Coumarins from the Herb Cnidium monnieri and Chemically Modified Derivatives as Antifoulants against Balanus albicostatus and Bugula neritina Larvae
Abstract
:1. Introduction
2. Results and Discussion
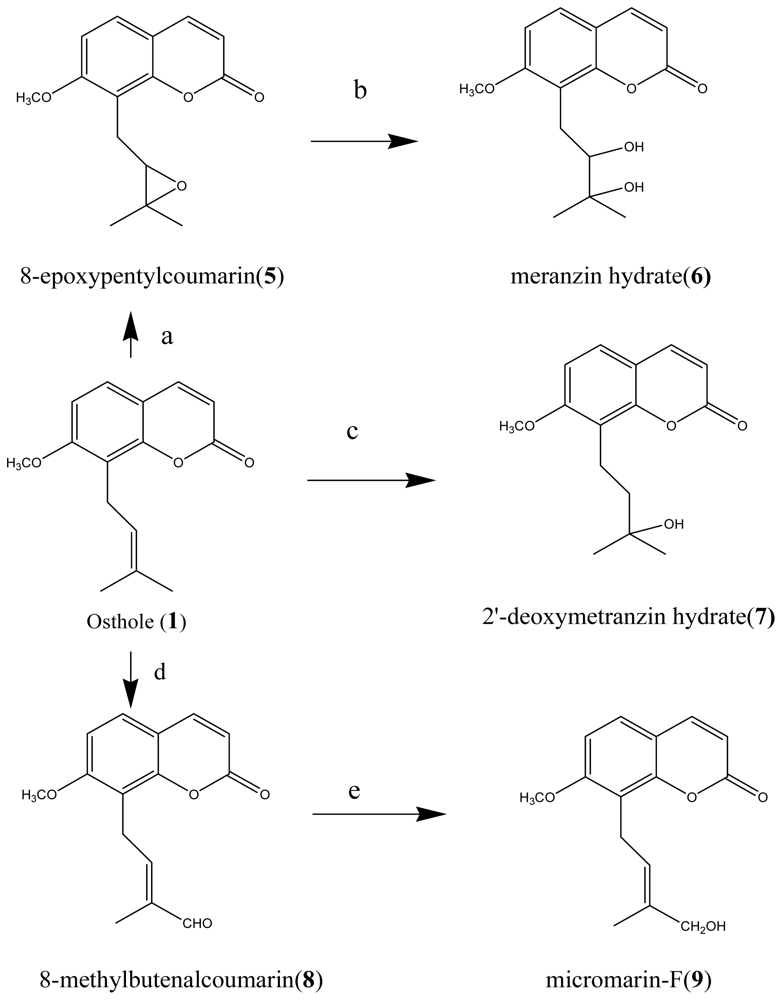
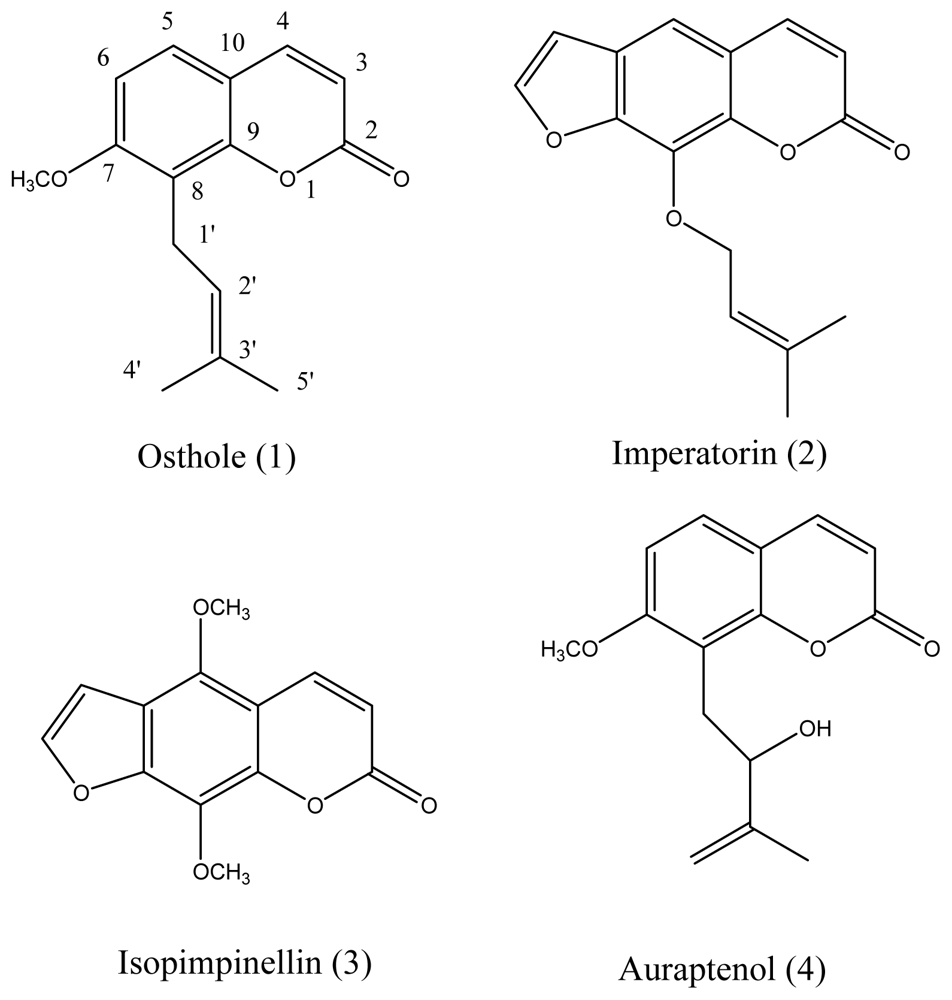
2.1. Isolation, Synthesis and Identification of These Compounds
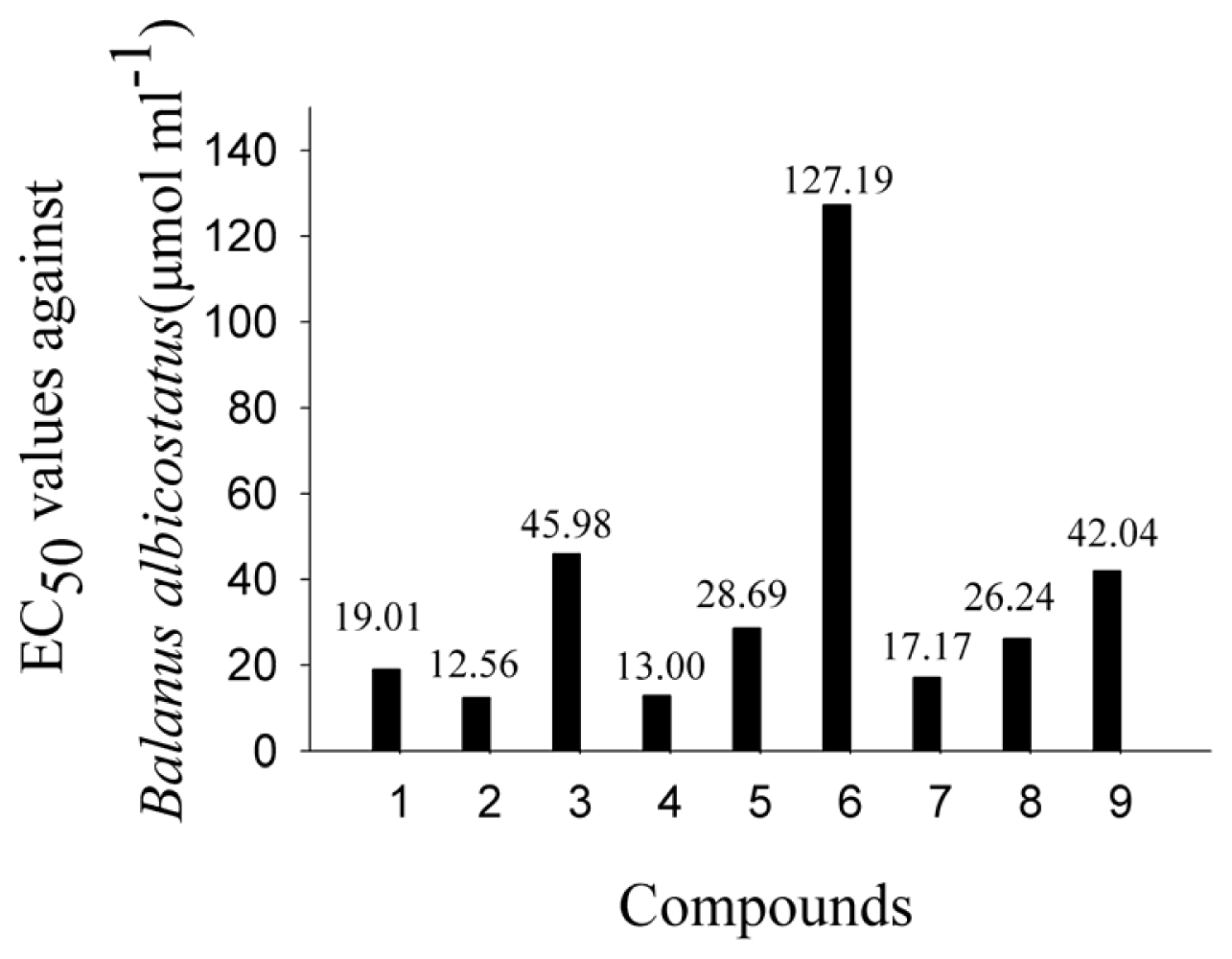
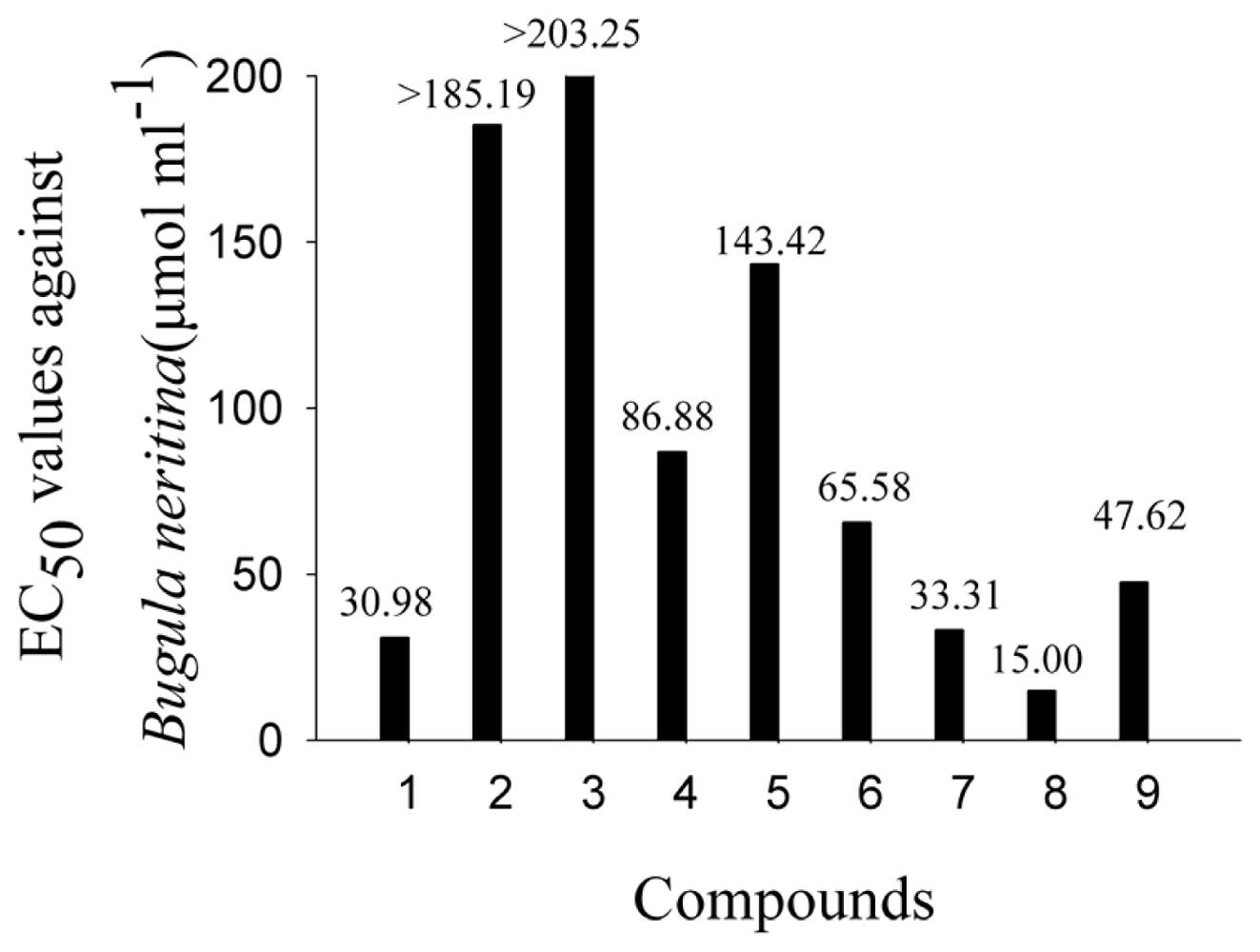
2.2. AF Activity of Compounds
2.3. Impact of the Functional Groups on Antilarval Settlement Activities
3. Experimental Section
3.1. Plant Material and Extraction
3.2. Isolation of Bioactive Compounds and Structural Identification
3.3. Chemical Synthesis of Osthole Derivatives
3.4. AF Assay
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Lewis, J.A. Marine biofouling and its prevention on underwater surfaces. Mater. Forum 1998, 22, 41–61. [Google Scholar]
- Rittschof, D. Natural product antifoulants: One perspective on the challenges related to coatings developments. Biofouling 2000, 15, 119–127. [Google Scholar]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat 2004, 50, 75–104. [Google Scholar]
- Krug, P.J. Defense of Benthic Invertebrates against Surface Colonization by Larvae: A Chemical Arms Race. In Antifouling Compounds; Fusetani, N., Clare, A.S., Eds.; Springer: Berlin, Germany, 2006; pp. 1–53. [Google Scholar]
- Ralston, E.; Swain, G. Bioinspiration-the solution for biofouling control? Bioinspir. Biomim 2009, 4, 1–9. [Google Scholar]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar]
- Abbott, A.; Abel, P.D.; Arnold, D.W.; Milne, A. Cost-benefit analysis of the use of TBT: The case for a treatment approach. Sci. Total Environ 2000, 258, 5–19. [Google Scholar]
- Champ, M.A. A review of organotin regulatory strategies pending actions, related costs and benefits. Sci. Total Environ 2000, 258, 21–71. [Google Scholar]
- Rittschof, D. Fouling and Natural Products as Antifoulants. In Recent Advances in Marine Biotechnology; Fingerman, M., Nagabhushanam, R., Thompson, M.F., Eds.; Oxford & IBH Publishing Company: New Delhi, India, 1999; pp. 245–257. [Google Scholar]
- Rittschof, D. Natural Product Antifoulants and Coatings Developments. In Marine Chemical Ecology; McClintock, J.B., Baker, B.J., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 543–566. [Google Scholar]
- Fusetani, N. Biofouling and antifouling. Nat. Prod. Rep 2004, 21, 94–104. [Google Scholar]
- Sears, M.A.; Gerhart, D.J.; Rittschof, D. Antifouling agents from marine sponge Lissodendoryx isodictyalis Carter. J. Chem. Ecol 1990, 16, 791–799. [Google Scholar]
- Fusetani, N.; Hiroto, H.; Okino, T.; Tomono, Y.; Yoshimura, E. Antifouling activity of isocyanoterpenoids and related compounds isolated from a marine sponge and nudibranchs. J. Nat. Toxins 1996, 5, 249–259. [Google Scholar]
- Clare, A.S.; Rittschof, D.; Gerhart, D.J.; Hooper, I.R.; Bonaventura, J. Antisettlement and narcotic action of analogues of diterpene marine natural product antifoulants from octocorals. Mar. Biotechnol 1999, 1, 427–436. [Google Scholar]
- Cho, J.Y.; Choi, J.S.; Kang, S.E.; Kim, J.K.; Shin, H.W.; Hong, Y.K. Isolation of antifouling active pyroglutamic acid, triethyl citrate and di-n-octylphthalate from the brown seaweed Ishige okamurae. J. Appl. Electrochem 2005, 17, 431–435. [Google Scholar]
- Qi, S.H.; Zhang, S.; Qian, P.Y.; Xiao, Z.H.; Li, M.Y. Ten new antifouling briarane diterpenoids from the South China Sea gorgonian Junceella juncea. Tetrahedron 2006, 62, 9123–9130. [Google Scholar]
- Qi, S.H.; Zhang, S.; Yang, L.H.; Qian, P.Y. Antifouling and antibacterial compounds from the gorgonians Subergorgia suberosa and Scripearia gracillis. Nat. Prod. Res 2008, 22, 154–166. [Google Scholar]
- Raveendran, T.V.; Limna Mol, V.P.; Parameswaran, P.S. Natural Product Antifoulants from the octocorals of Indian waters. Int. Biodeter. Biodegr 2011, 65, 265–268. [Google Scholar]
- Qian, P.Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2010, 26, 223–234. [Google Scholar]
- Feng, D.Q.; Ke, C.H.; Lu, C.Y.; Li, S.J. Herbal plants as a promising source of natural antifoulants: Evidence from barnacle settlement inhibition. Biofouling 2009, 25, 181–190. [Google Scholar]
- Zhou, X.J.; Zhang, Z.; Xu, Y.; Jin, C.L.; He, H.P.; Hao, X.J.; Qian, P.Y. Flavone and isoflavone derivatives of terrestrial plants as larval settlement inhibitors of the barnacle Balanus amphitrite. Biofouling 2009, 25, 69–76. [Google Scholar]
- Yang, S.L.; Zhang, B.G.; Chen, J.; Li, X.E.; Wei, J.H. Strategies for the cultivation of Chinese medicinal plants for the everlasting development and utilization of this great treasure house. Chin. Trad. Herb. Drugs 1999, 30, 870–873. [Google Scholar]
- Chen, Y.; Zhang, G.; Yu, Z. The advancement in the chemical and pharmacological study of the fruits of Cnidium monnieri. J. Shenyang Pharm. Univ 2006, 23, 256–260. [Google Scholar]
- Utinomi, H. Comments on some new and already known cirripedes with emended taxa, with special reference to the parietal structure. Publ. Seto. Mar. Biol. Lab 1967, 15, 199–237. [Google Scholar]
- Newman, W.A.; Ross, A. Revision of balanomorph barnacles, including catalog of the species. Mem. San Diego Soc. Nat. Hist 1976, 9, 101–108. [Google Scholar]
- Lee, C.; Kim, C.H. Larval development of Balanus albicostatus Pilsbry (Cirripedia, Thoracica) reared in the laboratory. J. Exp. Mar. Biol. Ecol 1991, 147, 231–244. [Google Scholar]
- Woollacott, R.M.; Zimmer, R.L. Attachment and metamorphosis of the cheilo-ctenostome bryozoan Bugula neritina (Linne’). J. Morphol 1971, 134, 351–382. [Google Scholar]
- Walters, L.J. Post-settlement success of the arborescent bryozoan Bugula neritina (L.): The importance of structural complexity. J. Exp. Mar. Biol. Ecol 1992, 164, 55–71. [Google Scholar]
- Yu, X.J.; Yan, Y.; Gu, J.D. Attachment of the biofouling bryozoan Bugula neritina larvae affected by inorganic and organic chemical cues. Int. Biodeter. Biodegr 2007, 60, 81–89. [Google Scholar]
- Dahms, H.U.; Dobretsov, S.; Qian, P.Y. The effect of bacterial and diatom biofilms on the settlement of the bryozoan Bugula neritina. J. Exp. Mar. Biol. Ecol 2004, 313, 191–209. [Google Scholar]
- Feng, Y.; Zhang, L.; Cai, J.N.; Wang, Z.T.; Xu, L.S.; Zhang, Z.X. Analysis of Cnidium monnieri fruits in different regions of China. Talanta 2001, 53, 1155–1162. [Google Scholar]
- Yang, X.H.; Liu, S.J.; Yie, H.Z. The inspection and extract of active constituents from the fruits of Cnidium Monnieri (L.) Cusson. J. Guangzhou Med 2003, 34, 67–68. [Google Scholar]
- Fujioka, T.; Furumi, K.; Fujii, H.; Okabe, H.; Mihashi, K.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative constituents from umbelliferae plants. V. A new furanocoumarin and falcarindiol euranocoumarin ethers from the root of Angelica japonica. Chem. Pharm. Bull 1999, 47, 96–100. [Google Scholar]
- Elgamal, M.H.A.; Elewa, N.H.; Elkhrisy, E.A.M.; Duddeck, H. 13C Chemical shift and carbon-proton coupling constants of some furocoumarins and furochromones. Phytochemistry 1979, 18, 139–143. [Google Scholar]
- Harkar, S.; Razdan, T.K.; Waight, E.S. Steroids, chromone and coumarins from Angelica officinalis. Phytochemistry 1984, 23, 419–426. [Google Scholar]
- Chang, C.T.; Doong, S.L.; Tsai, I.L.; Chen, I.S. Coumarins and anti-HBV constituents from Zanthoxylum schionifolium. Phytochemistry 1997, 45, 1419–1422. [Google Scholar]
- Cai, J.N.; Basnet, P.; Wang, Z.T.; Komatsu, K.; Xu, L.S.; Tani, T. Coumarins from the fruits of Cnidium monnieri. J. Nat. Prod 2000, 63, 485–488. [Google Scholar]
- Concannon, S.; Ramachandran, V.N.; Smyth, W.F. A study of the electrospray ionisation and ion trap fragmentation of hemiterpenoid and dimeric coumarin derivatives. Rapid Commun. Mass Spectrom 2000, 14, 2260–2270. [Google Scholar]
- Koul, S.K.; Dhar, K.L.; Thakur, R.S. Regiospecific oxidation of osthol [7-methoxy-8-(isopentenyl)coumarin] with selenium dioxide. Indian J. Chem 1978, 17, 285–287. [Google Scholar]
- Ito, C.; Otsuka, T.; Ruangrungsi, N.; Furukawa, H. Chemical constituents of Micromelum minutum. Isolation and structural elucidation of new coumarins. Chem. Pharm. Bull 2000, 48, 334–338. [Google Scholar]
- Rittschof, D.; Lai, C.H.; Kok, L.M.; Teo, S.L.M. Pharmaceuticals as antifoulants: Concept and principles. Biofouling 2003, 19, 207–212. [Google Scholar]
- Hitotsuyanagi, Y.; Kojima, H.; lkuta, H.; Takeya, K.; ltokawa, H. Identification and structure—activity relationship studies of osthol, a cytotoxic principle from Cnidium Monnieri. Bioorg. Med. Chem. Lett 1996, 6, 1791–1794. [Google Scholar]
- Lin, J.R.; Wang, Y.C.; Xu, Q.H.; Jin, R.H.; Tian, W.S. Formal synthesis of 1α,25-dihydroxyvitamin D3 by utilizing the intact skeleton of diosgenin. Acta Chem. Sinica 2007, 65, 1685–1692. [Google Scholar]
- Bryan, J.P.; Kreider, L.; Qian, P.Y. Settlement of the serpulid polychaete Hydroides elegans (Haswell) on the arborescent bryozoan Bugula neritina (L.): Evidence of a chemically mediated relationship. J. Exp. Mar. Biol. Ecol 1998, 220, 171–190. [Google Scholar]
- Hamilton, M.A.; Russo, R.C.; Thurston, R.V. Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1978, 12, 417–417, (Correction to: Hamilton M.A., Russo R.C., Thurston R.V. (1977)). [Google Scholar]
| Tested sample | B. albicostatus | B. neritina | ||
|---|---|---|---|---|
| EC50(μg mL−1) | LC50(μg mL−1) | LC50/EC50 | EC50(μg mL−1) | |
| osthole (1) | 4.64 | 37.42 | 8.06 | 7.56 |
| imperatorin (2) | 3.39 | 49.93 | 14.7 | >50 |
| isopimpinellin (3) | 11.31 | >50 | UD | >50 |
| auraptenol (4) | 3.38 | 39.47 | 11.7 | 22.59 |
| 8-epoxypentylcoumarin (5) | 7.46 | >50 | >6.7 | 37.29 |
| Meranzin hydrate (6) | 35.36 | >100 | UD | 18.23 |
| 2′-deoxymetranzin hydrate (7) | 4.67 | >50 | >10.7 | 9.06 |
| 8-methylbutenalcoumarin (8) | 6.77 | >100 | >14.8 | 3.87 |
| micromarin-F (9) | 10.93 | 33.36 | 3.1 | 12.38 |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Z.-C.; Feng, D.-Q.; Ke, C.-H. Coumarins from the Herb Cnidium monnieri and Chemically Modified Derivatives as Antifoulants against Balanus albicostatus and Bugula neritina Larvae. Int. J. Mol. Sci. 2013, 14, 1197-1206. https://doi.org/10.3390/ijms14011197
Wang Z-C, Feng D-Q, Ke C-H. Coumarins from the Herb Cnidium monnieri and Chemically Modified Derivatives as Antifoulants against Balanus albicostatus and Bugula neritina Larvae. International Journal of Molecular Sciences. 2013; 14(1):1197-1206. https://doi.org/10.3390/ijms14011197
Chicago/Turabian StyleWang, Zhan-Chang, Dan-Qing Feng, and Cai-Huan Ke. 2013. "Coumarins from the Herb Cnidium monnieri and Chemically Modified Derivatives as Antifoulants against Balanus albicostatus and Bugula neritina Larvae" International Journal of Molecular Sciences 14, no. 1: 1197-1206. https://doi.org/10.3390/ijms14011197
APA StyleWang, Z. -C., Feng, D. -Q., & Ke, C. -H. (2013). Coumarins from the Herb Cnidium monnieri and Chemically Modified Derivatives as Antifoulants against Balanus albicostatus and Bugula neritina Larvae. International Journal of Molecular Sciences, 14(1), 1197-1206. https://doi.org/10.3390/ijms14011197




