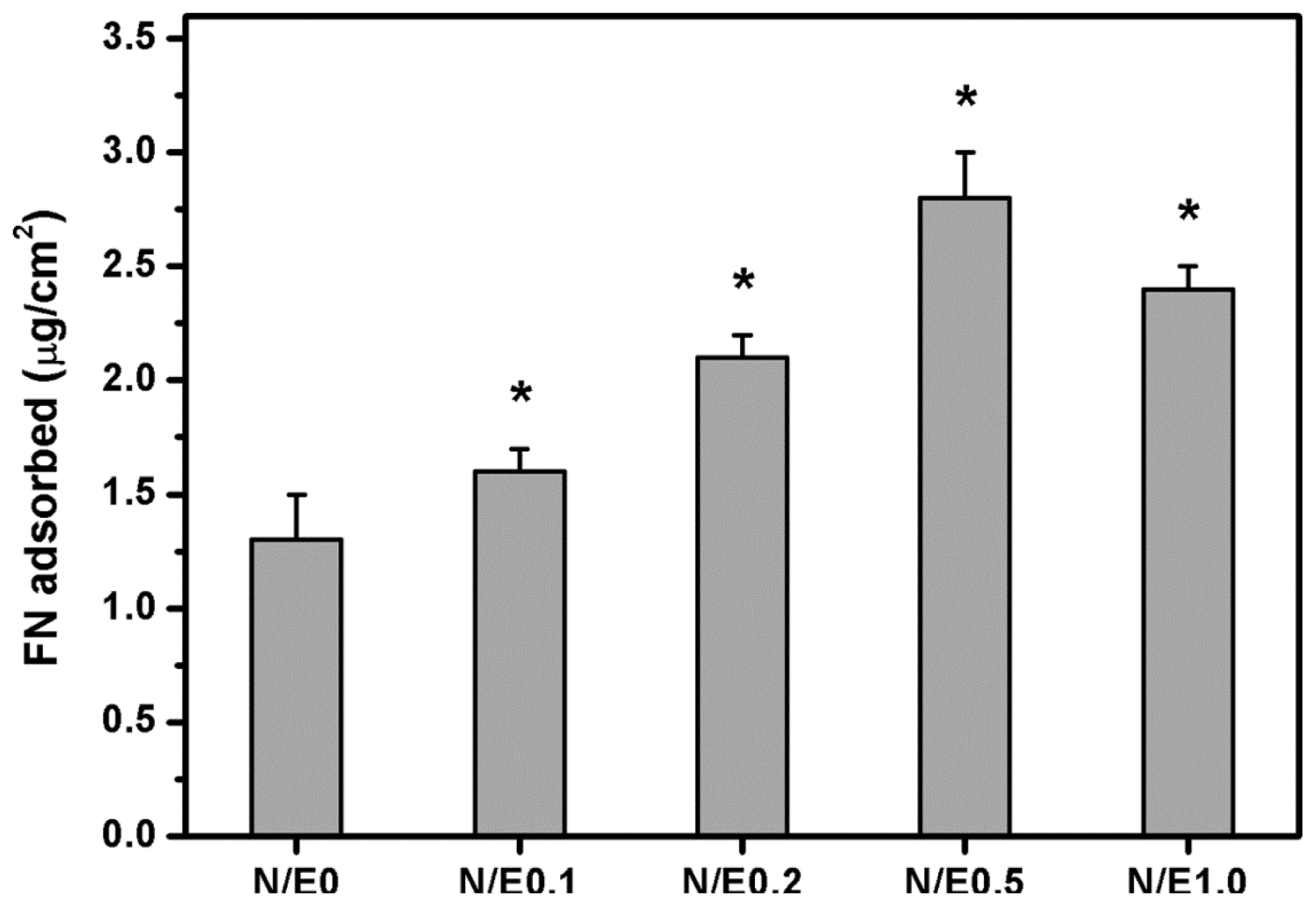
2.7. Protein Adsorption Studies
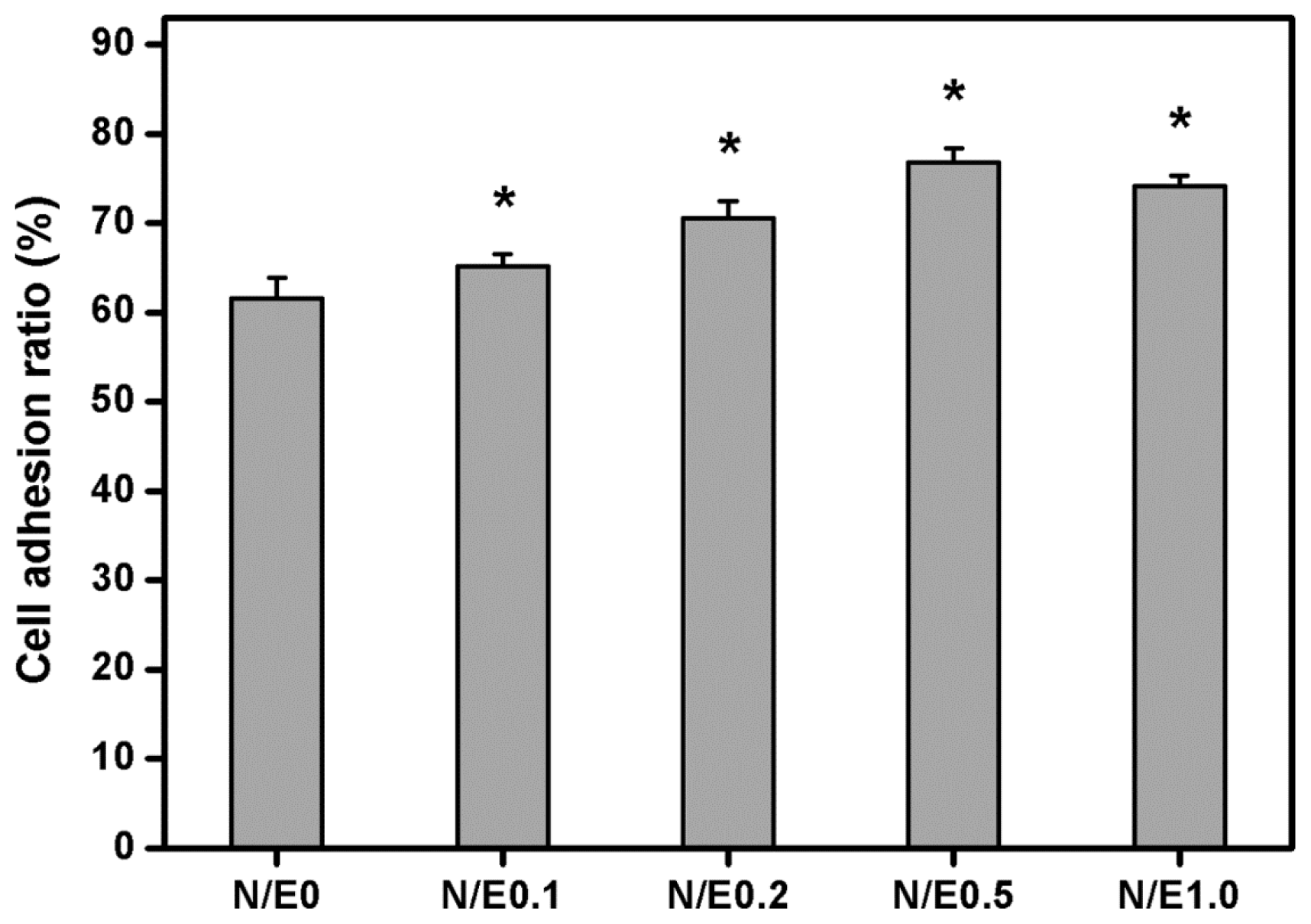
2.8. Cell Adhesion Assays
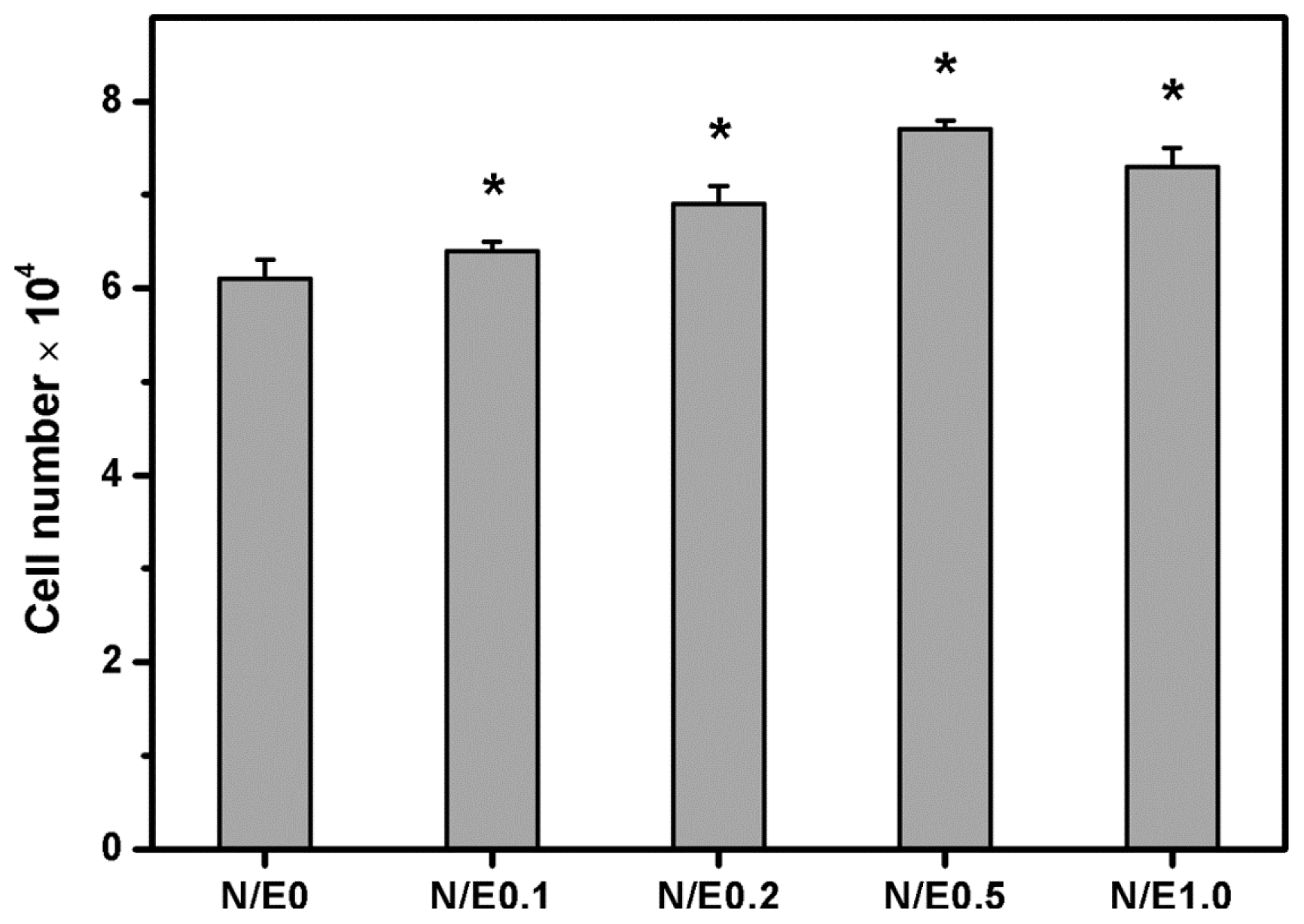
2.9. Cell Proliferation Assays
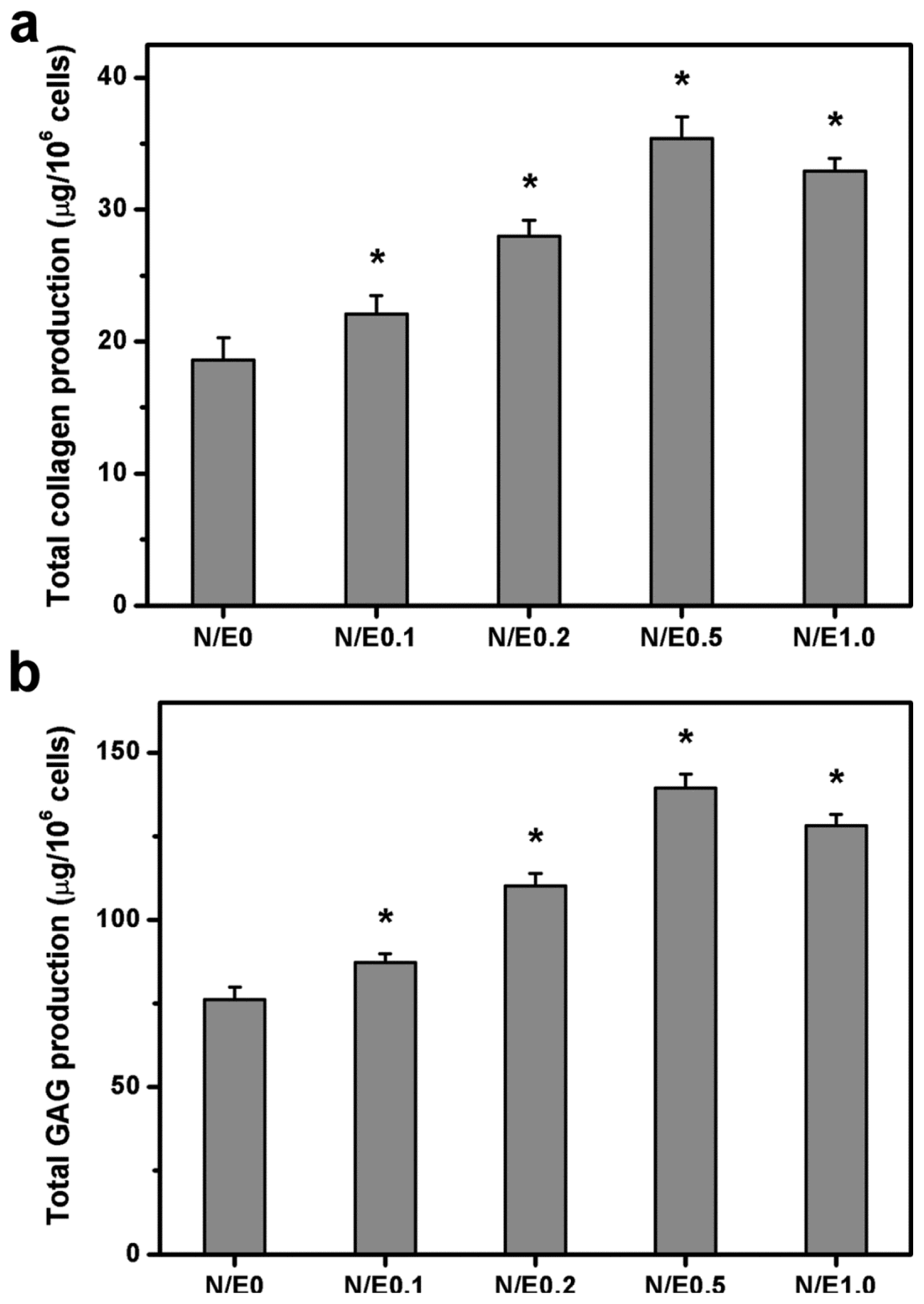
2.10. Extracellular Matrix Production Assays
3. Experimental Section
3.1. Materials
3.2. Modification of Porous Gelatin Scaffolds with Chondroitin Sulfate
3.3. Fourier Transform Infrared (FTIR) Spectroscopy
3.4. Dimethylmethylene Blue Assays
3.5. Water Content Measurements
3.6. Mechanical Tests
3.7. Glucose Permeation Studies
3.8. Isolation and Culture of Rabbit Corneal Keratocytes
3.9. In Vitro Biocompatibility Studies
3.10. Protein Adsorption Studies
3.11. Cell Adhesion Assays
3.12. Cell Proliferation Assays
3.13. Extracellular Matrix Production Assays
3.14. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-02036-s001.pdfAcknowledgments
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar]
- Lai, J.Y.; Hsiue, G.H. Functional biomedical polymers for corneal regenerative medicine. React. Funct. Polym 2007, 67, 1284–1291. [Google Scholar]
- Li, F.; Carlsson, D.; Lohmann, C.; Suuronen, E.; Vascotto, S.; Kobuch, K.; Sheardown, H.; Munger, R.; Nakamura, M.; Griffith, M. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc. Natl. Acad. Sci. USA 2003, 100, 15346–15351. [Google Scholar]
- Liu, Y.; Gan, L.; Carlsson, D.J.; Fagerholm, P.; Lagali, N.; Watsky, M.A.; Munger, R.; Hodge, W.G.; Priest, D.; Griffith, M. A simple, cross-linked collagen tissue substitute for corneal implantation. Invest. Ophthalmol. Vis. Sci 2006, 47, 1869–1875. [Google Scholar]
- Vrana, N.E.; Builles, N.; Justin, V.; Bednarz, J.; Pellegrini, G.; Ferrari, B.; Damour, O.; Hulmes, D.J.S.; Hasirci, V. Development of a reconstructed cornea from collagen-chondroitin sulfate foams and human cell cultures. Invest. Ophthalmol. Vis. Sci 2008, 49, 5325–5331. [Google Scholar]
- Lai, J.Y.; Ma, D.H.K.; Cheng, H.Y.; Sun, C.C.; Huang, S.J.; Li, Y.T.; Hsiue, G.H. Ocular biocompatibility of carbodiimide cross-linked hyaluronic acid hydrogels for cell sheet delivery carriers. J. Biomater. Sci. Polym. Ed 2010, 21, 359–376. [Google Scholar]
- Lai, J.Y.; Li, Y.T. Functional assessment of cross-linked porous gelatin hydrogels for bioengineered cell sheet carriers. Biomacromolecules 2010, 11, 1387–1397. [Google Scholar]
- Ma, D.H.K.; Lai, J.Y.; Cheng, H.Y.; Tsai, C.C.; Yeh, L.K. Carbodiimide cross-linked amniotic membranes for cultivation of limbal epithelial cells. Biomaterials 2010, 31, 6647–6658. [Google Scholar]
- Nakajima, N.; Ikada, Y. Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjug. Chem 1995, 6, 123–130. [Google Scholar]
- Lai, J.Y.; Hsieh, A.C. A gelatin-g-poly(N-isopropylacrylamide) biodegradable in situ gelling delivery system for the intracameral administration of pilocarpine. Biomaterials 2012, 33, 2372–2387. [Google Scholar]
- Lai, J.Y.; Li, Y.T.; Cho, C.H.; Yu, T.C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int. J. Nanomed 2012, 7, 1101–1114. [Google Scholar]
- Wang, H.J.; Di, L.; Ren, Q.S.; Wang, J.Y. Applications and degradation of proteins used as tissue engineering materials. Materials 2009, 2, 613–635. [Google Scholar]
- Kuijpers, A.J.; Engbers, G.H.M.; Krijgsveld, J.; Zaat, S.A.J.; Dankert, J.; Feijen, J. Cross-linking and characterisation of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. Ed 2000, 11, 225–243. [Google Scholar]
- Olde Damink, L.H.H.; Dijkstra, P.J.; van Luyn, M.J.A.; van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996, 17, 765–773. [Google Scholar]
- Lu, P.L.; Lai, J.Y.; Tabata, Y.; Hsiue, G.H. A methodology based on the “anterior chamber of rabbit eyes” model for noninvasively determining the biocompatibility of biomaterials in an immune privileged site. J. Biomed. Mater. Res. A 2008, 86, 108–116. [Google Scholar]
- Hsiue, G.H.; Lai, J.Y.; Chen, K.H.; Hsu, W.M. A novel strategy for corneal endothelial reconstruction with a bioengineered cell sheet. Transplantation 2006, 81, 473–476. [Google Scholar]
- Lai, J.Y.; Chen, K.H.; Hsiue, G.H. Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials. Transplantation 2007, 84, 1222–1232. [Google Scholar]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev 2007, 59, 207–233. [Google Scholar]
- Leonard, D.W.; Meek, K.M. Refractive indices of the collagen fibrils and extrafibrillar material of the corneal stroma. Biophys. J 1997, 72, 1382–1387. [Google Scholar]
- Monti, D.; Chetoni, P.; Burgalassi, S.; Najarro, M.; Saettone, M.F. Increased corneal hydration induced by potential ocular penetration enhancers: assessment by differential scanning calorimetry (DSC) and by desiccation. Int. J. Pharm 2002, 232, 139–147. [Google Scholar]
- Hsiue, G.H.; Lai, J.Y.; Lin, P.K. Absorbable sandwich-like membrane for retinal-sheet transplantation. J. Biomed. Mater. Res 2002, 61, 19–25. [Google Scholar]
- Lai, J.Y.; Lin, P.K.; Hsiue, G.H.; Cheng, H.Y.; Huang, S.J.; Li, Y.T. Low Bloom strength gelatin as a carrier for potential use in retinal sheet encapsulation and transplantation. Biomacromolecules 2009, 10, 310–319. [Google Scholar]
- Lu, P.L.; Lai, J.Y.; Ma, D.H.K.; Hsiue, G.H. Carbodiimide cross-linked hyaluronic acid hydrogels as cell sheet delivery vehicles: characterization and interaction with corneal endothelial cells. J. Biomater. Sci.-Polym. Ed 2008, 19, 1–18. [Google Scholar]
- Lai, J.Y. Solvent composition is critical for carbodiimide cross-linking of hyaluronic acid as an ophthalmic biomaterial. Materials 2012, 5, 1986–2002. [Google Scholar]
- Zeugolis, D.I.; Paul, G.R.; Attenburrow, G. Cross-linking of extruded collagen fibers—a biomimetic three-dimensional scaffold for tissue engineering applications. J. Biomed. Mater. Res. A 2009, 89, 895–908. [Google Scholar]
- Jayasuriya, A.C.; Scheinbeim, J.I.; Lubkin, V.; Bennett, G.; Kramer, P. Piezoelectric and mechanical properties in bovine cornea. J. Biomed. Mater. Res. A 2003, 66, 260–265. [Google Scholar]
- Chayakul, V.; Reim, M. The enzymatic activities in the alkali-burnt rabbit cornea. Graefes Arch. Clin. Exp. Ophthalmol 1982, 218, 145–148. [Google Scholar]
- Lai, J.Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci.-Mater. Med 2010, 21, 1899–1911. [Google Scholar]
- Lai, J.Y.; Li, Y.T.; Wang, T.P. In vitro response of retinal pigment epithelial cells exposed to chitosan materials prepared with different cross-linkers. Int. J. Mol. Sci 2010, 11, 5256–5272. [Google Scholar]
- Lai, J.Y.; Wang, T.P.; Li, Y.T.; Tu, I.H. Synthesis, characterization and ocular biocompatibility of potential keratoprosthetic hydrogels based on photopolymerized poly(2-hydroxyethyl methacrylate)-co-poly(acrylic acid). J. Mater. Chem 2012, 22, 1812–1823. [Google Scholar]
- Lai, J.Y. Biocompatibility of genipin and glutaraldehyde cross-linked chitosan materials in the anterior chamber of the eye. Int. J. Mol. Sci 2012, 13, 10970–10985. [Google Scholar]
- Strehin, I.; Nahas, Z.; Arora, K.; Nguyen, T.; Elisseeff, J. A versatile pH sensitive chondroitin sulfate-PEG tissue adhesive and hydrogel. Biomaterials 2010, 31, 2788–2797. [Google Scholar]
- Yamada, K.M.; Olden, K. Fibronectins-adhesive glycoproteins of cell surface and blood. Nature 1978, 275, 179–184. [Google Scholar]
- Wasylnka, J.A.; Moore, M.M. Adhesion of Aspergillus species to extracellular matrix proteins: evidence for involvement of negatively charged carbohydrates on the conidial surface. Infect. Immun 2000, 68, 3377–3384. [Google Scholar]
- Lai, J.Y.; Tu, I.H. Adhesion, phenotypic expression, and biosynthetic capacity of corneal keratocytes on surfaces coated with hyaluronic acid of different molecular weights. Acta Biomater 2012, 8, 1068–1079. [Google Scholar]
- Rammelt, S.; Illert, T.; Bierbaum, S.; Scharnweber, D.; Zwipp, H.; Schneiders, W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 2006, 27, 5561–5571. [Google Scholar]
- Zou, X.H.; Jiang, Y.Z.; Zhang, G.R.; Jin, H.M.; Hieu Nguyen, T.M.; Ouyang, H.W. Specific interactions between human fibroblasts and particular chondroitin sulfate molecules for wound healing. Acta Biomater 2009, 5, 1588–1595. [Google Scholar]
- Milev, P.; Monnerie, H.; Popp, S.; Margolis, R.K.; Margolis, R.U. The core protein of the chondroitin sulfate proteoglycan phosphacan is a high-affinity ligand of fibroblast growth factor-2 and potentiates its mitogenic activity. J. Biol. Chem 1998, 273, 21439–21442. [Google Scholar]
- Smith, S.M.L.; West, L.A.; Govindraj, P.; Zhang, X.; Ornitz, D.M.; Hassell, J.R. Heparan and chondroitin sulfate on growth plate perlecan mediate binding and delivery of FGF-2 to FGF receptors. Matrix Biol 2007, 26, 175–184. [Google Scholar]
- Van Susante, J.L.C.; Pieper, J.; Buma, P.; van Kuppevelt, T.H.; van Beuningen, H.; van der Kraan, P.M.; Veerkamp, J.H.; van den Berg, W.B.; Veth, R.P.H. Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials 2001, 22, 2359–2369. [Google Scholar]
- Bassleer, C.T.; Combal, J.P.A.; Bougaret, S.; Malaise, M. Effects of chondroitin sulfate and interleukin-1β on human articular chondrocytes cultivated in clusters. Osteoarthritis Cartilage 1998, 6, 196–204. [Google Scholar]
- Cao, H.; Xu, S.Y. EDC/NHS-crosslinked type II collagen-chondroitin sulfate scaffold: characterization and in vitro evaluation. J. Mater. Sci.-Mater. Med 2008, 19, 567–575. [Google Scholar]
- Lai, J.Y.; Li, Y.T. Influence of cross-linker concentration on the functionality of carbodiimide cross-linked gelatin membranes for retinal sheet carriers. J. Biomater. Sci.-Polym. Ed 2011, 22, 277–295. [Google Scholar]
- Lai, J.Y.; Lu, P.L.; Chen, K.H.; Tabata, Y.; Hsiue, G.H. Effect of charge and molecular weight on the functionality of gelatin carriers for corneal endothelial cell therapy. Biomacromolecules 2006, 7, 1836–1844. [Google Scholar]
- Lai, J.Y.; Li, Y.T. Evaluation of cross-linked gelatin membranes as delivery carriers for retinal sheets. Mater. Sci. Eng. C 2010, 30, 677–685. [Google Scholar]
- Lai, J.Y. Evaluation of cross-linking time for porous gelatin hydrogels on cell sheet delivery performance. J. Mech. Med. Biol 2011, 11, 967–981. [Google Scholar]
- Lai, J.Y.; Chen, K.H.; Hsu, W.M.; Hsiue, G.H.; Lee, Y.H. Bioengineered human corneal endothelium for transplantation. Arch. Ophthalmol 2006, 124, 1441–1448. [Google Scholar]
- Lai, J.Y. The role of bloom index of gelatin on the interaction with retinal pigment epithelial cells.


© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lai, J.-Y. Corneal Stromal Cell Growth on Gelatin/Chondroitin Sulfate Scaffolds Modified at Different NHS/EDC Molar Ratios. Int. J. Mol. Sci. 2013, 14, 2036-2055. https://doi.org/10.3390/ijms14012036
Lai J-Y. Corneal Stromal Cell Growth on Gelatin/Chondroitin Sulfate Scaffolds Modified at Different NHS/EDC Molar Ratios. International Journal of Molecular Sciences. 2013; 14(1):2036-2055. https://doi.org/10.3390/ijms14012036
Chicago/Turabian StyleLai, Jui-Yang. 2013. "Corneal Stromal Cell Growth on Gelatin/Chondroitin Sulfate Scaffolds Modified at Different NHS/EDC Molar Ratios" International Journal of Molecular Sciences 14, no. 1: 2036-2055. https://doi.org/10.3390/ijms14012036



