Agriculture and Bioactives: Achieving Both Crop Yield and Phytochemicals
Abstract
:Abbreviations
| ROS | Reactive Oxigen Species |
| SA | Salicylic Acid |
| JA | Jasmonic Acid |
| ET | Ethylene |
| SAR | Systemic Acquired Resistance |
| ISR | Induction of Systemic Resistance |
| IPP | Isopentenyl Diphosphate |
| MEP | Methylerythritol Phosphate |
| OA | Organic Agriculture |
| PCBs | Polychlorinated Biphenyls |
| GM | Genetically Modified |
| GMOs | Genetically Modified Organisms |
| MJ | Methyl Jasmonate |
| BTH | Benzothiadiazole |
| INA | 2,6-Dichloroisonicotinic acid |
| ASM | S-methylbenzo[1,2,3] thiadiazole-7-carbothiate (Acibenzolar-S-Methyl) |
| BABA | β-aminobutyric acid |
| PAA-Q | Poly (Acrylic acid)-Chitosan |
| BA | Benzoic Acid |
| ICM | Integrated Crop Management. |
1. Introduction
2. Agricultural Systems
3. Achievement Crop Yield for Fulfillment of Global Food Requirements
4. Phytochemical Enhancement for Health
| Active compounds | Potential health benefits | References |
|---|---|---|
| Polyphenols | Antiproliferative, antimutagenic, antioxidant, estrogenic, antimicrobial, anti-inflammatory, anticarcinogenic, cardioprotective, anti-itch, hypocholesterolemic, antidiabetic activity | [88,89,90,91,92,93,94,95,96,97] |
| Terpenes | Antioxidant activity, cancer prevention, cardioprotective activity, protection against eye diseases (cataracts, macular degeneration), antimicrobial, antidiabetic activity | [65,98,99,100,101] |
| Alkaloids | Antioxidant, antitumor, anticancer, anti-inflammatory activity, rheumatoid arthritis, hypertension | [102,103,104,105,106,107] |
5. An Approach to Sustainable Agriculture Using Elicitors
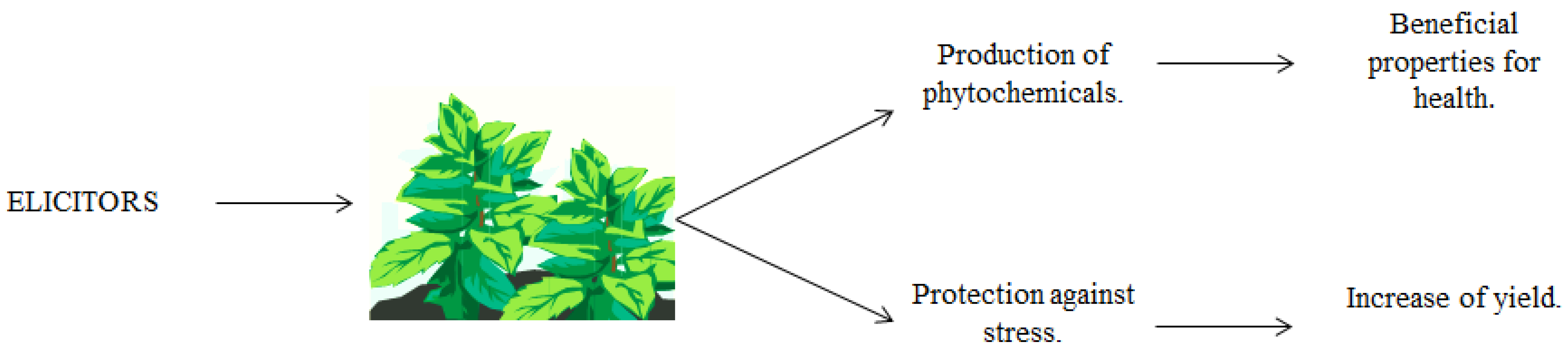
6. Conclusions
Acknowledgments
Conflict of Interest
References
- Davies, B.; Baulcombe, D.; Crute, I.; Dunwell, J.; Gale, M.; Jones, J.; Pretty, J.; Sutherlnd, W.; Toulmin, C. Reaping the Benefits: Science and the Sustainable Intensification of Global Agriculture; the Royal Society: London, UK, 2009. [Google Scholar]
- Hawkesworth, S.; Dangour, A.D.; Johnston, D.; Lock, K.; Poole, N.; Rushton, J.; Uauy, R.; Waage, J. Feeding the world healthily: The challenge of measuring the effects of agriculture on health. Phil. Trans. Roy. Soc. B 2010, 365, 3083–3097. [Google Scholar] [CrossRef]
- Ruhul Amin, A.R.M.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef]
- Jahangir, M.; Abdel-Farid, I.B.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Healthy and unhealthy plants: The effect of stress on the metabolism of Brassicaceae. Environ. Exp. Bot. 2009, 67, 23–33. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Azadi, H.; Schoonbeek, S.; Mahmoudi, H.; Derudder, B.; de Maeyer, P.; Witlox, F. Organic agriculture and sustainable food production system: main potentials. Agric. Ecosyst. Environ. 2011, 144, 92–94. [Google Scholar] [CrossRef]
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the demand for crop production: the challenge of yield decline in crops grown in short rotation. Biol. Rev. 2012, 87, 52–71. [Google Scholar]
- Frost, C.J.; Mescher, M.C.; Carlston, J.E.; de Moraes, C.M. Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef]
- Rea, G.; Antonacci, A.; Lambreva, M.; Margonelli, A.; Ambrosi, C.; Giardi, M.T. The NUTRA-SNACKS Project: Basic Research and Biotechnological Programs on Nutraceutic. In Bio-Farms for Nutraceuticals: Functional Food and Safety Control by Biosensors; Giardi, M.T., Rea, G., Berra, B., Eds.; Springer: New York, NY, USA, 2010; pp. 1–13. [Google Scholar]
- Kim, H.-J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2006, 54, 2327–2332. [Google Scholar] [CrossRef]
- Ramos-Solano, B.; Algar, E.; García-Villaraco, A.; García-Cristóbal, J.; Lucas García, J.A.; Gutierrez-Mañero, F.J. Biotic elicitation of isoflavone metabolism with plant growth promoting rhizobacteria in early stages of development in Glycine max var. Osumi. J. Agric. Food Chem. 2010, 58, 1484–1492. [Google Scholar]
- Xiong, L.; Schumaker, K.S.; Zhu, J.-K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [CrossRef]
- Godfray, J.H.C.; Bedington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: the challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar]
- Floros, J.D.; Newsome, R.; Fisher, W.; Barbosa-Cánovas, G.V.; Chen, H.; Dunne, C.P.; German, J.B.; Hall, R.L.; Heldman, D.R.; Karwe, M.V.; et al. Feeding the world today and tomorrow: The importance of food science and technology. Compr. Rev. Food Sci. F 2010, 9, 572–599. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations (FAO). The State of Food Insecurity in the World, Economic Crises-Impacts and Lessons Learned; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009.
- Wirsenius, S.; Azar, C.; Berndes, G. How much land is needed for global food production under scenarios of dietary changes and livestock productivity increases in 2030? Agric. Syst. 2010, 103, 621–638. [Google Scholar] [CrossRef]
- Bolognesi, C.; Merlo, F.D. Pesticides: Human Health Effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, MA, USA, 2011; pp. 438–453. [Google Scholar]
- McKinlay, R.; Plant, J.A.; Bell, J.N.B.; Voulvoulis, N. Endocrine disrupting pesticides: Implications for risk assessment. Environ. Int. 2008, 34, 168–183. [Google Scholar] [CrossRef]
- Nemecek, T.; Huguenin-Elie, O.; Dubois, D.; Gaillard, G.; Schaller, B.; Chervet, A. Life cycle assessment of Swiss farming systems: II. Extensive and intensive production. Agric. Syst. 2011, 104, 233–245. [Google Scholar] [CrossRef]
- Connor, D.J. Organic agriculture cannot feed the world. Field Crop Res. 2008, 106, 187–190. [Google Scholar] [CrossRef]
- Nemecek, T.; Duboi, D.; Huguenin-Elie, O.; Gaillard, G. Life cycle assessment of Swiss farming systems: I. Integrated and organic farming. Agric. Syst. 2011, 104, 217–232. [Google Scholar] [CrossRef]
- De Boer, I.J.M. Environmental impact assessment of conventional and organic milk production. Livest. Prod. Sci. 2003, 80, 69–77. [Google Scholar] [CrossRef]
- Wood, R.; Lenzen, M.; Dey, C.; Lundie, S. A comparative study of some environmental impacts of conventional and organic farming in Australia. Agric. Syst. 2006, 89, 324–348. [Google Scholar] [CrossRef]
- Archer, D.W.; Jaradat, A.A.; Johnson, J.M.-F.; Weyers, S.L.; Gesch, R.W.; Forcella, F.; Kludze, H.K. Crop productivity and economics during the transition to alternative cropping systems. Agron. J. 2007, 99, 1538–1547. [Google Scholar] [CrossRef]
- Gopinath, K.A.; Saha, S.; Mina, B.L.; Pande, H.; Srivastva, A.K.; Gupta, H.S. Bell pepper yield and soil properties during conversion from conventional to organic production in Indian Himalayas. Sci. Hortic-Amsterdam 2009, 122, 339–345. [Google Scholar] [CrossRef]
- De Ponti, T.; Rijk, B.; van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Del Amor, F.M. Growth, photosynthesis and chlorophyll fluorescence of sweet pepper plants as affected by the cultivation method. Ann. Appl. Biol. 2006, 148, 133–139. [Google Scholar] [CrossRef]
- Herencia, J.F.; García-Galavís, P.A.; Ruiz Dorado, J.R.; Maqueda, C. Comparison of nutritional quality of the crops grown in an organic and conventional fertilized soil. Sci. Hortic-Amsterdam 2011, 129, 882–888. [Google Scholar] [CrossRef]
- Huber, M.; Rembiałkowska, E.; Srednicka, D.; Bügel, S.; van de Vijver, L.P.L. Organic food and impact on human health: Assessing the status quo and prospects of research. NJAS-Wagen. J. Life Sci. 2011, 58, 103–109. [Google Scholar] [CrossRef]
- Pérez-López, A.J.; López-Nicolas, J.M.; Núñez-Delicado, E.; del Amor, F.M.; Carbonell-Barrachina, A.A. Effects of agricultural practices on color, carotenoids composition, and minerals contents of sweet pepper, c.v. Almuden. J. Agric. Food Chem. 2007, 55, 8158–8164. [Google Scholar]
- Kim, G.D.; Lee, Y.S.; Cho, J.Y.; Lee, Y.H.; Choi, K.J.; Lee, Y.; Han, T.H.; Lee, S.H.; Park, K.H.; Moon, J.H. Comparison of the content of bioactive substances and the inhibitory effects against rat plasma oxidation of conventional and organic hot pepper (Capsicum annuum L.). J. Agric. Food Chem. 2010, 58, 12300–12306. [Google Scholar]
- Luthria, D.; Singh, A.P.; Wilson, T.; Vorsa, N.; Banuelos, G.S.; Vinyard, B.T. Influence of conventional and organic agricultural practices on the phenolic content in eggplant pulp: Plant-to-plant variation. Food Chem. 2010, 121, 406–411. [Google Scholar] [CrossRef]
- Mózner, Z.; Tabi, A.; Csutora, M. Modifying the yield factor based on more efficient use of fertilizer-The environmental impacts of intensive and extensive agricultural practices. Ecol. Indic. 2012, 16, 58–66. [Google Scholar] [CrossRef]
- Casado, G.I.G.; de Molina, M.G. Preindustrial agriculture versus organic agriculture. The land cost of sustainability. Land Use Policy 2009, 26, 502–510. [Google Scholar] [CrossRef]
- Stefanelly, D.; Goodwin, I.; Jones, R. Minimal nitrogen and water use in horticulture: Effects on quality and content of selected nutrients. Food Res. Int. 2010, 43, 1833–1843. [Google Scholar] [CrossRef]
- Hanson, J.D.; Franzluebbers, A. Principles of integrated agricultural systems. Renew. Agric. Food Syst. 2008, 23, 263–264. [Google Scholar] [CrossRef]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Popkin, B.M. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006, 84, 289–298. [Google Scholar]
- Foresight. The Future of Food and Farming. Executive Summary; the Government Office for Science: London, UK, 2011.
- McGill, A.E.J. The potential effects of demands for natural and safe foods on global food security. Trends Food Sci. Tech. 2009, 20, 402–406. [Google Scholar]
- Middaugh, A.L.; Fisk, P.S.; Brunt, A.; Rhee, Y.S. Few associations between income and fruit and vegetable consumption. J. Nutr. Educ. Behav. 2012, 44, 196–203. [Google Scholar]
- Fresco, L.O. Challenges for food system adaptation today and tomorrow. Environ. Sci. Policy 2009, 12, 378–385. [Google Scholar]
- Vermeulen, S.J.; Aggarwal, P.K.; Ainslie, A.; Angelone, C.; Campbell, B.M.; Challinor, A.J.; Hansen, J.W.; Ingram, J.S.I.; Jarvis, A.; Kristjanson, P.; et al. Options for support to agriculture and food security under climate change. Environ.Sci. Policy 2012, 15, 136–144. [Google Scholar]
- Gerbens-Leenes, P.W.; Nonhebel, S.; Krol, M.S. Food consumption patterns and economic growth. Increasing affluence and the use of natural resources. Appetite 2010, 55, 597–608. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). How to Feed the World in 2050. In Proceedings of High Level Expert Forum, Rome, Italy, 12-13 October 2009; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- U.S. Department of Commerce. 2012 World POPClock Projection. Available online: http://www.census.gov/main/www/popclock.html (accessed on 27 August 2012).
- United Nations (UN). World Population Prospects; the 2010 Revision; Population Division, Department of Economic and Social Affairs, United Nations: New York, NY, USA, 2010.
- Food and Agriculture Organization of the United Nations (FAO). World Agriculture: Towards 2015/2030. An FAO Perspective; Bruinsma, J., Ed.; Food and Agriculture Organization of the United Nations/Earthscan: London, UK, 2003.
- Khan, S.; Hanjra, M.A. Footprints of water and energy inputs in food production–Global perspectives. Food Policy 2009, 34, 130–140. [Google Scholar] [CrossRef]
- The Royal Society. Sustainable Biofuels: Prospects and Challenges; the Royal Society: London, UK, 2008. [Google Scholar]
- Mullie, P.; Clarys, P.; Hulens, M.; Vansant, G. Dietary patterns and socioeconomic position. Eur. J. Clin. Nutr. 2010, 64, 231–238. [Google Scholar] [CrossRef]
- World Health Organization/Food and Agriculture Organization of the United Nations (WHO/FAO). Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO technical report series 916; World Health Organization: Geneva, Switzerland, 2003.
- World Health Organization (WHO). Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Rome, Italy, 2011.
- Falguera, V.; Aliguer, N.; Falguera, M. An integrated approach to current trends in food consumption: moving toward functional and organic products? Food Control 2012, 26, 274–281. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Tech. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Szakály, Z.; Szente, V.; Kövér, G.; Polereczki, Z.; Szigeti, O. The influence of lifestyle on health behavior and preference for functional foods. Appetite 2012, 58, 406–413. [Google Scholar] [CrossRef]
- Swaminathan, M.S. Can science and technology feed the world in 2025? Field Crop. Res. 2007, 104, 3–9. [Google Scholar] [CrossRef]
- Spiertz, H. Food production, crops and sustainability: restoring confidence in science and technology. Curr. Opin. Environ. Sustain. 2010, 2, 439–443. [Google Scholar] [CrossRef]
- European Commision DG Environment. Integrated Crop Management Systems in the EU; Amended Final Report for European Commission DG Environment; Agra CEAS Consulting: Brussels, Belgium, 2002.
- Hassimotto, N.M.A.; Genovese, M.I.; Lajolo, F.M. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J. Agric. Food Chem. 2005, 53, 2928–2935. [Google Scholar] [CrossRef]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Total and specific fruit and vegetable consumption and risk of stroke: a prospective study. Atherosclerosis 2012. [Google Scholar] [CrossRef]
- Raaman, N. Categories of Phytochemicals. In Phytochemical Techniques; New India Publishing Agency: New Delhi, India, 2006; pp. 197–274. [Google Scholar]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar]
- Heredia, J.B.; Cisneros-Zevallos, L. The effects of exogenous ethylene and methyl jasmonate on the accumulation of phenolic antioxidants in selected whole and wounded fresh produce. Food Chem. 2009, 115, 1500–1508. [Google Scholar] [CrossRef]
- Yahia, E.M.; Ornelas-Paz, J.J. Chemistry, Stability and Biological Actions of Carotenoids. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; de la Rosa, L.A., Álvarez-Parrilla, E., González-Aguilar, G.A., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 177–222. [Google Scholar]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P.J.; Menichini, F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Matsufuji, H.; Ishikawa, K.; Nunomura, O.; Chino, M.; Takeda, M. Anti-oxidant content of different coloured sweet peppers, white, green, yellow, orange and red (Capsicum annuum L.). Int. J. Food Sci. Tech. 2007, 42, 1482–1488. [Google Scholar] [CrossRef]
- Marín, A.; Gil, M.I.; Flores, P.; Hellín, P.; Selma, M.V. Microbial quality of bioactive constituents of sweet peppers from sustainable production systems. J. Agric. Food Chem. 2008, 56, 11334–11341. [Google Scholar]
- Roussos, P.A. Phytochemicals and antioxidant capacity of orange (Citrus sinensis (1.) Osbeck cv. Salustiana) juice produced under organic and integrated farming system in Greece. Sci. Hort. 2011, 129, 253–258. [Google Scholar] [CrossRef]
- Shilpa, K.; Varun, K.; Lakshmi, B.S. An alternate method of natural drug production: Eliciting secondary metabolite production using plant cell culture. J. Plant Sci. 2010, 5, 222–247. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar]
- Modolo, L.V.; Reichert, A.I.; Dixon, R.A. Introduction to the Different Classes of Biosynthetic Enzymes. In Plant-Derived Natural Products; Osbourns, A.E., Lanzottim, V., Eds.; Springer: New York, NY, USA, 2009; pp. 143–163. [Google Scholar]
- Jeong, G.-T.; Park, D.-H. Enhancement of growth and secondary metabolite biosynthesis: Effect of elicitors derived from plants and insects. Biotechnol. Bioprocess Eng. 2005, 10, 73–77. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Züst, T.; Joseph, B.; Shimizu, K.K.; Kliebenstein, D.J.; Turnbull, L.A. Using knockout mutant to reveal the growth cost of defensive traits. Proc. R. Soc. B 2011, 278, 2598–2603. [Google Scholar] [CrossRef]
- Bajguz, A.; Tretyn, A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 2003, 62, 1027–1046. [Google Scholar]
- Bent, A.F.; Mackey, D. Elicitors, effectors and R genes: The new paradigm and a lifetime supply of questions. Ann. Rev. Phytopathol. 2007, 45, 399–436. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A renaissance of elicitors: perception of Microbe–associated molecular patterns and danger signals by pattern-recognition. Ann. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Gijzen, M. Phytochemical diversity: The sounds of silent metabolism. Plant Sci. 2009, 176, 161–169. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2006, 120, 175–188. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–233. [Google Scholar]
- Ferrari, S. Biological Elicitors of Plant Secondary Metabolites: Mode of Action and Use in the Production of Nutraceutics. In Bio-Farms for Nutraceuticals: Functional Food and Safety Control by Biosensors; Giardi, M.T., Rea, G., Berra, B., Eds.; Springer: New York, NY, USA, 2010; pp. 152–166. [Google Scholar]
- Bari, R.; Jone, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Chithrashree, A.C.; Udayashankar, A.C.; Chandra Nayaka, S.; Reddy, M.S.; Srinivas, C. Plant growth-promoting rhizobacteria mediate induced systemic resistance in rice against bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae. Biol. Control 2011, 59, 114–122. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; van der Graaff, E.; Roitsch, T. Phytoalexin transgenics in crop protection-fairy tale with a happy end? Plant Sci. 2012, 195, 54–70. [Google Scholar] [CrossRef]
- Issa, A.Y.; Volate, S.R.M.; Wargovich, M.J. The role of phytochemicals in inhibition of cancer and inflammation: new directions and perspectives. J. Food Compos. Anal. 2006, 19, 40–419. [Google Scholar]
- Bernal, J.; Mendiola, J.A.; Ibáñez, E.; Cifuentes, A. Advanced analysis of nutraceuticals. J. Pharm. Biomed. 2011, 55, 758–774. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Cardador-Martínez, A.; Castaño-Tostado, E.; Loarca-Piña, G. Antimutagenic activity of natural phenolic compounds present in the common bean (Phaseolus vulgaris) against aflatoxin B1. Food Addit. Contam. 2002, 19, 62–69. [Google Scholar] [CrossRef]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P.M. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef]
- Sur, R.; Nigam, A.; Grote, D.; Liebel, F.; Southall, M.D. Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Arch. Dermatol. Res. 2008, 300, 569–574. [Google Scholar] [CrossRef]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Veloz-García, R.; Marín-Martínez, R.; Veloz-Rodríguez, R.; Rodríguez-Guerra, R.; Torres-Pacheco, I.; González Chavira, M.M.; Anaya-López, J.L.; Guevara-Olvera, L.; Feregrino-Pérez, A.A.; Loarca-Piña, G.; et al. Antimicrobial activities of cascalote (Caesalpinia cacalaco) phenolics-containing extract against fungus Colletotrichum lindemuthianum. Ind. Crop. Prod. 2010, 31, 134–138. [Google Scholar] [CrossRef]
- Jiao, R.; Zhang, Z.; Yu, H.; Huang, Y.; Chen, Z.-Y. Hypocholesterolemic activity of grape seed proanthocyanidin is mediated by enhancement of bile acid excretion and up-regulation of CYP7A1. J. Nutr. Biochem. 2010, 21, 1134–1139. [Google Scholar]
- Feregrino-Pérez, A.A.; Torres-Pacheco, I.; Vargas-Hernández, M.; Munguía-Fragozo, P.V.; Loarca-Piña, G.F.; Mendoza-Díaz, S.O.; Ocampo-Velázquez, R.V.; Rico-García, E.; Guevara-González, R.G. Antioxidant and antimutagenic activities of Acacia pennatula pods. J. Sci. Ind. Res. India 2011, 70, 859–864. [Google Scholar]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Mathabe, M.C.; Hussein, A.A.; Nikolova, R.V.; Basson, A.E.; Meyer, J.J.; Lall, N. Antibacterial activities and cytotoxicity of terpenoids isolated from Spirostachys africana. J. Ethnopharmacol. 2008, 116, 194–197. [Google Scholar] [CrossRef]
- Lage, H.; Duarte, N.; Coburger, C.; Hilgeroth, A.; Ferreira, M.J.U. Antitumor activity of terpenoids against classical and atypical multidrug resistant cancer cells. Phytomedicine 2010, 17, 441–448. [Google Scholar] [CrossRef]
- Patil, R.; Patil, R.; Ahirwar, B.; Ahirwar, D. Current status of Indian medicinal plant with antidiabetic potential: a review. Asian Pac. J. Trop. Biomed. 2011, 1, S291–S298. [Google Scholar]
- Herraiz, T.; Galisteo, J. Tetrahydro-β-carboline alkaloids occur in fruits and fruit juice. Activity as antioxidant and radical scavengers. J. Agric. Food Chem. 2003, 51, 7156–7161. [Google Scholar] [CrossRef]
- Wang, C.; Dai, Y.; Yang, J.; Chou, G.; Wang, C.; Wang, Z. Treatment with total alkaloids from Radix Linderae reduces inflammation and joint destruction in type II collagen-induced model for rheumatoid arthritis. J. Ethnopharmacol. 2007, 111, 322–328. [Google Scholar] [CrossRef]
- Yang, C.-W.; Chuang, T.-H.; Wu, P.-L.; Huang, W.-H.; Lee, S.-J. Anti-inflammatory effects of 7-methoxycryptopleurine and structure–activity relations of phenanthroindolizidines and phenanthroquinolizidines. Biochem. Biophys. Res. Commun. 2007, 354, 942–948. [Google Scholar] [CrossRef]
- Baunbæk, D.; Trinkler, N.; Ferandin, Y.; Lozach, O.; Ploypradith, P.; Rucirawat, S.; Ishibashi, F.; Iwao, M.; Meijer, L. Anticancer alkaloid lamellarins inhibit protein kinases. Mar. Drugs 2008, 6, 514–527. [Google Scholar] [CrossRef]
- Kabashima, H.; Miura, N.; Shimizu, M.; Shinoda, W.; Wang, X.; Wang, Z.; Takahashi, S.; Harada, T.; Maruyama, H.; Tashiro, S.; et al. Preventive impact of alkaloids with anti-cancer effect extracted from natural herb and the derivatives. Webmed Central Preventive Med. 2010, 1, MC00519. [Google Scholar]
- Monteiro, F.S.; Silva, A.C.L.; Martins, I.R.R.; Correia, A.C.C.; Basílio, I.J.L.D.; Agra, M.F.; Bhattacharyya, J.; Silva, B.A. Vasorelaxant action of the total alkaloid fraction obtained from Solanum paludosum Moric. (Solanaceae) involves NO/cGMP/PKG pathway and potassium channels. J. Ethnopharmacol. 2012, 141, 895–900. [Google Scholar] [CrossRef]
- Capanoglu, E. The potential of priming in food production. Trends Food Sci. Tech. 2010, 21, 399–407. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Heijari, J.; Nerg, A.M.; Vuorinen, M.; Kainulainen, P. Potential for the use of exogenous chemical elicitors in disease and insect pest management of conifer seedling production. Open For. Sci. J. 2009, 2, 17–24. [Google Scholar]
- Montesano, M.; Brader, G.; Palva, E.T. Pathogen derived elicitors: Searching for receptors in plants. Mol. Plant Pathol. 2003, 4, 73–79. [Google Scholar] [CrossRef]
- Saw, N.M.M.T.; Riedel, H.; Kütük, O.; Ravichandran, K.; Smetanska, I. Effect of elicitors and precursors on the synthesis of anthocyanin in grape Vitis vinifera cell culture. Energy Rec. J. 2010, 1, 189–192. [Google Scholar] [CrossRef]
- Ortega-Ortiz, H.; Benavides-Mendoza, A.; Mendoza-Villarreal, R.; Ramírez-Rodríguez, H.; de Alba Romenus, K. Enzymatic activity in tomato fruits as a response to chemical elicitors. J. Mex. Chem. Soc. 2007, 51, 141–144. [Google Scholar]
- Walters, D.; Walsh, D.; Newton, A.; Lyon, G. Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Terry, L.A.; Joyce, D.C. Elicitors of induced disease resistance in postharvest horticultural crops: A brief review. Postharvest Biol. Tec. 2004, 32, 1–13. [Google Scholar] [CrossRef]
- Rohwer, C.L.; Erwin, J.E. Horticultural applications of jasmonates: A review. J. Hortic. Sci. Biotech. 2008, 83, 283–304. [Google Scholar]
- Nadarajah, K.; Turner, J.G. The role of jasmonate in plant pathogen interactions in Arabidopsis thaliana. J. Teknologi 2003, 39, 9–16. [Google Scholar]
- Bautista-Baños, S.; Hernández-López, M.; Bosquez-Molina, E.; Wilson, C.L. Effects of chitosan and plant extracts on grown of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Prot. 2003, 22, 1087–1092. [Google Scholar] [CrossRef]
- Boonlertnirun, S.; Meechoui, S.; Sarobol, E. Physiological and morphological responses of field corn seedlings to chitosan under hypoxic conditions. Sci. Asia 2010, 36, 89–93. [Google Scholar]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Eswaranandam, S.; Salyer, J.; Chen, P.; Lee, S.O. Effect of elicitor spray at different reproductive stages on saponin content of soybean. J. Food Sci. 2012, 77, H81–H86. [Google Scholar] [CrossRef]
- Puthusseri, B.; Divya, P.; Lokesh, V.; Neelwarne, B. Enhancement of folate content and its stability using food grade elicitors in coriander (Coriandrum sativum L.). Plant Food Hum. Nutr. 2012, 67, 162–170. [Google Scholar] [CrossRef]
- Chen, H.; Jones, D.; Howe, G. Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett. 2006, 580, 2540–2546. [Google Scholar]
- Warabieda, W.; Olszak, R.W. Effect of exogenous methyl jasmonate on numerical growth of the population of the two-spotted spider mite (Tetranychus urticae Koch.) on strawberry plants and young apple trees. J. Plant Prot. Res. 2010, 50, 541–544. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, Y.J.; Lee, S.C.; Hong, J.K.; Hwang, B.K. Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiol. 2007, 145, 890–904. [Google Scholar] [CrossRef]
- Yoshioka, H.; Mase, K.; Yoshioka, M.; Kobayashi, M.; Asai, S. Regulatory mechanisms of nitric oxide and reactive oxygen species generation and their role in plant immunity. Nitric Oxide-Biol. Chem. 2011, 25, 216–221. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Bindschedler, L.V.; Blee, K.A.; Butt, V.S.; Davies, D.R.; Gardner, S.L.; Gerrish, C.; Minibayeva, F. The apoplastic oxidative burst in response to biotic stress in plants: A three-component system. J. Exp. Bot. 2002, 53, 1367–1376. [Google Scholar] [CrossRef]
- Tierranegra-García, N.; Salinas-Soto, P.; Torres-Pacheco, I.; Ocampo-Velázquez, R.V.; Rico-García, E.; Mendoza-Diaz, S.O.; Feregrino-Pérez, A.A.; Mercado-Luna, A.; Vargas-Hernández, M.; Soto-Zarazúa, G.M.; Guevara-González, R.G. Effect of foliar salicylic acid and methyl jasmonate applications on protection against pill-bugs in lettuce plants (Lactuca sativa). Phytoparasitica 2011, 39, 137–144. [Google Scholar] [CrossRef]
- Paradikovic, N.; Vinkovic, T.; Vinkovi Vrcek, I.; Zuntar, I.; Bojic, M.; Medic-Saric, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar]
- Benavides-Mendoza, A.; Burgos-Limón, D.; Ortega-Ortiz, H.; Ramírez, H. El ácido benzoico y el poliácido acrílico-quitosán en la calidad y el rendimiento del tomate cultivado en suelo calcáreo. Terra Latinoamericana 2007, 25, 261–268, in Spanish. [Google Scholar]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Iriti, M.; Giulia, C.; Sara, V.; Ilaria, M.; Soave, C.; Fico, G.; Faoro, F. Chitosan-induced ethylene-independent resistance does not reduce crop yield in bean. Biol. Control 2010, 54, 241–247. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2005, 53, 3696–3701. [Google Scholar] [CrossRef]
- Cho, M.H.; No, H.K.; Prinyawiwatkul, W. Chitosan treatments affected growth and selected quality of sunflower sprout. J. Food Sci. 2008, 73, S70–S77. [Google Scholar]
- Bishnoi, U.R.; Payyavula, R.S.; Kumar, S. Enhancing disease resistance and yield in tomato and canola with plant activator. Res. Crop. 2004, 23, 268–273. [Google Scholar]
- Boonlertnirun, S.; Boonraung, C.; Suvanasara, R. Application of chitosan in rice production. J. Met. Mater. Miner. 2008, 18, 47–52. [Google Scholar]
- Mejía-Teniente, L.; Torres-Pacheco, I.; González-Chavira, M.M.; Ocampo-Velázquez, R.V.; Herrera-Ruiz, G.; Chapa-Oliver, A.M.; Guevara-González, R.G. Use of elicitors as an approach for sustainable agriculture. Afr. J. Biotechnol. 2010, 9, 9155–9162. [Google Scholar]
- Conrath, U. Priming of induced plant defense responses. Adv. Bot. Res. 2009, 51, 361–395. [Google Scholar]
- Heijari, J.; Nerg, A.-M.; Kainulainen, P.; Viiri, H.; Vuorinen, M.; Holopainen, J.K. Application of methyl jasmonate reduces growth, but increases chemical defence and resistance against Hylobius abietis in Scots pine seedlings. Entomol. Exp. Appl. 2005, 115, 117–124. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Nissinen, A.; Holopainen, J.K. The response of Plutella xylostella and its parasitoid Cotesia plutellae to volatile compounds. J. Chem. Ecol. 2005, 31, 1969–1984. [Google Scholar] [CrossRef]
- Cipollini, D.; Purringon, C.B.; Bergelson, J. Costs of induced responses in plants. Basic Appl. Ecol. 2003, 4, 79–85. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
García-Mier, L.; Guevara-González, R.G.; Mondragón-Olguín, V.M.; Del Rocío Verduzco-Cuellar, B.; Torres-Pacheco, I. Agriculture and Bioactives: Achieving Both Crop Yield and Phytochemicals. Int. J. Mol. Sci. 2013, 14, 4203-4222. https://doi.org/10.3390/ijms14024203
García-Mier L, Guevara-González RG, Mondragón-Olguín VM, Del Rocío Verduzco-Cuellar B, Torres-Pacheco I. Agriculture and Bioactives: Achieving Both Crop Yield and Phytochemicals. International Journal of Molecular Sciences. 2013; 14(2):4203-4222. https://doi.org/10.3390/ijms14024203
Chicago/Turabian StyleGarcía-Mier, Lina, Ramón G. Guevara-González, Víctor M. Mondragón-Olguín, Beatriz Del Rocío Verduzco-Cuellar, and Irineo Torres-Pacheco. 2013. "Agriculture and Bioactives: Achieving Both Crop Yield and Phytochemicals" International Journal of Molecular Sciences 14, no. 2: 4203-4222. https://doi.org/10.3390/ijms14024203




