Self-Assembly, Surface Activity and Structure of n-Octyl-β-D-thioglucopyranoside in Ethylene Glycol-Water Mixtures
Abstract
:1. Introduction
2. Results and Discussion
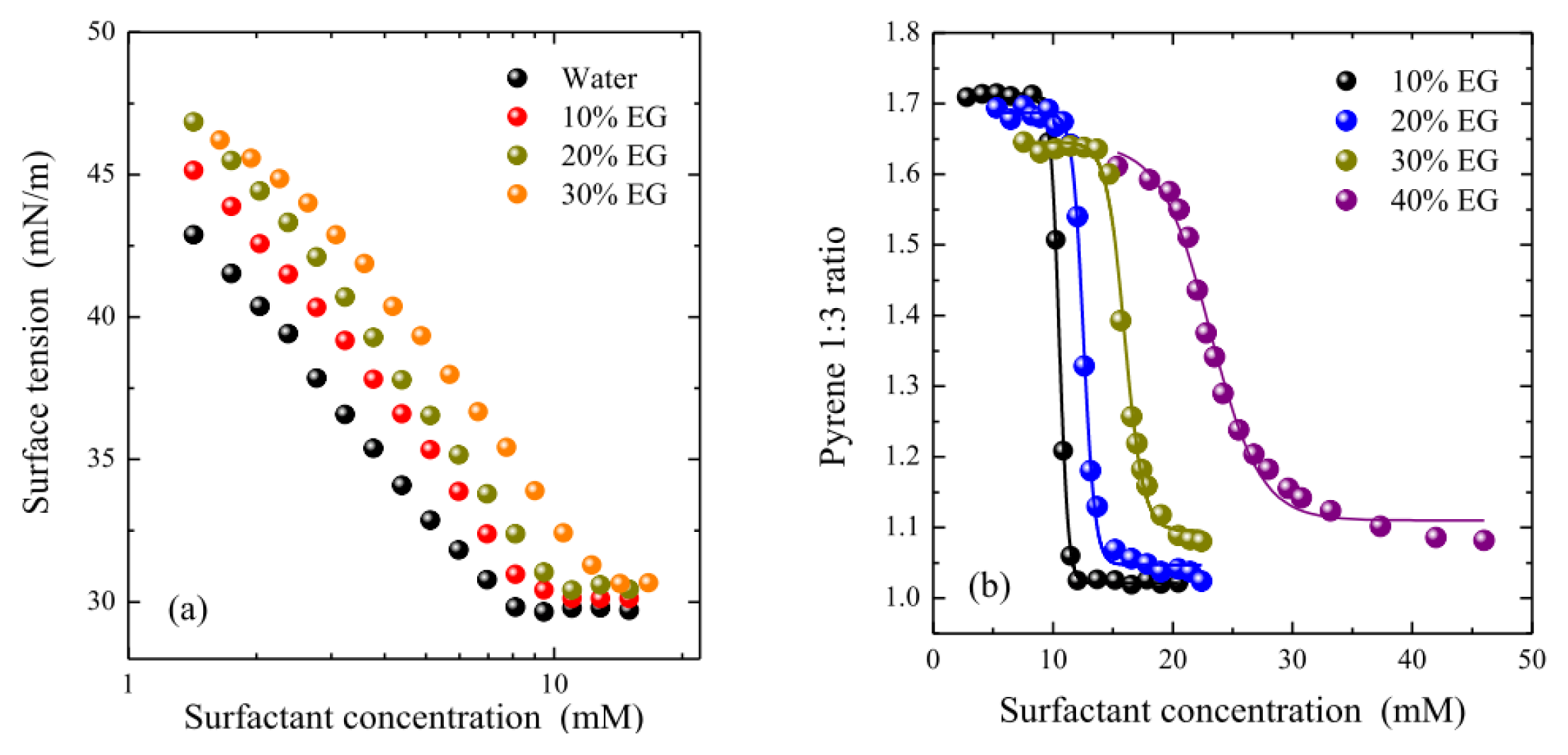
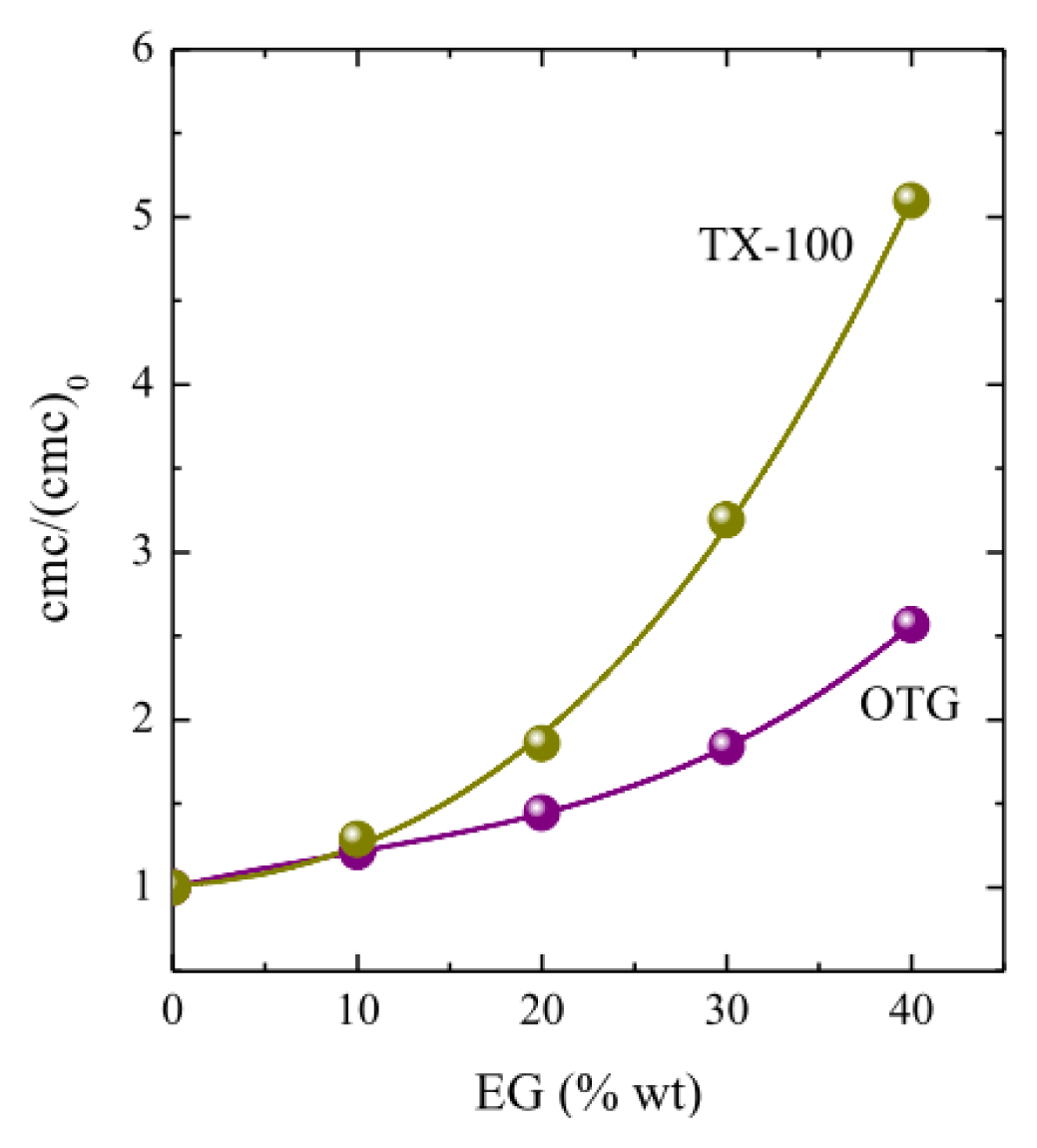
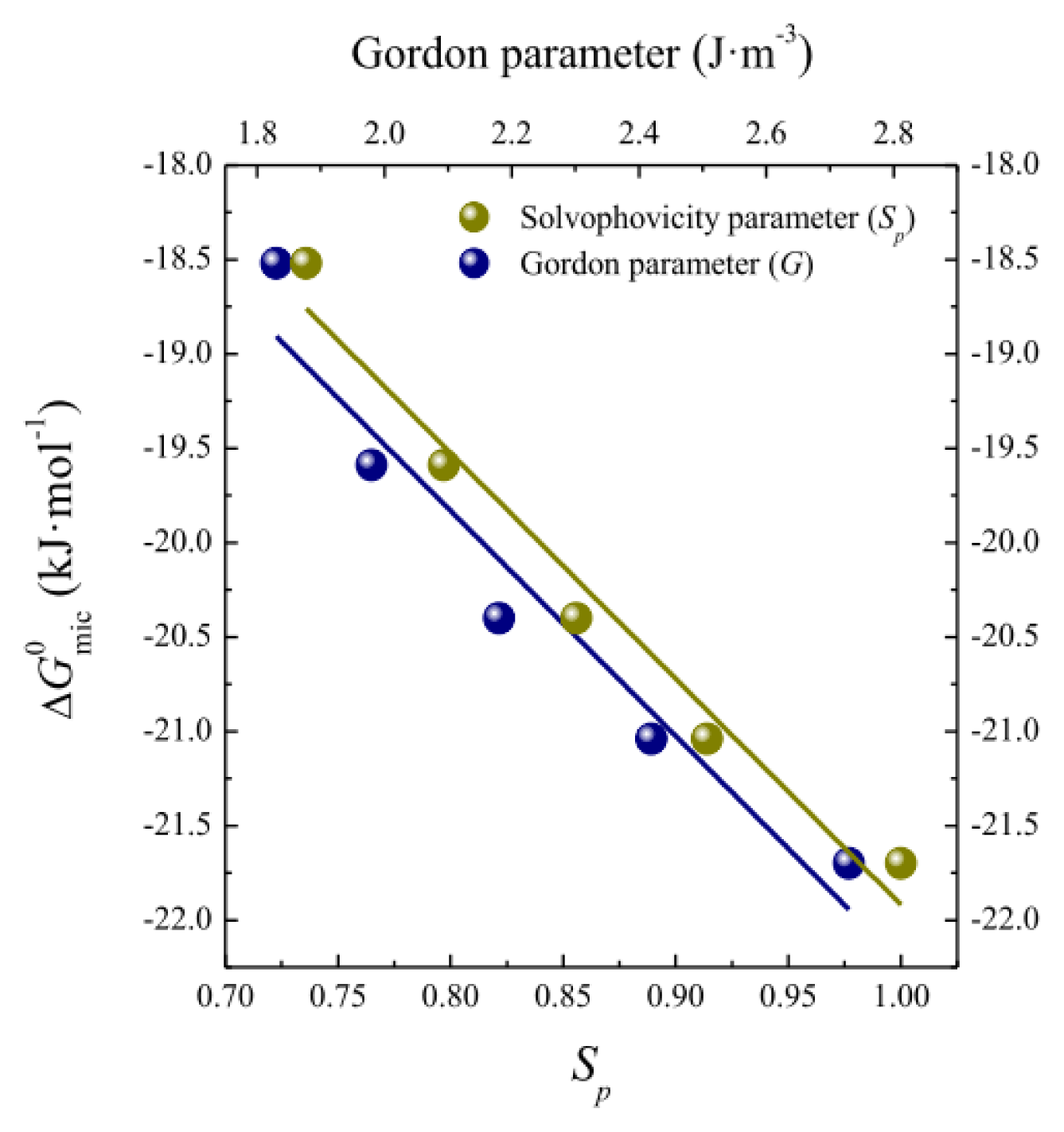
2.1. Micellization and Adsorption Properties
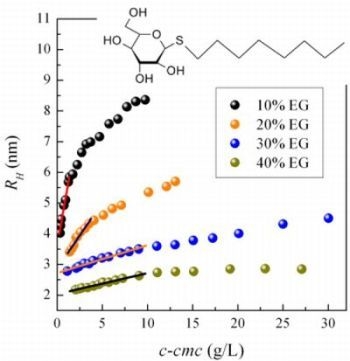
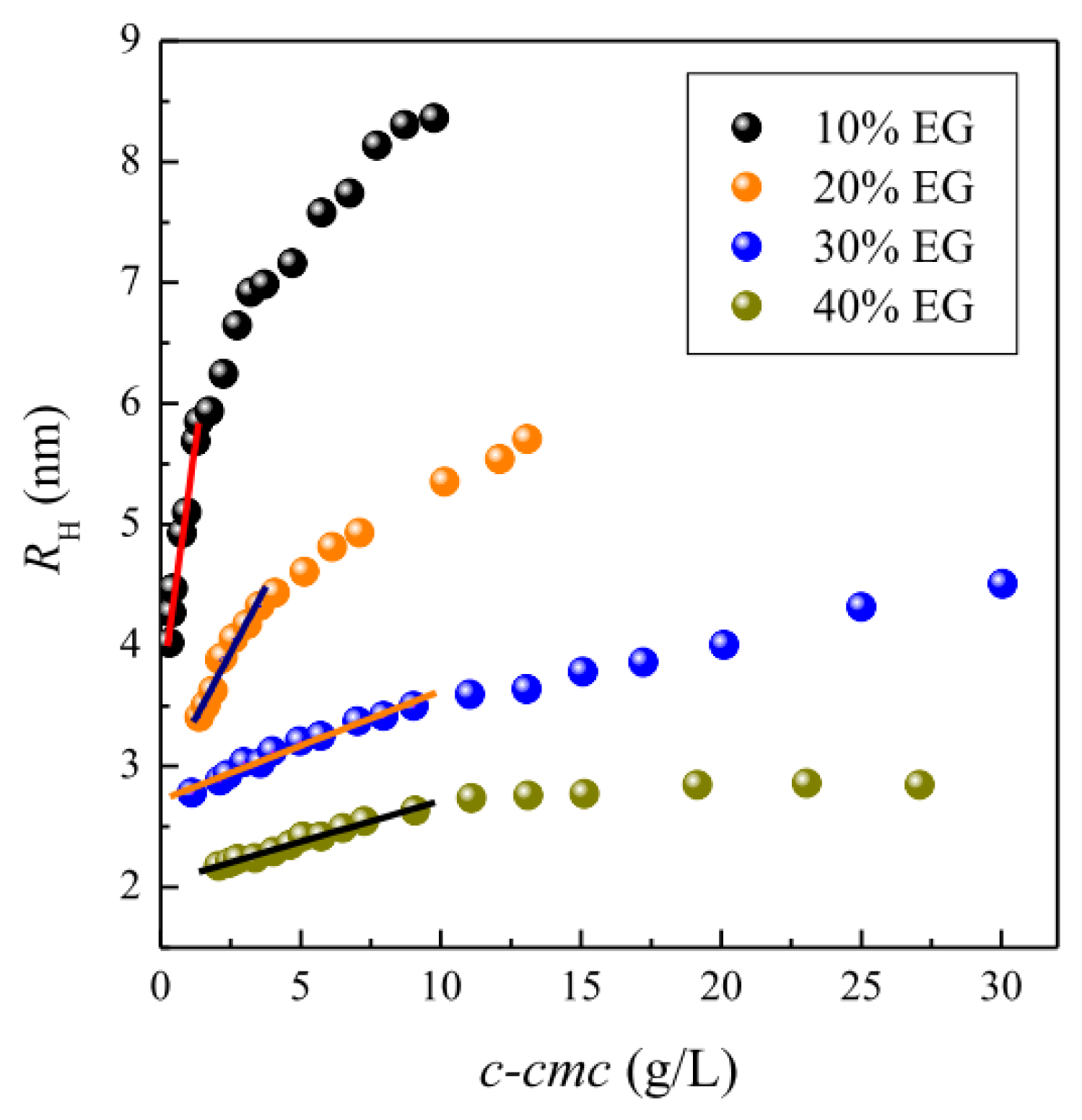
2.2. Micellar Size
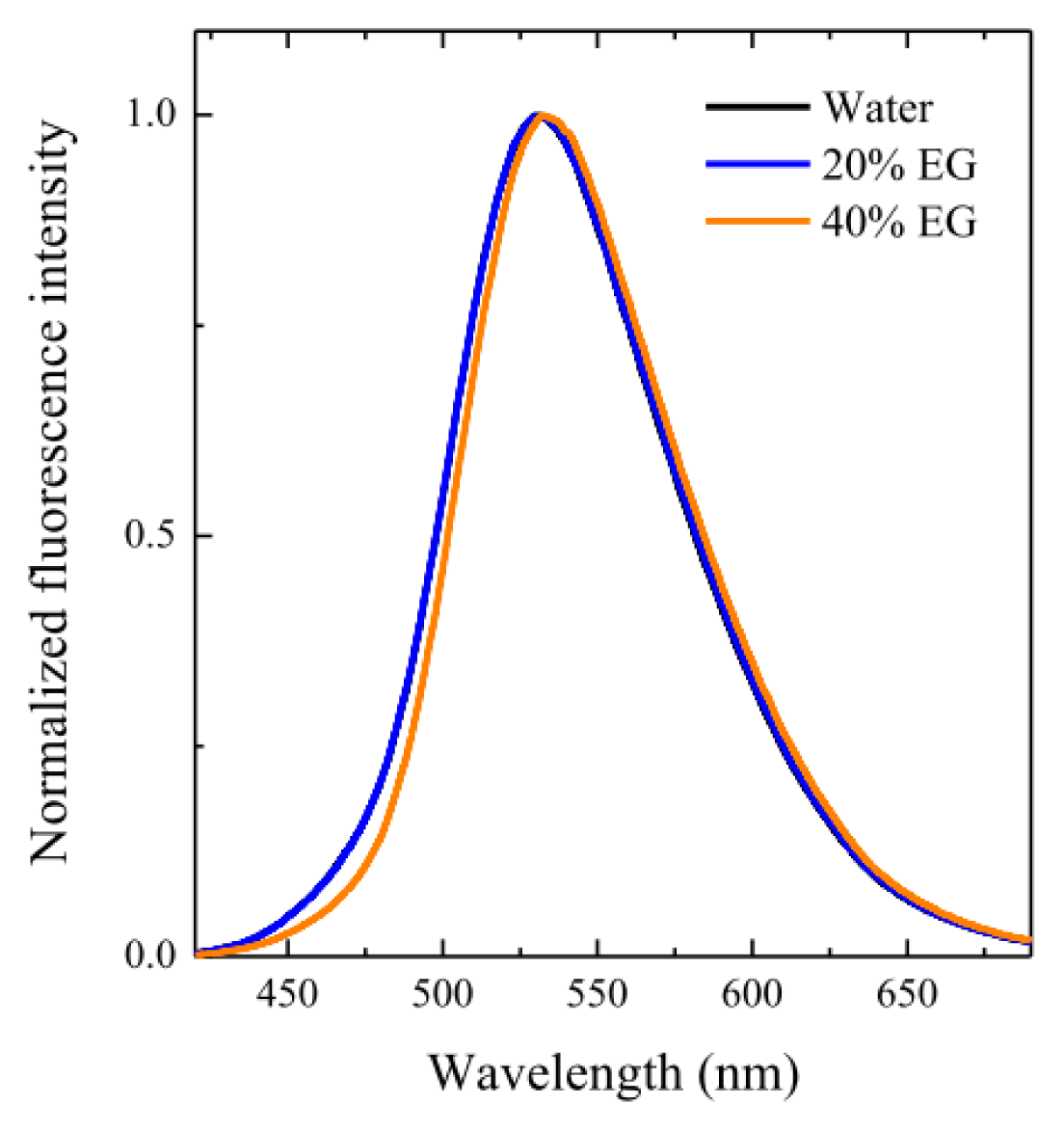
2.3. Micellar Microstructure
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Surface Tension
3.2.2. Light Scattering
3.2.3. Fluorescence Spectroscopy
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Ramadan, M.S.; Evans, D.F.; Lumry, R. Why micelles form in water and hydrazine—A reexamination of the origins of hydrophobicity. J. Phys. Chem 1983, 87, 4538–4543. [Google Scholar]
- Evans, D.F.; Miller, D.D. Organized Solutions and Their Manifestations in Polar Solvents. In Organized Solutions: Surfactants in Science and Technology; Friberg, S.E., Lindman, B., Eds.; Marcel Dekker Inc: New York, NY, USA, 1992; Volume 44, pp. 33–45. [Google Scholar]
- Sedov, I.A.; Stolov, M.A.; Solomonov, B.N. Solvophobic effects and relationships between the Gibbs energy and enthalpy for the salvation process. J. Phys. Org. Chem 2011, 24, 1088–1094. [Google Scholar]
- Ray, A. Solvophobic interactions and micelle formation in structure forming nonaqueous solvents. Nature 1971, 231, 313–314. [Google Scholar]
- Cantú, L.; Corti, M.; Degiorgio, V.; Hoffmann, H.; Ulbrichts, W. Nonionic micelles in mixed water-glycerol solvent. J. Colloid Interface Sci 1987, 116, 384–389. [Google Scholar]
- Backlund, S.; Bergenstahl, B.; Molander, O.; Wärnheim, T. Aggregation of tetradecyltrimethyl-ammonium bromide in water, 1,2-ethanediol, and their mixtures. J. Colloid Interface Sci 1989, 131, 393–401. [Google Scholar]
- Sjöberg, M.; Henriksson, U.; Wärnheim, T. 2H nuclear magnetic relaxation of [1,1-2H]hexadecyl-trimetylammonium bromide in micellar solutions of nonaqueous polar solvents and their mixtures with water. Langmuir 1990, 6, 1205–1211. [Google Scholar]
- Tarisawa, N.; Thomason, M.; Bloor, D.M.; Wyn-Jones, E. Ultrasonic relaxation and electrochemical studies of the micellization of sodium decyl sulfate and decyltrimethylammonium bromide in glycerol/water mixtures. J. Colloid Interface Sci 1993, 157, 77–81. [Google Scholar]
- Bakshi, M.S. Micelle formation by anionic and cationic surfactants in binary aqueous solvents. J. Chem. Soc. Faraday Trans 1993, 89, 4323–4326. [Google Scholar]
- Palepu, R.; Gharibi, H.; Bloor, D.M.; Wyn-Jones, E. Electrochemical studies associated with the micellization of cationic surfactants in aqueous mixtures of ethylene glycol and glycerol. Langmuir 1993, 9, 110–112. [Google Scholar]
- Gracie, K.; Turner, D.; Palepu, R. Thermodynamic properties of micellization of sodium dodecyl sulfate in binary mixtures of ethylene glycol with water. Can. J. Chem 1996, 74, 1616–1625. [Google Scholar]
- Aramaki, K.; Olsson, U.; Yamaguchi, Y.; Kunieda, H. Effect of water-soluble alcohols on surfactant aggregation in the C12EO8 system. Langmuir 1999, 15, 6226–6232. [Google Scholar]
- Nagarajan, R.; Wang, C.-C. Theory of surfactant aggregation in water/ethylene glycol mixed solvents. Langmuir 2000, 16, 5242–5251. [Google Scholar]
- Glenn, K.M.; Moroze, S.; Palepu, R.; Bhattacharya, S.C. Effect of ethylene glycol on the thermodynamic and micellar properties of Tween 40, 60, and 80. J. Dispersion Sci. Technol 2005, 26, 79–86. [Google Scholar]
- D’Errico, G.; Ciccarelli, D.; Ortona, O. Effect of glycerol on micelle formation by ionic and nonionic surfactants at 25 °C. J. Colloid Interface Sci 2005, 286, 747–754. [Google Scholar]
- Rodríguez, A.; Graciani, M.M.; Muñoz, M.; Moyá, M.L. Water-ethylene glycol alkyltrimethylam-monium bromide micellar solutions as reaction media: Study of spontaneous hydrolysis of phenyl chloroformate. Langmuir 2003, 19, 7206–7213. [Google Scholar]
- Graciani, M.M.; Rodríguez, A.; Muñoz, M.; Moyá, M.L. Micellar solutions of sulfobetaine surfactants in water-ethylene glycol mixtures: surface tension, fluorescence, spectroscopic, conductometric, and kinetic studies. Langmuir 2005, 21, 7161–7169. [Google Scholar]
- Graciani, M.M.; Muñoz, M.; Robina, I.; Moyá, M.L. Effect of ethylene glycol addition on the aggregation and micellar growth of Gemini surfactants. Langmuir 2006, 22, 9519–9525. [Google Scholar]
- Graciani, M.M.; Muñoz, M.; Rodríguez, A.; Moyá, M.L. Water−N,N-dimethylformamide alkyltrimethylammonium bromide micellar solutions: thermodynamic, structural, and kinetic studies. Langmuir 2005, 21, 3303–3310. [Google Scholar]
- Rodríguez, A.; Graciani, M.M.; Moyá, M.L. Effects of addition of polar organic solvents on micellization. Langmuir 2008, 24, 12785–12792. [Google Scholar]
- Rodríguez, A.; Graciani, M.M.; Angulo, M.; Moyá, M.L. Effects of organic solvent addition on the aggregation and micellar growth of cationic dimeric surfactant 12-3-12,2Br. Langmuir 2007, 23, 11496–11505. [Google Scholar]
- Rodríguez, A.; Graciani, M.M.; Fernández, G.; Moyá, M.L. Effects of glycols on the thermodynamic and micellar properties of TTAB in water. J. Colloid Interface Sci 2009, 338, 207–215. [Google Scholar]
- Rodríguez, A.; Graciani, M.M.; Cordobés, F.; Moyá, M.L. Water-ethylene glycol cationic dimeric micellar solution: Aggregation, micellar growth, and characteristics as reaction media. J. Phys. Chem. B 2009, 113, 7767–7779. [Google Scholar]
- Moyá, M.L.; Rodríguez, A.; Graciani, M.M.; Fernández, G. Role of the solvophobic effect on micellization. J. Colloid Interface Sci 2007, 316, 787–795. [Google Scholar]
- Alexandridis, P.; Yang, L. SANS investigation of polyether block copolymer micelle structure in mixed solvents of water and formamide, ethanol, or glycerol. Macromolecules 2000, 33, 5574–5587. [Google Scholar]
- Lin, Y.N.; Alexandridis, P. Small-angle neutron scattering characterization of micelles formed by poly(dimethysiloxane)-graf-polyether copolymers in mixed polar solvents. J. Phys. Chem. B 2002, 106, 12124–12132. [Google Scholar]
- Ivanova, R.; Lindman, B.; Alexandridis, P. Effect of pharmaceutically acceptable glycols on the stability of the liquid crystalline gels formed by poloxamer 407 in water. J. Colloid Interface Sci 2002, 252, 226–235. [Google Scholar]
- Lin, Y.; Alexandridis, P. Cosolvent effects on the micellization of an amphiphilic siloxane graft copolymer in aqueous solutions. Langmuir 2002, 18, 4220–4231. [Google Scholar]
- Sarkar, B.; Lam, S.; Alexandridis, P. Micellization of alkyl-propoxy-ethoxylate surfactants in water-polar organic solvent mixtures. Langmuir 2010, 26, 10532–10540. [Google Scholar]
- Sarkar, B.; Ravi, V.; Alexandridis, P. Micellization of amphiphilic block copolymers in binary and ternary solvent mixtures. J. Colloid Interface Sci 2013, 390, 137–146. [Google Scholar]
- Alexandridis, P.; Yang, L. Micellization of polyoxyalkylene block copolymers in formamide. Macromolecules 2000, 33, 3382–3391. [Google Scholar]
- Seguin, C.; Eastoe, J.; Clapperton, R.; Heenan, R.K.; Grillo, I. Alternative non-aqueous water-miscible solvents for surfactants. Colloids Surf. A 2006, 282–283, 134–142. [Google Scholar]
- Seguin, C.; Eastoe, J.; Rogers, S.; Hollamby, M.; Dalgliesh, R.M. Unexpected adsorption behavior of nonionic surfactants from glycol solvents. Langmuir 2006, 22, 11187–11192. [Google Scholar]
- Seguin, C.; Eastoe, J.; Heenan, R.K.; Grillo, I. Controlling aggregation of nonionic surfactants using mixed glycol media. Langmuir 2007, 23, 4199–4202. [Google Scholar]
- Carnero Ruiz, C. Thermodynamics of micellization of tetradecyltrimethylammonium bromide in ethylene glycol-water mixtures. Colloid Polym. Sci 1999, 277, 701–707. [Google Scholar]
- Carnero Ruiz, C. Fluorescence anisotropy of probes solubilized in micelles of tetradecyltrimethyl-ammonium bromide: Effect of ethylene glycol added. J. Colloid Interface Sci 2000, 221, 262–267. [Google Scholar]
- Carnero Ruiz, C.; Molina-Bolívar, J.A.; Aguiar, J.; Maclsaac, G.; Moroze, S.; Palepu, R. Thermodynamic and structural studies of Triton X-100 micelles in ethylene glycol−water mixed solvents. Langmuir 2001, 17, 6831–6840. [Google Scholar]
- Molina-Bolívar, J.A.; Aguiar, J.; Peula-García, J.M.; Carnero Ruiz, C. Photophysical and light scattering studies on the aggregation behavior of Triton X-100 in formamide-water mixed solvents. Mol. Phys 2002, 100, 3259–3269. [Google Scholar]
- Aguiar, J.; Molina-Bolívar, J.A.; Peula-García, J.M.; Carnero Ruiz, C. Thermodynamics and micellar properties of tetradecyltrimethylammonium bromide in formamide-water mixtures. J. Colloid Interface Sci 2002, 255, 382–390. [Google Scholar]
- Carnero Ruiz, C.; Molina-Bolívar, J.A.; Aguiar, J.; MacIsaac, G.; Moroze, S.; Palepu, R. Effect of ethylene glycol on the thermodynamic and micellar properties of Tween 20. Colloid Polym. Sci 2003, 281, 531–541. [Google Scholar]
- Carnero Ruiz, C.; López-Díaz, L.; Aguiar, J. Self-assembly of tetradecyltrimethylammonium bromide in glycerol aqueous mixtures: A thermodynamic and structural study. J. Colloid Interface Sci 2007, 305, 293–300. [Google Scholar]
- Carnero Ruiz, C.; López-Díaz, L.; Aguiar, J. Micellization of sodium dodecyl sulfate in glycerol aqueous mixtures. J. Dispersion Sci. Technol 2008, 29, 266–273. [Google Scholar]
- Sohrabi, B.; Bazyari, A.; Hashemianzadeh, M. Effect of ethylene glycol on micellization and surface properties of Gemini surfactant solutions. Colloids Surf. A 2010, 364, 87–93. [Google Scholar]
- Wang, J.; Zhang, L.; Wang, H.; Wu, C. Aggregation behavior modulation of 1-dodecyl-3-methylimidazolium bromide by organic solvents in aqueous solution. J. Phys. Chem. B 2011, 115, 4955–4962. [Google Scholar]
- Pan, A.; Naskar, B.; Prameela, G.K.S.; Kumar, B.V.N.P.; Mandal, A.B.; Bhattacharya, S.C.; Moulik, S.P. Amphiphile behavior in mixed solvent media I: Self-aggregation and ion association of sodium dodecylsulfate in 1,4-dioxane-water and methanol-water media. Langmuir 2012, 28, 13830–13843. [Google Scholar]
- Peyre, V.; Bouguerra, S.; Testard, F. Micellization of dodecyltrimethylammonium bromide in water-dimethylsulfoxide mixtures: A multi-length scale approach in a model system. J. Colloid Interface Sci 2013, 389, 164–174. [Google Scholar]
- Das, D.; Dey, J.; Chandra, A.K.; Thapa, U.; Ismail, K. Aggregation behavior of sodium dioctylsulfosuccinate in aqueous ethylene glycol medium. A case of hydrogen bonding between surfactant and solvent and its manifestation in the surface tension isotherm. Langmuir 2012, 28, 15762–15769. [Google Scholar]
- Mcauley, W.J.; Jones, D.J.; Kett, V.L. Characterization of the interaction of lactate dehydrogenase with Tween-20 using isothermal titration calorimetry, interfacial rheometry and surface tension measurements. J. Pharm. Sci 2009, 98, 2659–2669. [Google Scholar]
- Hill, K.; Rhode, O. Sugar-based surfactants for consumer products and technical applications. Fett/Lipid 1999, 101, 25–33. [Google Scholar]
- Söderman, O.; Johansson, I. Polyhydroxyl-based surfactants and their physico-chemical properties and applications. Curr. Opin. Colloid Interface Sci 2000, 4, 391–401. [Google Scholar]
- Stubenrauch, C. Sugar surfactants—Aggregation, interfacial, and adsorption phenomena. Curr. Opin. Colloid Interface Sci 2001, 6, 160–170. [Google Scholar]
- Molina-Bolívar, J.A.; Carnero Ruiz, C. Self-Assembly and Micellar Structures of Sugar-Based Surfactants: Effect of Temperature and Salt Addition. In Sugar-Based Surfactants: Fundamentals and Applications; Carnero Ruiz, C., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 61–104. [Google Scholar]
- Arnold, T.; Linke, D. Phase separation in the isolation and purification of membrane proteins. BioTechniques 2007, 43, 427–440. [Google Scholar]
- Saito, S.; Tsuchiya, T. Characteristics of n-octyl-β-d-thioglucopyranoside, a new non-ionic detergent useful for membrane biochemistry. Biochem. J 1984, 222, 829–832. [Google Scholar]
- Chami, M.; Pehau-Arnaudet, G.; Lambert, O.; Ranck, J.-L.; Lèvy, D.; Rigaud, J.-L. Use of octyl-β-thioglucopyranoside in two-dimensional crystallization of membrane proteins. J. Struct. Biol 2001, 133, 64–74. [Google Scholar]
- Wenk, M.R.; Seelig, J. Interaction of octyl-β-thioglucopyranoside with lipid membranes. Biophys. J 1997, 73, 2565–2574. [Google Scholar]
- Azusa, A.; Sonoyama, M. Solubilization and structural stability of bacteriorhodopsin with a mild nonionic detergent, n-octyl-β-d-thioglucoside. Biosci. Biotechnol. Biochem 2011, 75, 376–378. [Google Scholar]
- Molina-Bolívar, J.A.; Aguiar, J.; Peula-García, J.M.; Carnero Ruiz, C. Surface activity, micelle formation, and growth of n-octyl-β-d-thioglucopyranoside in aqueous solutions at different temperatures. J. Phys. Chem. B 2004, 108, 12813–12820. [Google Scholar]
- Molina-Bolívar, J.A.; Hierrezuelo, J.M.; Carnero Ruiz, C. Effect of NaCl on the self-aggregation of n-octyl-β-d-thioglucopyranoside in aqueous medium. J. Phys. Chem. B 2006, 110, 12086–12095. [Google Scholar]
- Hierrezuelo, J.M.; Molina-Bolívar, J.A.; Carnero Ruiz, C. Micellar properties of a mixed surfactant system constituted by n-octyl-β-d-thioglucopyranoside and sodium dodecyl sulphate. Colloids Surf. A 2005, 264, 29–36. [Google Scholar]
- Carnero Ruiz, C.; Molina-Bolívar, J.M. Characterization of mixed non-ionic surfactants n-octyl-β-d-thioglucoside and octaethylene-glycol monododecyl ether: Micellization and microstructure. J. Colloid Interface Sci 2011, 361, 178–185. [Google Scholar]
- Naous, M.; Aguiar, J.; Carnero Ruiz, C. Synergism in mixtures of n-octyl-β-d-thioglucoside and different n-alkyltrimethylammonium bromides: Effect of the alkyl chain length. Colloid Polym. Sci 2012, 290, 1041–1051. [Google Scholar]
- Carnero Ruiz, C. Rotational dynamics of Coumarin 153 in non-ionic mixed micelles of n-octyl-β-d-thioglucoside and Triton X-100. Photochem. Photobiol. Sci 2012, 11, 1331–1338. [Google Scholar]
- Molina-Bolívar, J.A.; Carnero Ruiz, C. Micellar size and phase behavior in n-octyl-β-d-thioglucoside/Triton X-100 mixtures: The effect of NaCl addition. Fluid Phase Equilib 2012, 327, 58–64. [Google Scholar]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc 1977, 99, 2039–2044. [Google Scholar]
- Aguiar, J.M.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci 2003, 258, 116–122. [Google Scholar]
- Stubenrauch, C.; Claesson, P.M.; Rutland, M.; Manev, E.; Johansson, I.; Pedersen, J.S.; Langenvin, D.; Blunk, D.; Bain, C.D. Mixtures of n-dodecyl-β-d-maltoside and hexaoxyethylene dodecyl ether—Surface properties, bulk properties, foam films, and foams. Adv. Colloid Interface Sci 2010, 155, 5–18. [Google Scholar]
- Tyrode, E.; Johnson, C.M.; Kumpulainen, A.; Rutland, M.W.; Claesson, P.M. Hydration state of nonionic surfactant monolayers at the liquid/vapor interface: Structure determination by vibrational sum frequency spectroscopy. J. Am. Chem. Soc 2005, 127, 16848–16859. [Google Scholar]
- Abraham, M.H.; Grellier, P.L.; McGill, R.A. A quantitative measure of solvent solvophobic effect. J. Chem. Soc. Perkin Trans. 1988, 339–345. [Google Scholar]
- Rosen, M.J. Surfactants and Interfacial Phenomena; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Kumpulainen, A.J.; Tyrode, E.C.; Eriksson, J.C. Soluble Monolayers of Sugar-Based Surfactants at the Air-Solution Interface. In Sugar-Based Surfactants: Fundamentals and Applications; Carnero Ruiz, C., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 153–206. [Google Scholar]
- Brunetti, S.; Roux, D.; Bellocq, A.M.; Fourche, G.; Bothord, P. Micellar interactions in water-in-oil microemulsions. II. Light-scattering determination of the 2nd virial-coefficient. J. Phys. Chem 1983, 87, 1028–1034. [Google Scholar]
- Frindi, M.; Michels, B.; Zana, R. Ultrasonic absorption studies of surfactant exchange between micelles and bulk phase in aqueous micellar solutions of nonionic surfactants with a short alkyl chain. III. Surfactants with a sugar head group. J. Phys. Chem 1992, 96, 8137–8141. [Google Scholar]
- Valiente, M.; Cortés, A.B.; Gradzielski, M.; Noirez, L.; Schweins, R. A SANS investigation of micelles in mixtures of cetyltrimethylammonium bromide (CTAB)/octyl-β-glucopyranoside (C8G1) in water/glycerol solvent. Colloids Surf. A 2011, 375, 117–123. [Google Scholar]
- Yousefi, A.; Javadian, S.; Gharibi, H.; Kakemam, J.; Rashidi-Alavijeh, M. Cosolvent effects on the spontaneous formation of nanorod vesicles in cationic mixtures in the rich cationic region. J. Phys. Chem. B 2011, 115, 8112–8121. [Google Scholar]
- Li, X.; Mya, K.Y.; Ni, X.; He, C.; Leong, K.W.; Li, J. Dynamic and static light scattering studies on self-aggregation behavior of biodegradable amphiphilic poly(ethylente oxide)-poly [(R)-3-hydroxybutyrate]-Poly(ethylene oxide) triblock copolymers in aqueous solution. J. Phys. Chem. B 2006, 110, 5920–5926. [Google Scholar]
- Wei, L.; Jinfeng, L.; Jie, G.; Bohua, L.; Yu, X.; Yanchun, M.; Yongsheng, Y.; Huaiwen, C.; Jianxin, D.; Hao, W.; et al. The fine-tuning of thermosensitive and degradable polymer micelles for enhancing intracellular uptake and drug release in tumors. Biomaterials 2011, 32, 3832–3844. [Google Scholar]
- Yao, J.H.; Mya, K.Y.; Li, X.; Parameswaran, M.; Xu, Q.-H.; Loh, K.P.; Chen, Z.-K. Light scattering and luminescence studies of self-aggregation behavior of amphiphilic copolymer micelles. J. Phys. Chem. B 2008, 112, 749–755. [Google Scholar]
- Naik, S.S.; Ray, J.G.; Savin, D.A. Temperature and pH-responsive self-assembly of poly(propylene oxide)-b-poly(lysine) block copolymers in aqueous solutions. Langmuir 2011, 27, 7231–7240. [Google Scholar]
- Yang, L.; Qib, X.; Liu, P.; El Ghzaouia, A.; Li, S. Aggregation behavior of self-assembling polylactide/poly(ethylene glycol) micelles for sustained drug delivery. Int. J. Pharm 2010, 394, 43–49. [Google Scholar]
- Yoshimura, T.; Esumi, K. Physicochemical properties of anionic triple-chain surfactants in alkaline solutions. J. Colloid Interface Sci 2004, 276, 450–455. [Google Scholar]
- Christov, N.C.; Denkov, N.D.; Kralchevsky, P.A.; Ananthapadmanabhan, K.P.; Lips, A. Synergistic sphere-to-rod micelle formation in mixed solutions of sodium dodecyl sulphate and cocoamidopropyl betaine. Langmuir 2004, 20, 565–571. [Google Scholar]
- Wagner, B.D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar]
- Hierrezuelo, J.M.; Carnero Ruiz, C. Rotational diffusion of coumarin 153 in nanoscopic micellar environments of n-dodecyl-β-d-maltoside and n-dodecyl-hexaethylene-glycol mixtures. J. Phys. Chem. A 2012, 116, 12476–12485. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed; Springer: New York, NY, USA, 2006. [Google Scholar]
- Quitevis, E.L.; Marcus, A.H.; Fayer, M.D. Dynamics of ionic lipophilic probes in micelles: Picosecond fluorescence depolarization measurements. J. Phys. Chem 1993, 97, 5762–5769. [Google Scholar]
- Maiti, N.C.; Krishna, M.M.G.; Britto, P.J.; Periasamy, N. Fluorescence dynamics of dye probes in micelles. J. Phys. Chem. B 1997, 101, 11051–11060. [Google Scholar]
- Dutt, G.B. Are the experimentally determined microviscosities of the micelles probe dependent? J. Phys. Chem. B 2004, 108, 3651–3657. [Google Scholar]
- Horng, M.L.; Gardecki, J.A.; Maroncelli, M. Rotational dynamics of coumarin 153: Time-dependent friction, dielectric friction, and other nonhydrodynamic effects. J. Phys. Chem. A 1997, 101, 1030–1047. [Google Scholar]
- Jin, H.; Baker, G.A.; Arzhantsev, S.; Dong, J.; Maroncelli, M. Solvation and rotational dynamics of coumarin 153 in ionic liquids: Comparisons to conventional solvents. J. Phys. Chem. B 2007, 111, 7291–7302. [Google Scholar]
- Banerjee, P.; Pramanik, S.; Sarkar, A.; Bhattacharya, S.C. Modulated photophysics of 3-pyrazolyl-2-pyrazoline derivative entrapped in micellar assembly. J. Phys. Chem. B 2008, 112, 7211–7219. [Google Scholar]
- Provencher, S.W. CONTIN—A general-purpose constrained regularization program for inverting noisy linear algebraic and integral-equations. Comput. Phys. Commun 1982, 27, 229–242. [Google Scholar]
- Pecora, R. Dynamic Light Scattering, Applications of Photon Correlation Spectroscopy; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Imae, T. Aqueous sodium-halide solutions of nonionic and cationic surfactants with a consolute phase-boundary light-scattering behavior. Langmuir 1989, 5, 205–210. [Google Scholar]
- Shukla, A.; Rehage, H. Zeta potentials and Debye screening lengths of aqueous, viscoelastic surfactant solutions (Cetyltrimethylammonium Bromide/Sodium Salicylate system). Langmuir 2008, 24, 8507–8513. [Google Scholar]



| EG (% wt) | (cmc) (mM) a | −ΔGmic0 (kJ/mol) | Γmax 103 (mmol/m2) | Amin Å2/molecule | Πcmc (mN/m) | −ΔGads0 (kJ/mol) |
|---|---|---|---|---|---|---|
| 0 | 7.9 (8.7) | 22.0 | 3.1 | 52.9 | 42.3 | 35.3 |
| 10 | 9.4 (10.5) | 21.4 | 3.4 | 49.4 | 37.7 | 32.5 |
| 20 | 10.1 (12.6) | 21.0 | 3.6 | 46.5 | 33.8 | 30.5 |
| 30 | 13.2 (16.0) | 20.2 | 3.5 | 48.0 | 30.4 | 28.9 |
| 40 | 18.1 (22.3) | 19.2 | 4.0 | 41.6 | 27.2 | 26.0 |
| EG (% wt) | η (mPas) | Nagg | R0 (nm) | −A2 × 109 (mol m3 g−2) | −kD (L g−1) | REFF (nm) | ρ (g cm−3) |
|---|---|---|---|---|---|---|---|
| 0 | 0.89 | 114 | 3.5 | 7.3 | 0.75 | 9.6 | 0.32 |
| 10 | 1.13 | 99 | 3.6 | 4.7 | 0.27 | 7.6 | 0.26 |
| 20 | 1.34 | 95 | 2.9 | 2.6 | 0.09 | 6.0 | 0.48 |
| 30 | 1.82 | 82 | 2.7 | 0.6 | 0.03 | 3.4 | 0.51 |
| 40 | 2.42 | 45 | 2.0 | 0.7 | 0.02 | 2.4 | 0.69 |
| %wt EG | r0 | β | τslow (ns) | τfast (ns) | χ2 | 〈τr〉 (ns) |
|---|---|---|---|---|---|---|
| 0 | 0.288 | 0.40 ± 0.01 | 3.77 ± 0.12 | 0.66 ± 0.01 | 1.04 | 1.90 ± 0.18 |
| 10 | 0.281 | 0.41 ± 0.01 | 3.63 ± 0.11 | 0.61 ± 0.01 | 1.05 | 1.90 ± 0.17 |
| 20 | 0.280 | 0.41 ± 0.01 | 3.39 ± 0.11 | 0.62 ± 0.01 | 1.03 | 1.76 ± 0.16 |
| 30 | 0.274 | 0.41 ± 0.01 | 3.22 ± 0.10 | 0.59 ± 0.01 | 1.16 | 1.66 ± 0.17 |
| 40 | 0.244 | 0.40 ± 0.02 | 2.93 ± 0.10 | 0.52 ± 0.01 | 1.04 | 1.45 ± 0.16 |
| %wt EG | 〈τr〉 (ns) | lang;τr〉P (ns) | τM (ns) | ηm (mPa s) |
|---|---|---|---|---|
| 0 | 1.90 | 1.90 | 1800 | 32.6 |
| 10 | 1.90 | 1.90 | 1181 | 32.6 |
| 20 | 1.76 | 1.78 | 131.5 | 30.5 |
| 30 | 1.66 | 1.72 | 50.6 | 29.5 |
| 40 | 1.45 | 1.57 | 19.7 | 26.9 |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ruiz, C.C.; Molina-Bolívar, J.A.; Hierrezuelo, J.M.; Liger, E. Self-Assembly, Surface Activity and Structure of n-Octyl-β-D-thioglucopyranoside in Ethylene Glycol-Water Mixtures. Int. J. Mol. Sci. 2013, 14, 3228-3253. https://doi.org/10.3390/ijms14023228
Ruiz CC, Molina-Bolívar JA, Hierrezuelo JM, Liger E. Self-Assembly, Surface Activity and Structure of n-Octyl-β-D-thioglucopyranoside in Ethylene Glycol-Water Mixtures. International Journal of Molecular Sciences. 2013; 14(2):3228-3253. https://doi.org/10.3390/ijms14023228
Chicago/Turabian StyleRuiz, Cristóbal Carnero, José Antonio Molina-Bolívar, José Manuel Hierrezuelo, and Esperanza Liger. 2013. "Self-Assembly, Surface Activity and Structure of n-Octyl-β-D-thioglucopyranoside in Ethylene Glycol-Water Mixtures" International Journal of Molecular Sciences 14, no. 2: 3228-3253. https://doi.org/10.3390/ijms14023228