The Effect of Long-Term Storage on the Physiochemical and Bactericidal Properties of Electrochemically Activated Solutions
Abstract
:1. Introduction
2. Results and Discussion
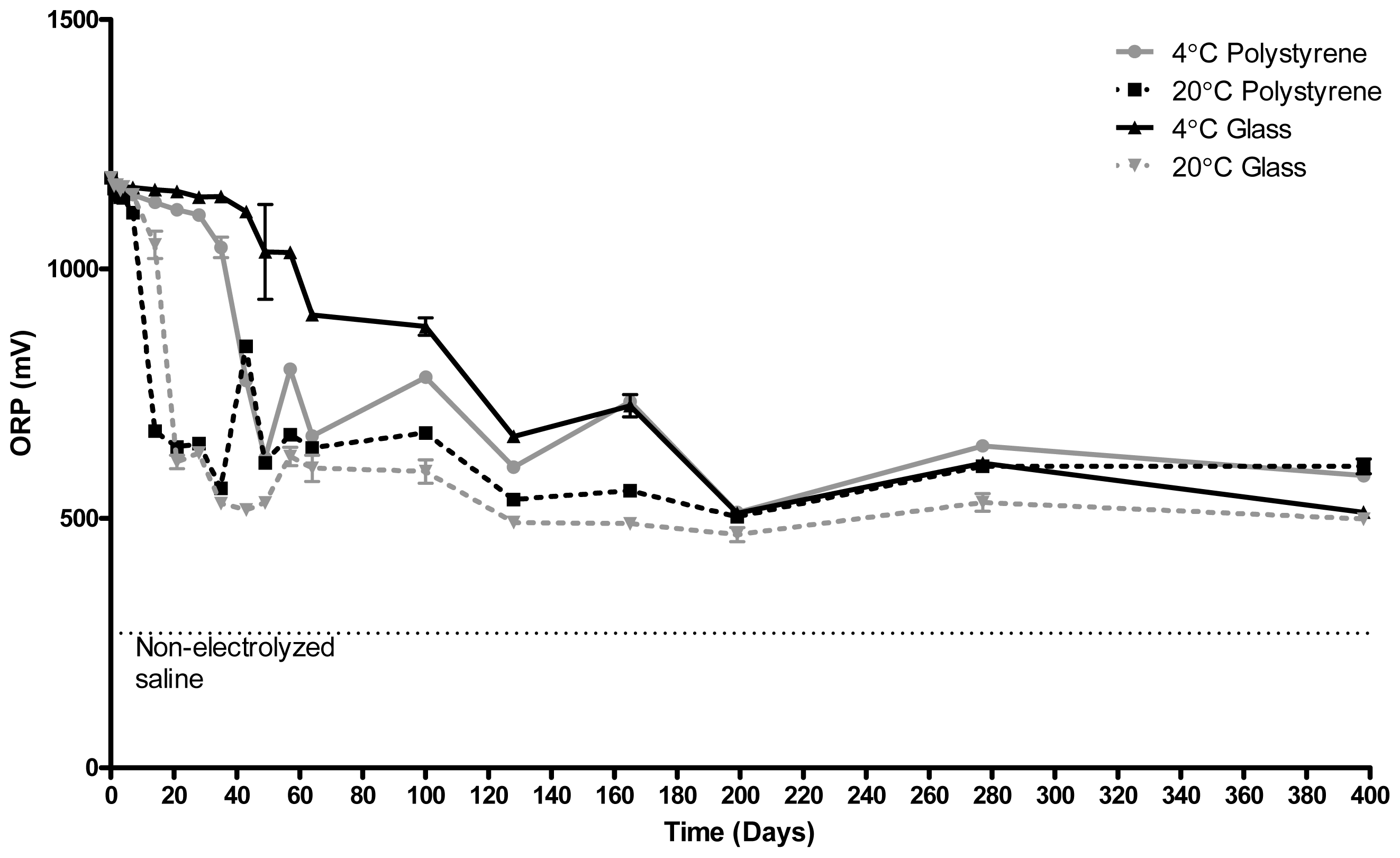
2.1. Redox Potential (ORP)
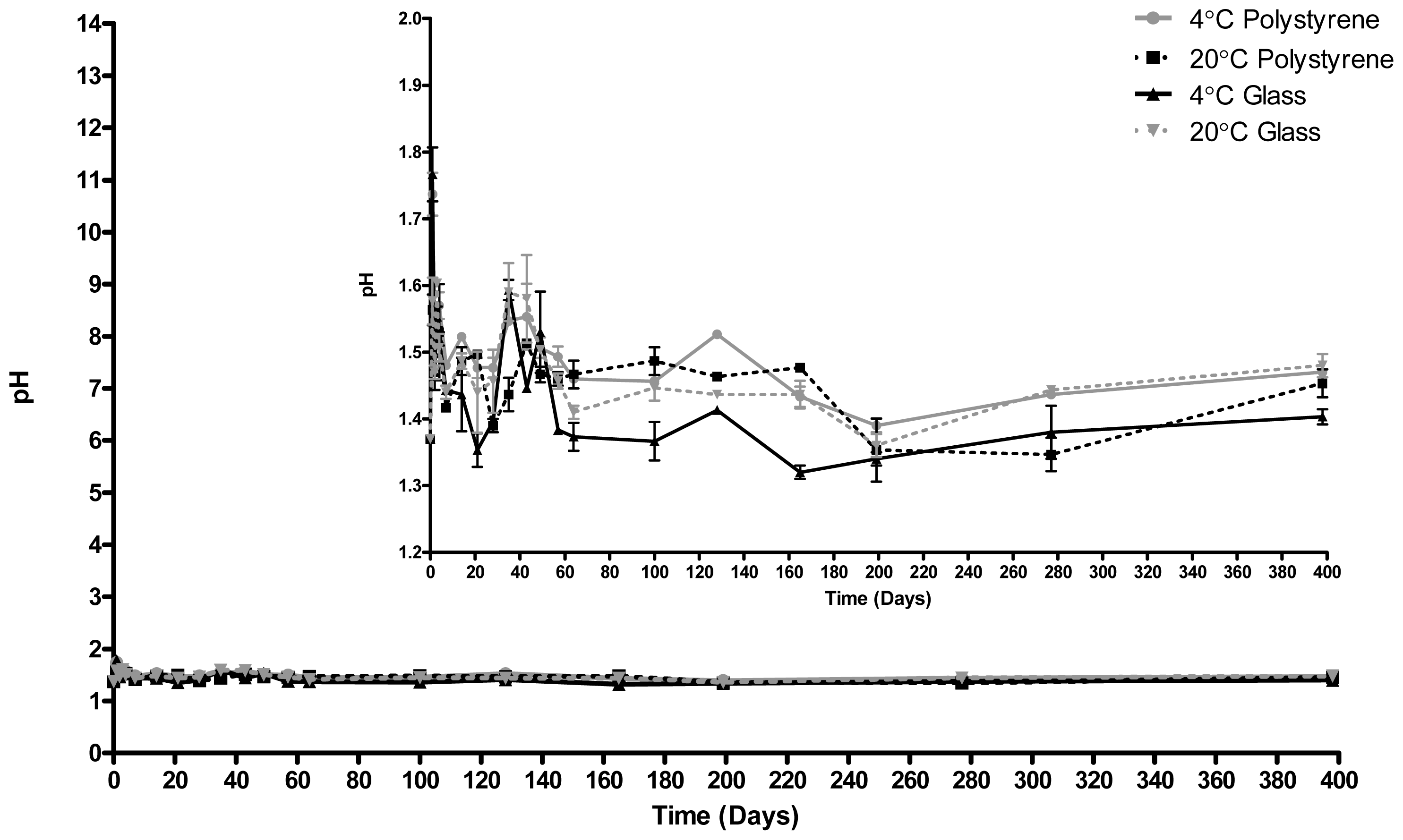
2.2. pH
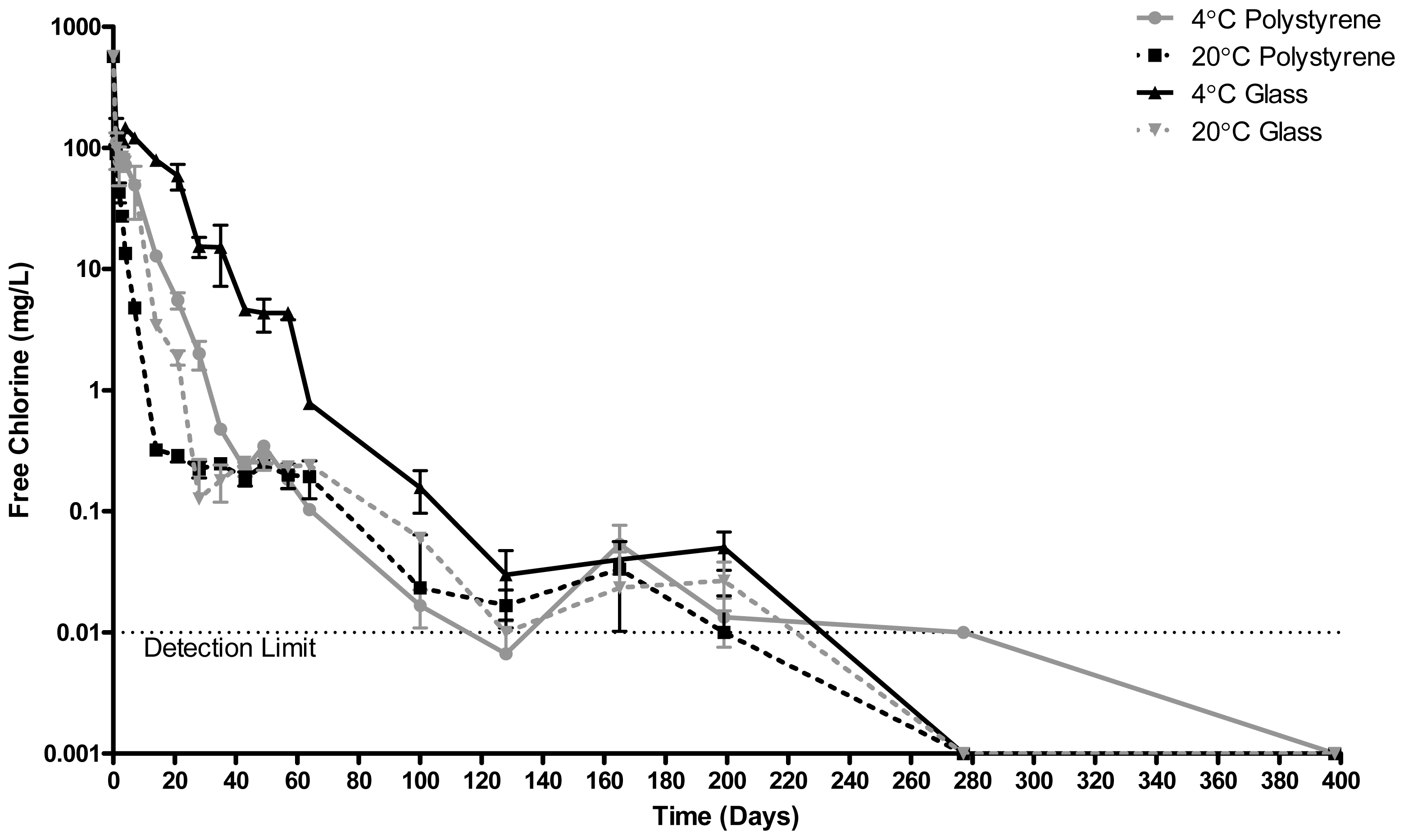
2.3. Free Chlorine
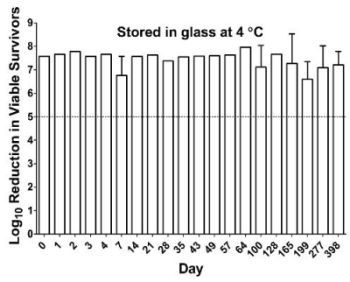
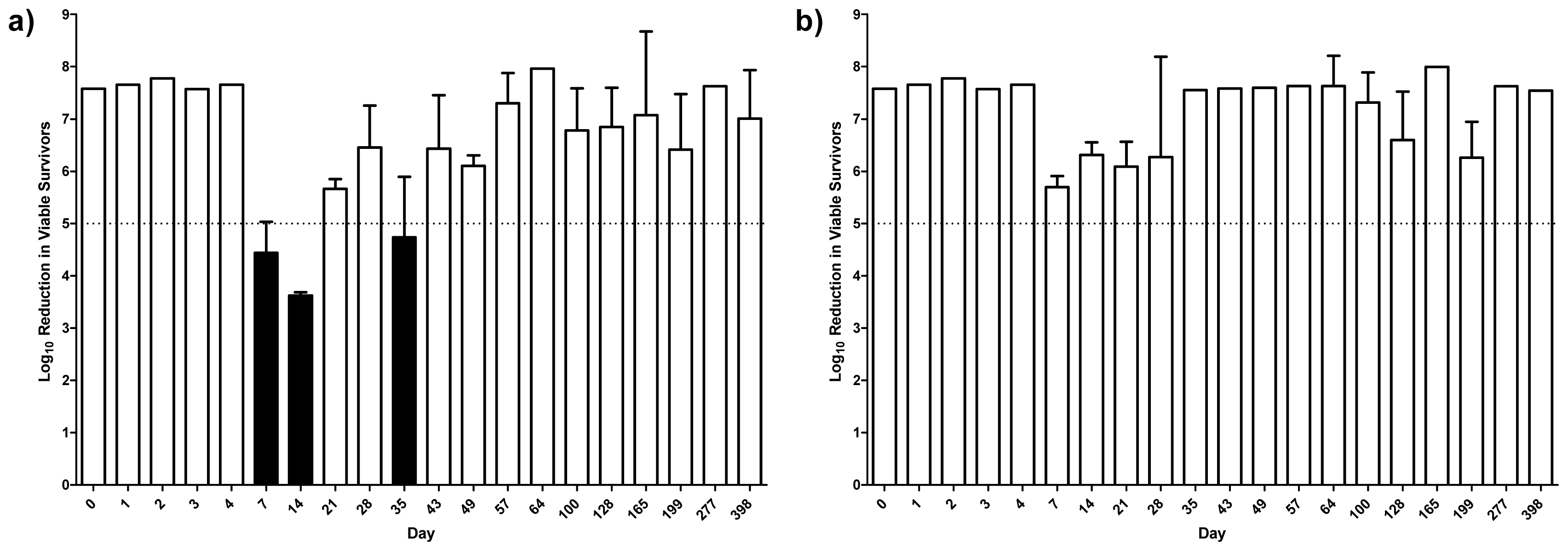
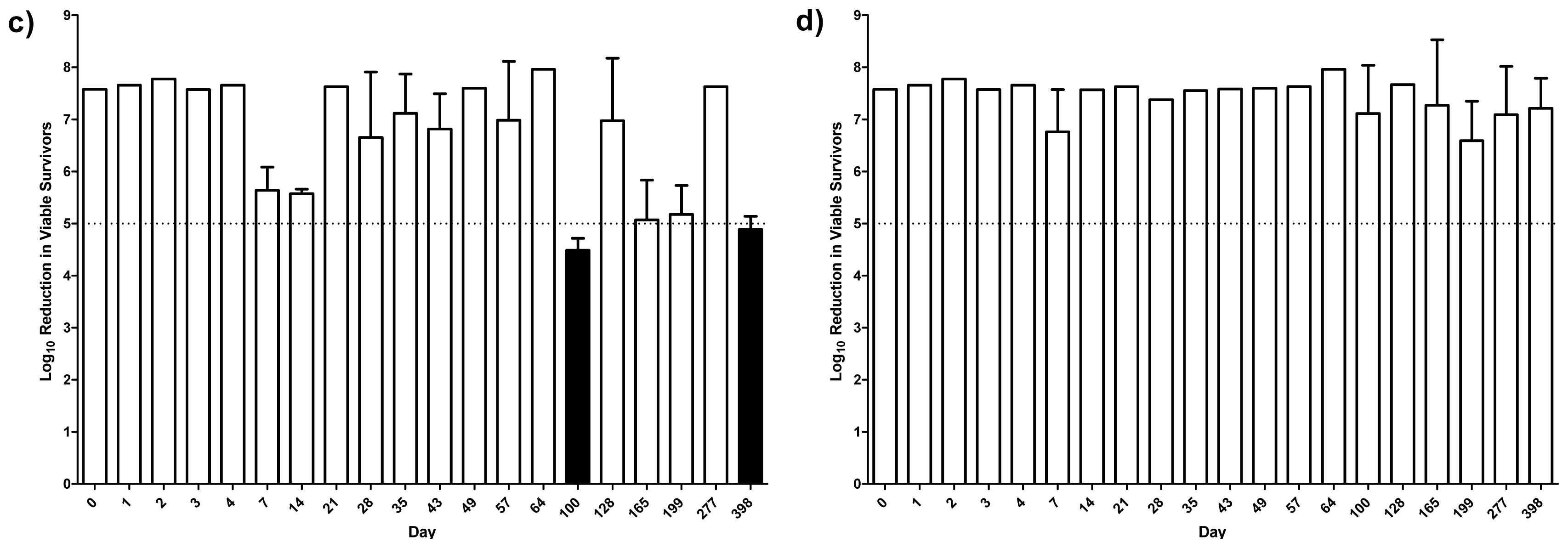
2.4. Antibacterial Activity
2.5. Discussion
3. Experimental Section
3.1. Solutions and Storage Conditions
3.2. Physiochemical Properties
3.3. Bactericidal Activity
3.4. Statistical Analysis
4. Conclusions
- Conflict of InterestThe authors declare no conflict of interest.
References
- Sharma, R.R.; Demirci, A. Treatment of Escherichia coli O157:H7 inoculated alfalfa seeds and sprouts with electrolyzed oxidizing water. Int. J. Food Microbiol 2003, 86, 231–237. [Google Scholar]
- Venczel, L.V.; Arrowood, M.; Hurd, M.; Sobsey, M.D. Inactivation of cryptosporidium parvum oocysts and clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine. Appl. Environ. Microbiol 1997, 63, 1598–1601. [Google Scholar]
- Cloete, T.E.; Thantsha, M.S.; Maluleke, M.R.; Kirkpatrick, R. The antimicrobial mechanism of electrochemically activated water against Pseudomonas aeruginosa and Escherichia coli as determined by SDS-PAGE analysis. J. Appl. Microbiol 2009, 107, 379–384. [Google Scholar]
- Thorn, R.M.; Lee, S.W.; Robinson, G.M.; Greenman, J.; Reynolds, D.M. Electrochemically activated solutions: Evidence for antimicrobial efficacy and applications in healthcare environments. Eur. J. Clin. Microbiol. Infect. Dis 2012, 31, 641–653. [Google Scholar]
- Robinson, G.M.; Lee, S.W.; Greenman, J.; Salisbury, V.C.; Reynolds, D.M. Evaluation of the efficacy of electrochemically activated solutions against nosocomial pathogens and bacterial endospores. Lett. Appl. Microbiol 2010, 50, 289–294. [Google Scholar]
- Shetty, N.; Srinivasan, S.; Holton, J.; Ridgway, G.L. Evaluation of microbicidal activity of a new disinfectant: Sterilox 2500 against Clostridium difficile spores, Helicobacter pylori, vancomycin resistant enterococcus species, Candida albicans and several mycobacterium species. J. Hosp. Infect 1999, 41, 101–105. [Google Scholar]
- Kim, C.; Hung, Y.C.; Brackett, R.E. Efficacy of electrolyzed oxidizing (EO) and chemically modified water on different types of foodborne pathogens. Int. J. Food Microbiol 2000, 61, 199–207. [Google Scholar]
- Rogers, J.V.; Ducatte, G.R.; Choi, Y.W.; Early, P.C. A preliminary assessment of Bacillus anthracis spore inactivation using an electrochemically activated solution (ECASOL). Lett. Appl. Microbiol 2006, 43, 482–488. [Google Scholar]
- Park, G.W.; Boston, D.M.; Kase, J.A.; Sampson, M.N.; Sobsey, M.D. Evaluation of liquid- and fog-based application of sterilox hypochlorous acid solution for surface inactivation of human norovirus. Appl. Environ. Microbiol 2007, 73, 4463–4468. [Google Scholar]
- Tagawa, M.; Yamaguchi, T.; Yokosuka, O.; Matsutani, S.; Maeda, T.; Saisho, H. Inactivation of a hepadnavirus by electrolysed acid water. J. Antimicrob. Chemother 2000, 46, 363–368. [Google Scholar]
- Morita, C.; Sano, K.; Morimatsu, S.; Kiura, H.; Goto, T.; Kohno, T.; Hong, W.; Miyoshi, H.; Iwasawa, A.; Nakamura, Y.; et al. Disinfection potential of electrolyzed solutions containing sodium chloride at low concentrations. J. Virol. Methods 2000, 85, 163–174. [Google Scholar]
- Xiong, K.; Liu, H.; Liu, R.; Li, L. Differences in fungicidal efficiency against Aspergillus flavus for neutralized and acidic electrolyzed oxidizing waters. Int. J. Food Microbiol 2010, 137, 67–75. [Google Scholar]
- Zeng, X.; Ye, G.; Tang, W.; Ouyang, T.; Tian, L.; Ni, Y.; Li, P. Fungicidal efficiency of electrolyzed oxidizing water on Candida albicans and its biochemical mechanism. J. Biosci. Bioeng 2011, 112, 86–91. [Google Scholar]
- Buck, J.W.; van Iersel, M.W.; Oetting, R.D.; Hung, Y.-C. In vitro fungicidal activity of acidic electrolyzed oxidizing water. Plant Dis 2002, 86, 278–281. [Google Scholar]
- Suzuki, T.; Itakura, J.; Watanabe, M.; Ohta, M.; Sato, Y.; Yamaya, Y. Inactivation of Staphylococcal enterotoxin-A with an electrolyzed anodic solution. J. Agric. Food Chem 2002, 50, 230–234. [Google Scholar]
- Huang, Y.; Hung, Y.; Hsu, S.; Huang, Y.; Hwang, D. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar]
- Koide, S.; Takeda, J.; Shi, J.; Shono, H.; Atungulu, G.G. Disinfection efficacy of slightly acidic electrolyzed water on fresh cut cabbage. Food Control 2009, 20, 294–297. [Google Scholar]
- Guentzel, J.L.; Liang Lam, K.; Callan, M.A.; Emmons, S.A.; Dunham, V.L. Reduction of bacteria on spinach, lettuce, and surfaces in food service areas using neutral electrolyzed oxidizing water. Food Microbiol 2008, 25, 36–41. [Google Scholar]
- Rahman, S.M.E.; Ding, T.; Oh, D. Inactivation effect of newly developed low concentration electrolyzed water and other sanitizers against microorganisms on spinach. Food Control 2010, 21, 1383–1387. [Google Scholar]
- Landa-Solis, C.; González-Espinosa, D.; Guzmán-Soriano, B.; Snyder, M.; Reyes-Terán, G.; Torres, K.; Gutierrez, A.A. Microcyntm: A novel super-oxidized water with neutral pH and disinfectant activity. J. Hosp. Infect 2005, 61, 291–299. [Google Scholar]
- Rutala, W.A.; Weber, D.J. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin. Microbiol. Rev 1997, 10, 597–610. [Google Scholar]
- Dufault, R.; LeBlanc, B.; Schnoll, R.; Cornett, C.; Schweitzer, L.; Wallinga, D.; Hightower, J.; Patrick, L.; Lukiw, W.J. Mercury from chlor-alkali plants: Measured concentrations in food product sugar. Environ. Health 2009, 8, 2. [Google Scholar]
- Robinson, G.M.; Tonks, K.M.; Thorn, R.M.; Reynolds, D.M. Application of bacterial bioluminescence to assess the efficacy of fast-acting biocides. Antimicrob. Agents Chemother 2011, 55, 5214–5220. [Google Scholar]
- Marais, J.T.; Williams, W.P. Antimicrobial effectiveness of electro-chemically activated water as an endodontic irrigation solution. Int. Endod. J 2001, 34, 237–243. [Google Scholar]
- Selkon, J.B.; Cherry, G.W.; Wilson, J.M.; Hughes, M.A. Evaluation of hypochlorous acid washes in the treatment of chronic venous leg ulcers. J. Wound Care 2006, 15, 33–37. [Google Scholar]
- Ayebah, B.; Hung, Y. Electrolyzed water and its corrosiveness on various surface materials commonly found in food processing facilities. J. Food Process Eng 2005, 28, 247–264. [Google Scholar]
- Koseki, S.; Yoshida, K.; Isobe, S.; Itoh, K. Efficacy of acidic electrolyzed water for microbial decontamination of cucumbers and strawberries. J. Food Prot 2004, 67, 1247–1251. [Google Scholar]
- Ozer, N.P.; Demirci, A. Electrolyzed oxidizing water treatment for decontamination of raw salmon inoculated with Escherichia coli O157:H7 and Listeria monocytogenes Scott A and response surface modeling. J. Food Eng 2006, 72, 234–241. [Google Scholar]
- Park, H.; Hung, Y.; Brackett, R.E. Antimicrobial effect of electrolyzed water for inactivating campylobacter jejuni during poultry washing. Int. J. Food Microbiol 2002, 72, 77–83. [Google Scholar]
- Fabrizio, K.A.; Cutter, C.N. Stability of electrolyzed oxidizing water and its efficacy against cell suspensions of Salmonella typhimurium and Listeria monocytogenes. J. Food Prot 2003, 66, 1379–1384. [Google Scholar]
- Thantsha, M.S.; Cloete, T.E. The effect of sodium chloride and sodium bicarbonate derived anolytes, and anolyte-catholyte combination on biofilms. Water SA 2006, 32, 237–242. [Google Scholar]
- Tanaka, N.; Fujisawa, T.; Daimon, T.; Fujiwara, K.; Yamamoto, M.; Abe, T. The effect of electrolyzed strong acid aqueous solution on hemodialysis equipment. Artif. Organs 1999, 23, 1055–1062. [Google Scholar]
- Selkon, J.B.; Babb, J.R.; Morris, R. Evaluation of the antimicrobial activity of a new super-oxidized water, sterilox, for the disinfection of endoscopes. J. Hosp. Infect 1999, 41, 59–70. [Google Scholar]
- Park, E.; Alexander, E.; Taylor, G.A.; Costa, R.; Kang, D. The decontaminative effects of acidic electrolyzed water for Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes on green onions and tomatoes with differing organic demands. Food Microbiol 2009, 26, 386–390. [Google Scholar]
- Nisola, G.M.; Yang, X.; Cho, E.; Han, M.; Lee, C.; Chung, W.J. Disinfection performances of stored acidic and neutral electrolyzed waters generated from brine solution. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng 2011, 46, 263–270. [Google Scholar]
- Len, S.V.; Hung, Y.C.; Chung, D.; Anderson, J.L.; Erickson, M.C.; Morita, K. Effects of storage conditions and ph on chlorine loss in electrolyzed oxidizing (EO) water. J. Agric. Food Chem 2002, 50, 209–212. [Google Scholar]
- Hsu, S.; Kao, H. Effects of storage conditions on chemical and physical properties of electrolyzed oxidizing water. J. Food Eng 2004, 65, 465–471. [Google Scholar]
- Kunigk, L.; Schramm, L.J.; Kunigk, C.J. Hypochlorous acid loss from neutral electrolyzed water and sodium hypochlorite solutions upon storage. Braz. J. Food Technol 2008, 11, 153–158. [Google Scholar]
- Cui, X.; Shang, Y.; Shi, Z.; Xin, H.; Cao, W. Physicochemical properties and bactericidal efficiency of neutral and acidic electrolyzed water under different storage conditions. J. Food Eng 2009, 91, 582–586. [Google Scholar]
- Hotta, K.; Kawaguchi, K.; Saitoh, F.; Saito, N.; Suzuki, K.; Ochi, K.; Nakayama, T. Antimicrobial activity of electrolyzed NaCl solutions: Effect on the growth of streptomyces Spp. Actinomycetologica 1994, 8, 51–56. [Google Scholar]
- Tomás-Callejas, A.; Martínez-Hernández, G.B.; Artés, F.; Artés-Hernández, F. Neutral and acidic electrolyzed water as emergent sanitizers for fresh-cut mizuna baby leaves. Postharvest Biol. Technol 2011, 59, 298–306. [Google Scholar]
- Park, H.; Hung, Y.; Chung, D. Effects of chlorine and pH on efficacy of electrolyzed water for inactivating Escherichia coli O157:H7 and Listeria monocytogenes. Int. J. Food Microbiol 2004, 91, 13–18. [Google Scholar]
- Venkitanarayanan, K.S.; Ezeike, G.O.; Hung, Y.C.; Doyle, M.P. Efficacy of electrolyzed oxidizing water for inactivating Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes. Appl. Environ. Microbiol 1999, 65, 4276–4279. [Google Scholar]
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Robinson, G.; Thorn, R.; Reynolds, D. The Effect of Long-Term Storage on the Physiochemical and Bactericidal Properties of Electrochemically Activated Solutions. Int. J. Mol. Sci. 2013, 14, 457-469. https://doi.org/10.3390/ijms14010457
Robinson G, Thorn R, Reynolds D. The Effect of Long-Term Storage on the Physiochemical and Bactericidal Properties of Electrochemically Activated Solutions. International Journal of Molecular Sciences. 2013; 14(1):457-469. https://doi.org/10.3390/ijms14010457
Chicago/Turabian StyleRobinson, Gareth, Robin Thorn, and Darren Reynolds. 2013. "The Effect of Long-Term Storage on the Physiochemical and Bactericidal Properties of Electrochemically Activated Solutions" International Journal of Molecular Sciences 14, no. 1: 457-469. https://doi.org/10.3390/ijms14010457