Hydration of AMP and ATP Molecules in Aqueous Solution and Solid Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dielectric Spectroscopy
2.2. Gravimetry
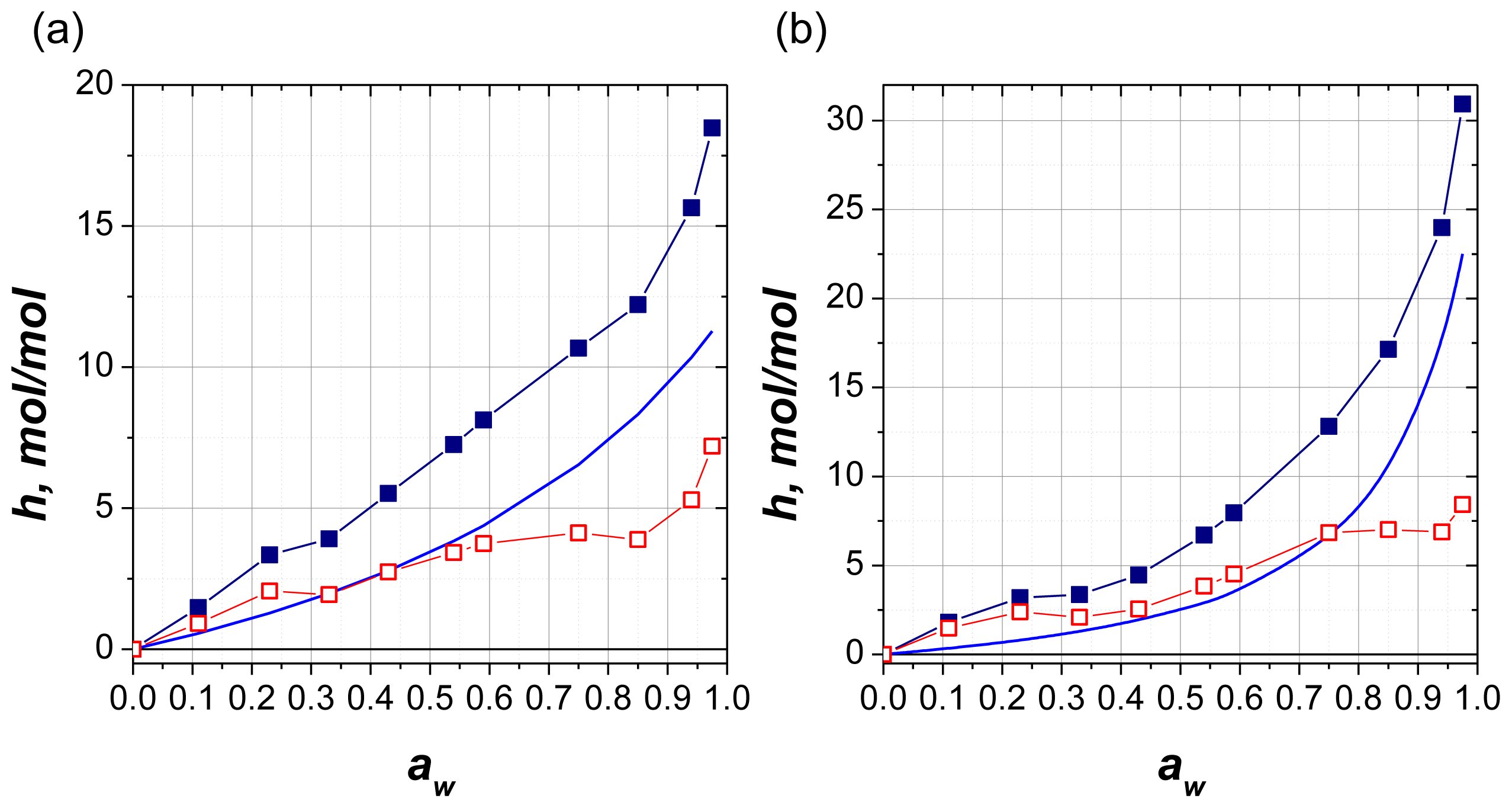
2.2.1. Water Sorption
2.2.2. Mechanical Losses
2.2.3. Sorption Centers
2.2.4. Conformational Transitions and Structure of Solid Film
2.2.5. Model of Hydration
2.2.6. Comparison with Solution
3. Experimental Section
3.1. Dielectric Spectroscopy
3.2. Gravimetry
4. Conclusions
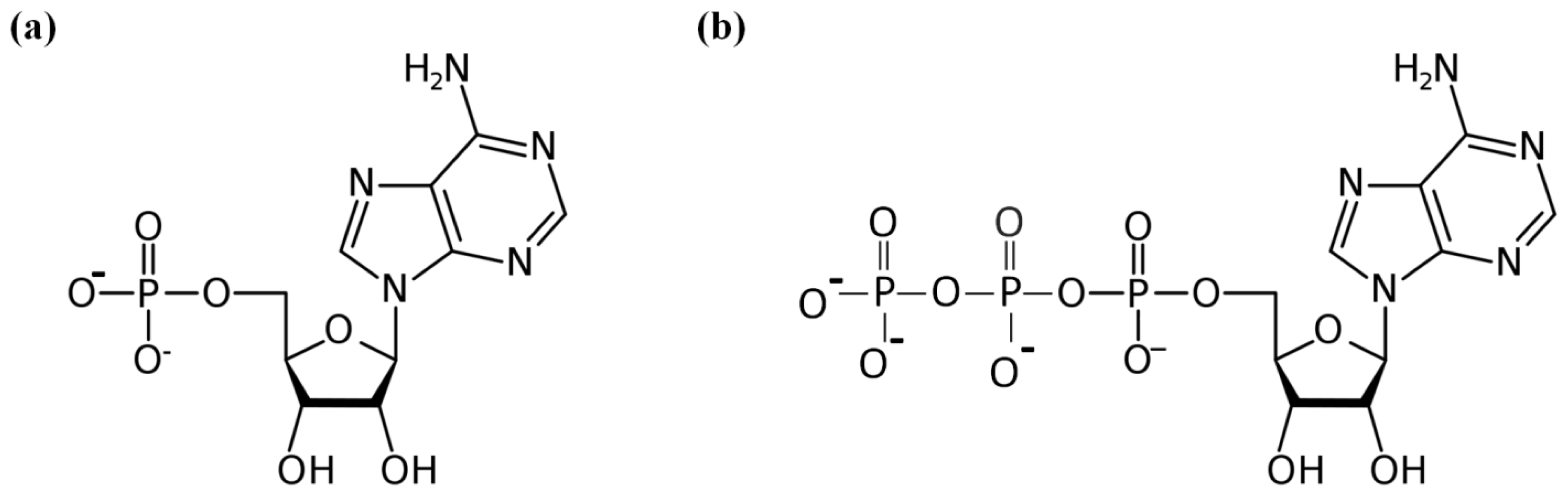
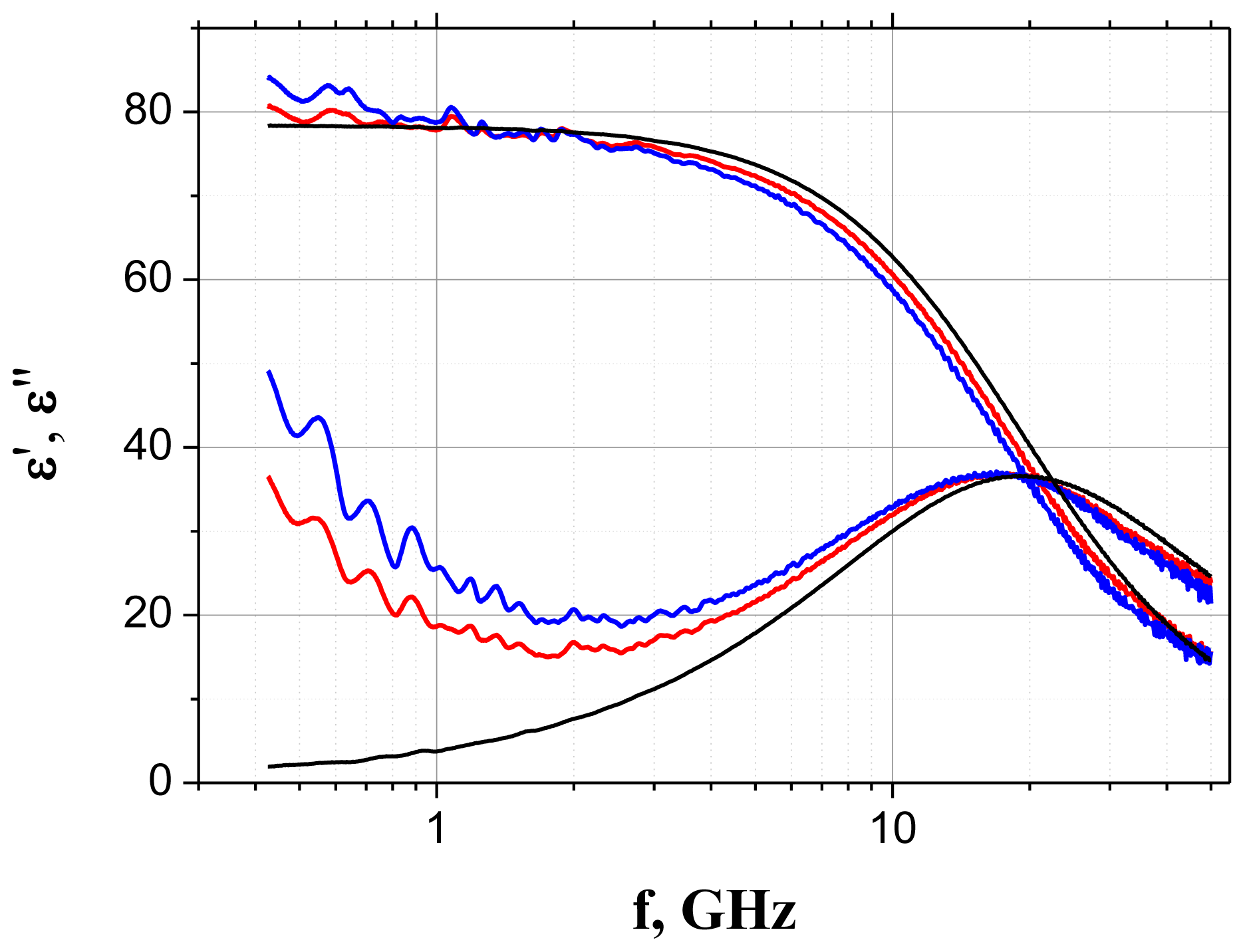

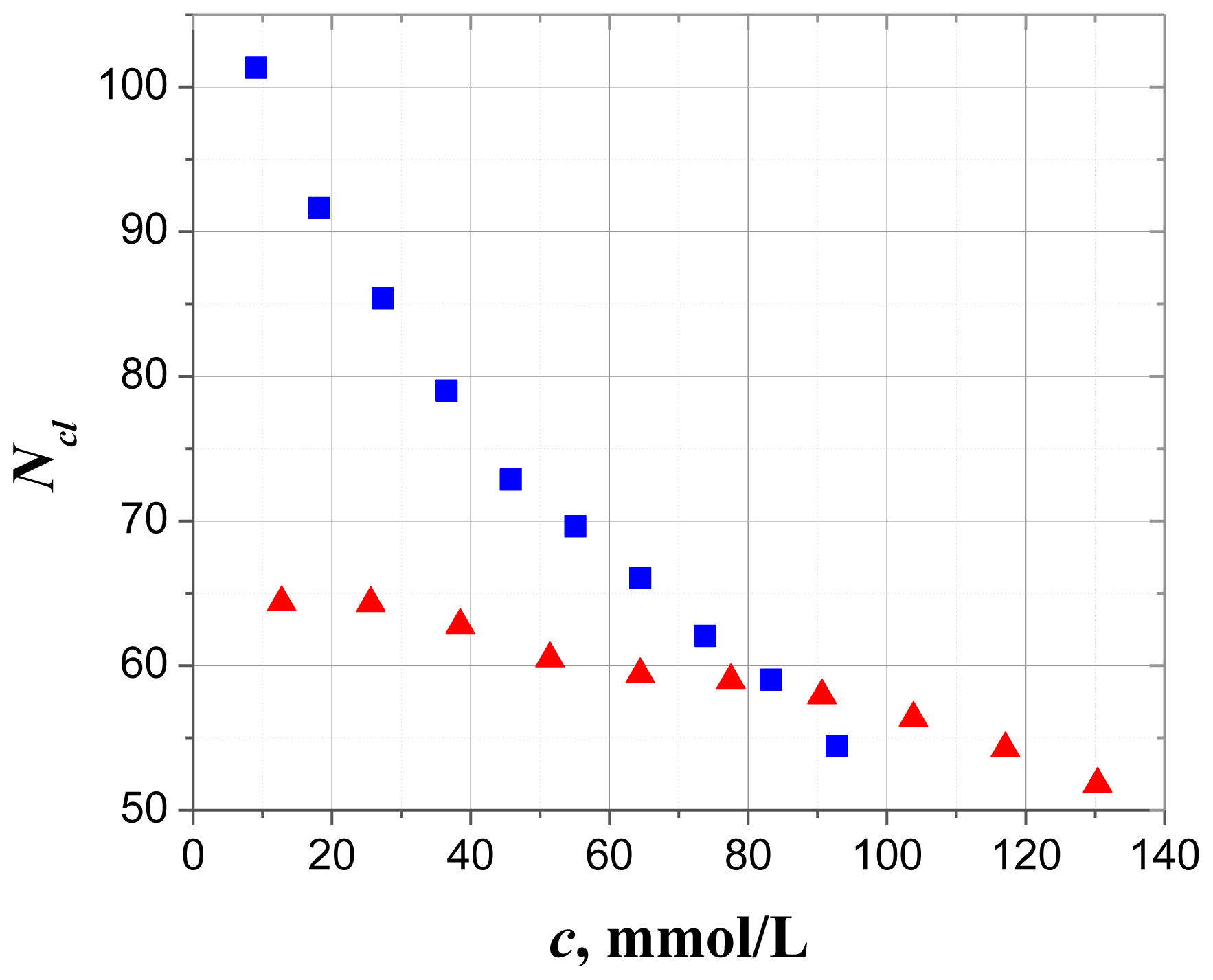
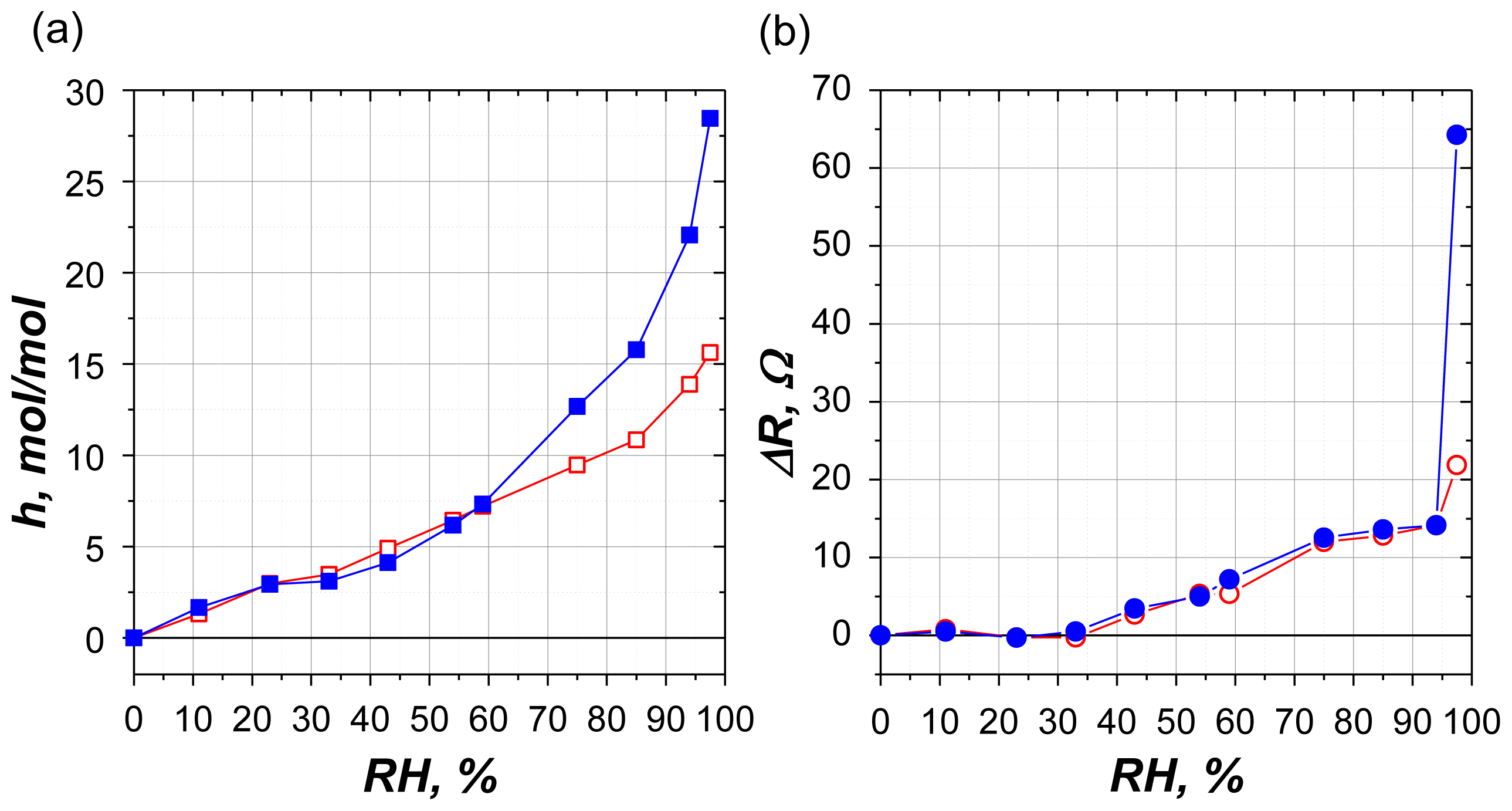

| Nucleotide | K4 | K5 | h1, mol H2O/mol nucleotide | h2, mol H2O/mol nucleotide | h3, mol H2O/mol nucleotide |
|---|---|---|---|---|---|
| AMP2− | 8 | 0.6 | 2 ± 0.2 | 4 ± 1 | 11 ± 3 |
| ATP4− | 3 | 0.905 | 2 ± 0.2 | 7 ± 1 | 22 ± 5 |
| Sample state | Ratio values of bound water |
|---|---|
| Solid films h2 ATP/h2 AMP | 1.8 |
| Solid films h3 ATP/h3 AMP | 2 |
| aSolution hATP/hAMP | 1.6 |
| bSolution hATP/hAMP | 2.1 |
Acknowledgments
Conflicts of Interest
References
- Ben-Naim, A. Molecular recognition—Viewed through the eyes of the solvent. Biophys. Chem 2002, 101–102, 309–319. [Google Scholar]
- Suzuki, M.; Shigematsu, J.; Fukunishi, Y.; Harada, Y.; Yanagida, T.; Kodama, T. Coupling of protein surface hydrophobicity change to ATP hydrolysis by myosin motor domain. Biophys. J 1997, 72, 18–23. [Google Scholar]
- Livshits, L.; Caduff, A.; Talary, M.S.; Lutz, H.U.; Hayashi, Y.; Puzenko, A.; Shendrik, A.; Feldman, Y. The role of GLUT1 in the sugar-induced dielectric response of human erythrocytes. J. Phys. Chem. B 2009, 113, 2212–2220. [Google Scholar]
- Hayashi, Y.; Livshits, L.; Caduff, A.; Feldman, Y. Dielectric spectroscopy study of specific glucose influence on human erythrocyte membranes. J. Phys. D Appl. Phys 2003, 36, 369. [Google Scholar] [CrossRef]
- Caduff, A.; Mueller, M.; Megej, A.; Dewarrat, F.; Suri, R.E.; Klisic, J.; Donath, M.; Zakharov, P.; Schaub, D.; Stahel, W.A.; et al. Characteristics of a multisensor system for non invasive glucose monitoring with external validation and prospective evaluation. Biosens. Bioelectron 2011, 26, 3794–3800. [Google Scholar]
- Zanon, M.; Sparacino, G.; Facchinetti, A.; Talary, M.S.; Mueller, M.; Caduff, A.; Cobelli, C. Non-Invasive continuous glucose monitoring with multi-sensor systems: A monte carlo-based methodology for assessing calibration robustness. Sensors 2013, 13, 7279–7295. [Google Scholar]
- Puzenko, A.; Ishai, P.B.; Feldman, Y. Cole-Cole broadening in dielectric relaxation and strange kinetics. Phys. Rev. Lett 2010, 105, 037601. [Google Scholar] [CrossRef]
- Levy, E.; Puzenko, A.; Kaatze, U.; Ishai, P.B.; Feldman, Y. Dielectric spectra broadening as the signature of dipole-matrix interaction. I. Water in nonionic solutions. J. Chem. Phys 2012, 136, 114502. [Google Scholar] [CrossRef]
- Levy, E.; Puzenko, A.; Kaatze, U.; Ben Ishai, P.; Feldman, Y. Dielectric spectra broadening as the signature of dipole-matrix interaction. II. Water in ionic solutions. J. Chem. Phys 2012, 136, 114503. [Google Scholar] [CrossRef]
- Puzenko, A.; Levy, E.; Shendrik, A.; Talary, M.S.; Caduff, A.; Feldman, Y. Dielectric spectra broadening as a signature for dipole-matrix interaction. III. Water in adenosine monophosphate/adenosine-5′-triphosphate solutions. J. Chem. Phys 137, 02. [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys 1941, 9, 341–351. [Google Scholar]
- Froehlich, H. Theory of Dielectrics: Dielectric Constant and Dielectric Loss, 2nd ed; Oxford University Press: Oxford, UK, 1958. [Google Scholar]
- Sugawara, Y.; Kamiya, N.; Iwasaki, H.; Ito, T.; Satow, Y. Humidity-controlled reversible structure transition of disodium adenosine 5′-triphosphate between dihydrate and trihydrate in a single crystal state. J. Am. Chem. Soc 1991, 113, 5440–5445. [Google Scholar]
- Sugawara, Y.; Iimura, Y.; Iwasaki, H.; Urabe, H.; Saito, H. Reversible crystal transition of guanosine between the dihydrate and anhydrous states coupled with adsorption-desorption process. J. Biomol. Struct. Dyn 1994, 11, 721–729. [Google Scholar]
- Urabe, H.; Sugawara, Y.; Kasuya, T. Humidity-dependent structural transition of guanosine and disodium adenosine 5′-triphosphate crystals studied by low-frequency Raman spectroscopy. Phys. Rev. B 1995, 51, 5666–5672. [Google Scholar]
- Kennard, O.; Isaacs, N.W.; Motherwell, W.D.S.; Coppola, J.C.; Wampler, D.L.; Larson, A.C.; Watson, D.G. The crystal and molecular structure of adenosine triphosphate. Proc. R. Soc. A Math. Phys. Eng. Sci 1971, 325, 401–436. [Google Scholar]
- Johannsmann, D. Studies of Viscoelasticity with the QCM. In Piezoelectric Sensors; Janshoff, P.D.A., Steinem, P.D.C., Eds.; Springer: Berlin, Germany, 2007; pp. 49–109. [Google Scholar]
- Axelrod, N.; Axelrod, E.; Gutina, A.; Puzenko, A.; Ishai, P.B.; Feldman, Y. Dielectric spectroscopy data treatment: I. Frequency domain. Meas. Sci. Technol 2004, 15, 755. [Google Scholar] [CrossRef]
- SIGMA Product, Adenosine 5′-triphosphate disodium salt; Product Number A 2383.
- Falk, M.; Hartman, K.A.; Lord, R.C. Hydration of deoxyribonucleic acid. II. An infrared study. J. Am. Chem. Soc 1963, 85, 387–391. [Google Scholar]
- Falk, M. Hydration of purines, pyrimidines, nucleosides, and nucleotides. Can. J. Chem 1965, 43, 314–318. [Google Scholar]
- Tajmir-Riahi, H.A.; Bertrand, M.J.; Theophanides, T. Synthesis, structure, proton-nuclear magnetic resonance, and Fourier transform infrared spectroscopy of several transition and nontransition metal–adenosine-5-triphosphate complexes. Can. J. Chem 1986, 64, 960–966. [Google Scholar]
- Shindo, Y. Stepwise conformational transition of crystalline disodium adenosine 5′-triphosphate with relative humidity as studied by high resolution solid state 13C and 31P NMR. J. Mol. Struct 2002, 602, 389–397. [Google Scholar]
- D’Arcy, R.L.; Watt, I.C. Analysis of sorption isotherms of non-homogeneous sorbents. Trans. Faraday Soc 1970, 66, 1236–1245. [Google Scholar]
- Mogami, G.; Wazawa, T.; Morimoto, N.; Kodama, T.; Suzuki, M. Hydration properties of adenosine phosphate series as studied by microwave dielectric spectroscopy. Biophys. Chem 2011, 154, 1–7. [Google Scholar]
- Agilent Technologies, 85070E Dielectric Probe Kit 200 MHz to 50 GHz: Technical Overview; Agilent Technologies: Santa Clara, CA, USA, 2012.
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Faizullin, D.; Zakharchenko, N.; Zuev, Y.; Puzenko, A.; Levy, E.; Feldman, Y. Hydration of AMP and ATP Molecules in Aqueous Solution and Solid Films. Int. J. Mol. Sci. 2013, 14, 22876-22890. https://doi.org/10.3390/ijms141122876
Faizullin D, Zakharchenko N, Zuev Y, Puzenko A, Levy E, Feldman Y. Hydration of AMP and ATP Molecules in Aqueous Solution and Solid Films. International Journal of Molecular Sciences. 2013; 14(11):22876-22890. https://doi.org/10.3390/ijms141122876
Chicago/Turabian StyleFaizullin, Dzhigangir, Nataliya Zakharchenko, Yuriy Zuev, Alexander Puzenko, Evgeniya Levy, and Yuri Feldman. 2013. "Hydration of AMP and ATP Molecules in Aqueous Solution and Solid Films" International Journal of Molecular Sciences 14, no. 11: 22876-22890. https://doi.org/10.3390/ijms141122876




