Comparative Analysis of the Symbiotic Efficiency of Medicago truncatula and Medicago sativa under Phosphorus Deficiency
Abstract
:Abbreviations
| AA | amino acid |
| ANA | apparent nitrogenase activity |
| ASN | asparagine |
| DAT | day after transplanting |
| DM | dry matter |
| EAC | electron allocation coefficient |
| FW | fresh weight |
| Hup | uptake hydrogenase |
| Lb | leghemoglobin |
| N2ase | nitrogenase |
| OA | organic acid |
| PBM | peribacteroid membrane |
| PH | phloem |
| RC | respiratory chain |
| Sm | Sinorhizobium meliloti |
| TNA | total nitrogenase activity |
| XY | xylem |
| YEM | yeast extract mannitol |
1. Introduction
2. Results
2.1. Effect of P-Deficiency on Plant Biomass Production
2.2. Effect of P-Deficiency on Nodulation
2.3. Nitrogen Fixation
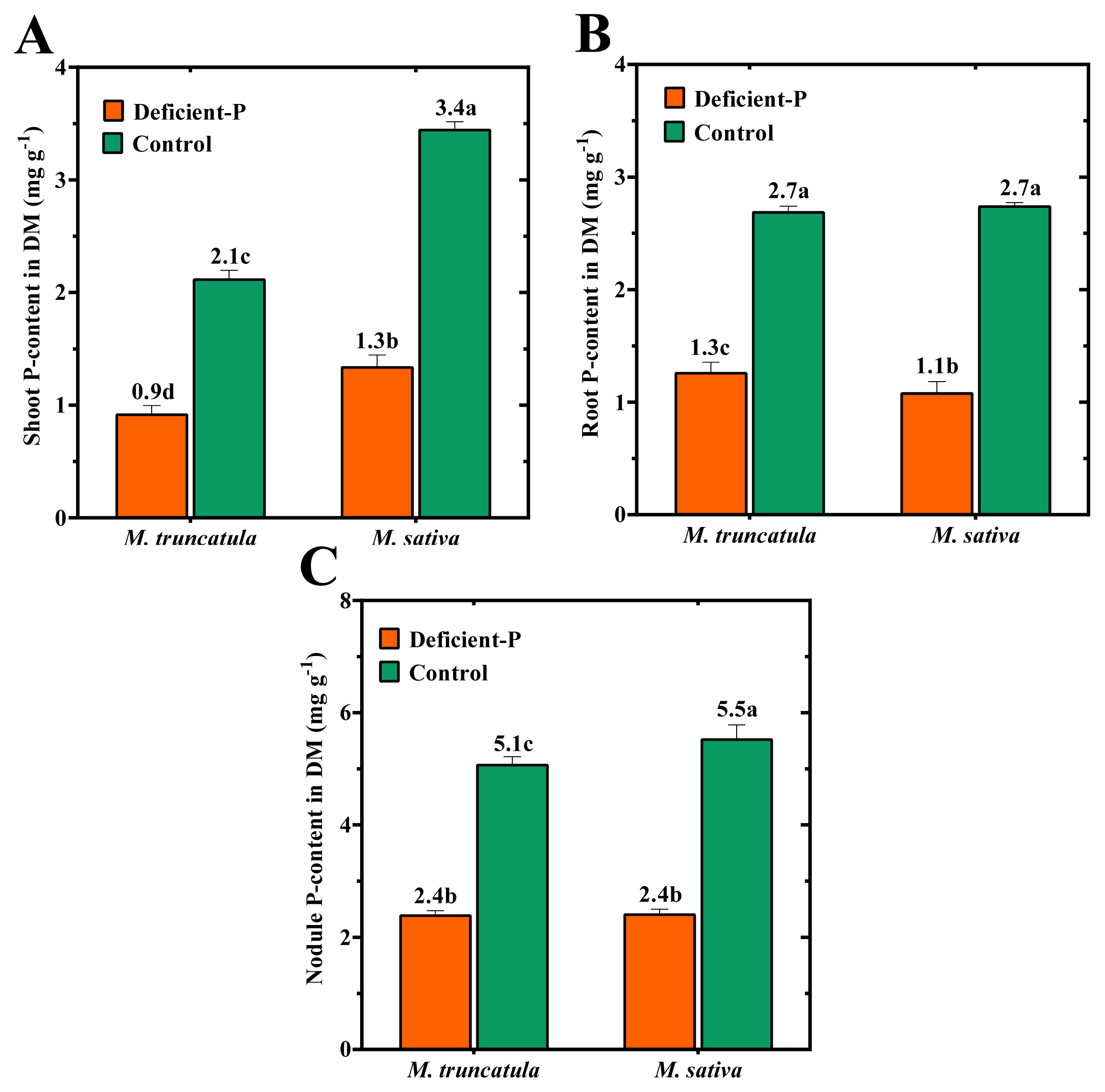
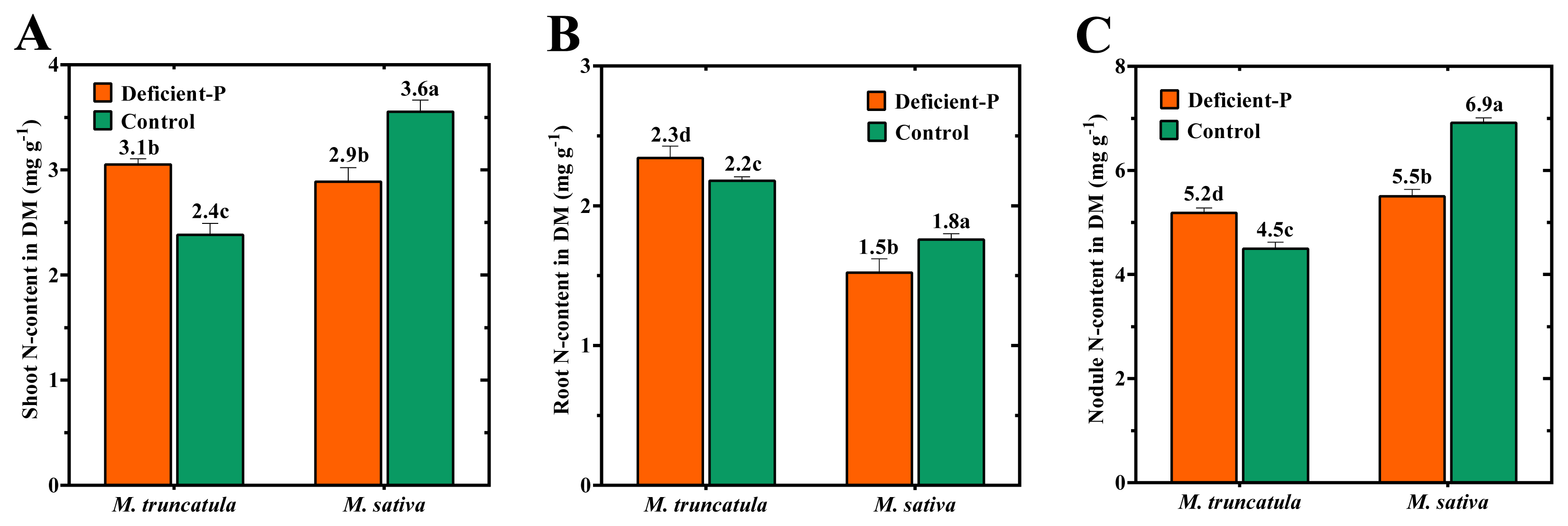
2.4. Effect of P-Deficiency on P- and N-Contents
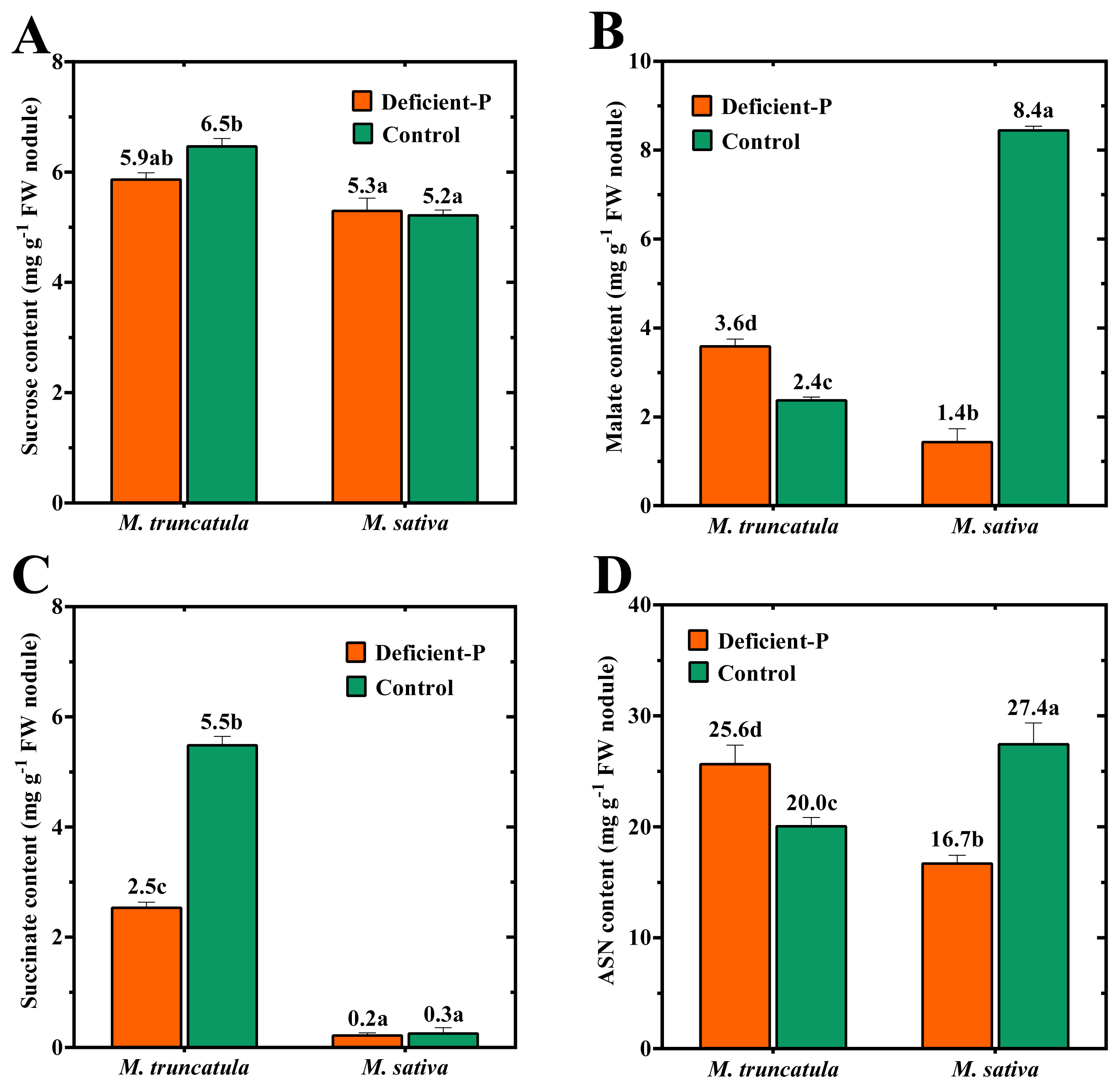
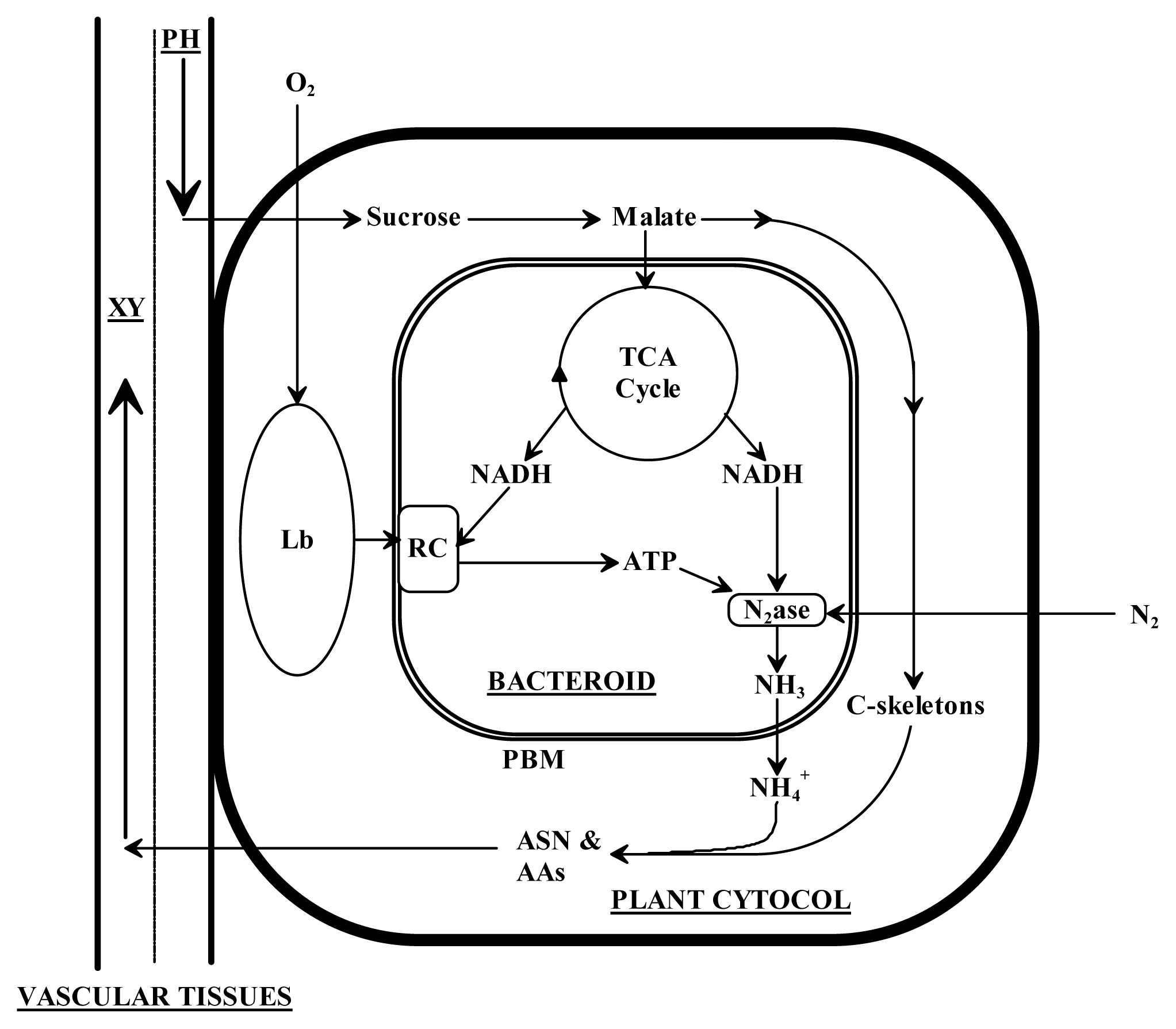
2.5. Major Nodule C- and N-Metabolites
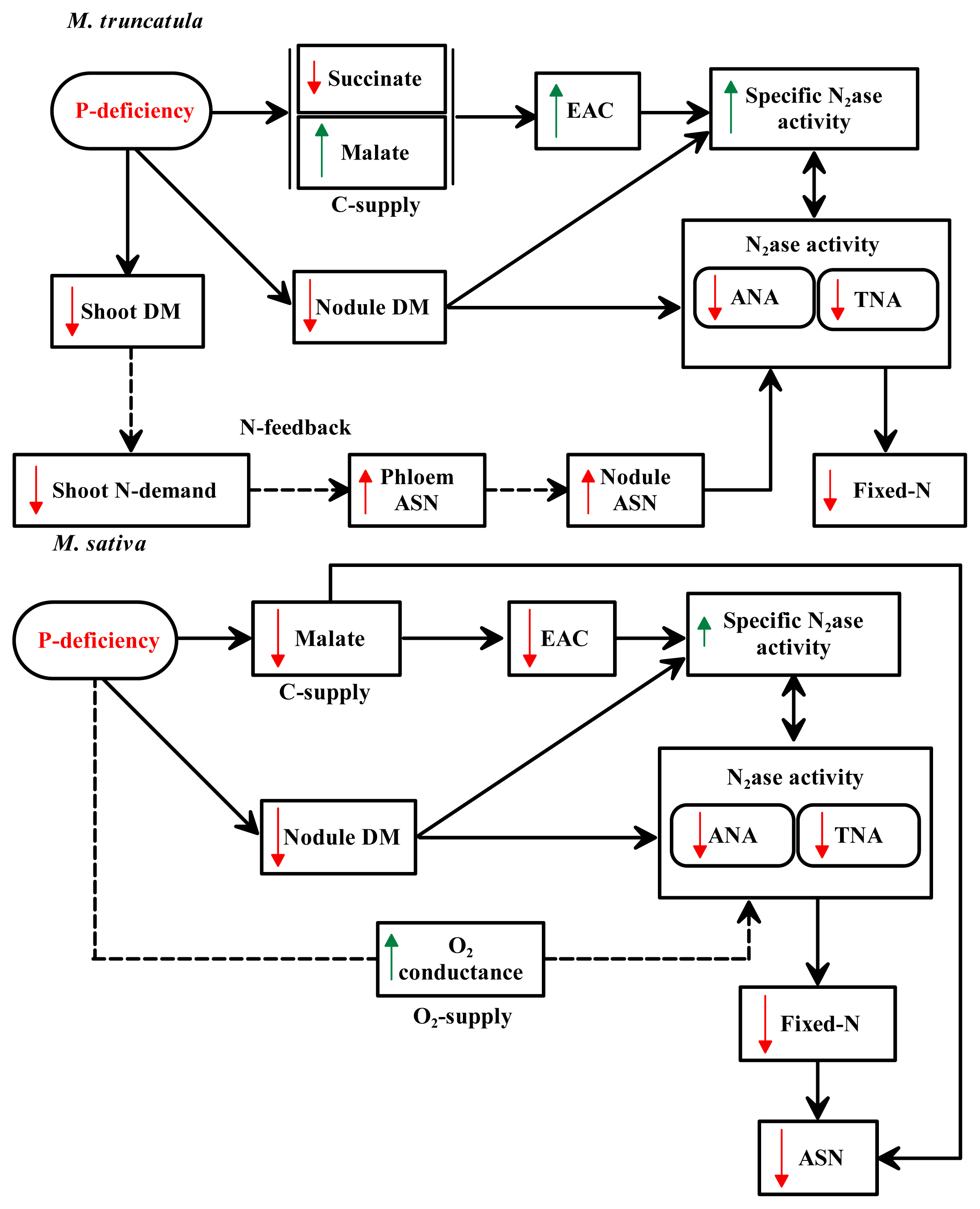
3. Discussion
4. Experimental Section
4.1. Plant Materials and Growth Conditions
4.2. Measurements of Nitrogenase Activity
4.3. HPLC Analyses
4.4. Determination of P- and N-Contents
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Ané, J.M.; Zhu, H.; Frugoli, J. Recent advances in Medicago truncatula genomics. Int. J. Plant Genomics 2008, 2008, 256597. [Google Scholar]
- Branca, A.; Paape, T.D.; Zhou, P.; Briskine, R.; Farmer, A.D.; Mudge, J.; Bharti, A.K.; Woodward, J.E.; May, G.D.; Gentzbittel, L.; et al. Whole-Genome nucleotide diversity, recombination, and linkage disequilibrium in the model legume Medicago truncatula. Proc. Natl. Acad. Sci. USA 2011, 108, E864–E870. [Google Scholar]
- Barker, D.G.; Bianchi, S.; Blondon, F.; Dattée, Y.; Duc, G.; Essad, S.; Flament, P.; Gallusci, P.; Génier, G.; Guy, P.; et al. Medicago truncatula, a model plant for studying the molecular genetics of the rhizobium-legume symbiosis. Plant Mol. Biol. Rep 1990, 8, 40–49. [Google Scholar]
- Cook, D.R. Medicago truncatula—A model in the making! Curr. Opin. Plant Biol 1999, 2, 301–304. [Google Scholar]
- Panara, F.; Calderini, O.; Porceddu, A. Medicago truncatula Functional Genomics—An Invaluable Resource for Studies on Agriculture Sustainability. In Functional Genomics; Meroni, G., Petrera, F., Eds.; InTech: Rijeka, Croatia, 2012; pp. 131–154. [Google Scholar]
- Young, N.D.; Debellé, F.; Oldroyd, G.E.D.; Geurts, R.; Cannon, S.B.; Udvardi, M.K.; Benedito, V.A.; Mayer, K.F.X.; Gouzy, J.; Schoof, H.; et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 2011, 480, 520–524. [Google Scholar]
- Pislariu, C.I.; Murray, J.D.; Wen, J.; Cosson, V.; Muni, R.R.D.; Wang, M.; Benedito, V.A.; Andriankaja, A.; Cheng, X.; Jerez, I.T.; et al. A Medicago truncatula tobacco retrotransposon insertion mutant collection with defects in nodule development and symbiotic nitrogen fixation. Plant Physiol 2012, 159, 1686–1699. [Google Scholar]
- Young, N.D.; Cannon, S.B.; Sato, S.; Kim, D.; Cook, D.R.; Town, C.D.; Roe, B.A.; Tabata, S. Sequencing the genespaces of Medicago truncatula and Lotus japonicus. Plant Physiol 2005, 137, 1174–1181. [Google Scholar]
- Young, N.D.; Udvardi, M. Translating Medicago truncatula genomics to crop legumes. Curr. Opin. Plant Biol 2009, 12, 193–201. [Google Scholar]
- Mochida, K.; Yoshida, T.; Sakurai, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. LegumeTFDB: An integrative database of Glycine max, Lotus japonicus and Medicago truncatula transcription factors. Bioinformatics 2010, 26, 290–291. [Google Scholar]
- Zhang, H.; Jin, J.; Tang, L.; Zhao, Y.; Gu, X.; Gao, G.; Luo, J. PlantTFDB 2.0: Update and improvement of the comprehensive plant transcription factor database. Nucleic Acids Res 2011, 39, D1114–D1117. [Google Scholar]
- Benedito, V.A.; Torres-Jerez, I.; Murray, J.D.; Andriankaja, A.; Allen, S.; Kakar, K.; Wandrey, M.; Verdier, J.; Zuber, H.; Ott, T.; et al. A gene expression atlas of the model legume Medicago truncatula. Plant J 2008, 55, 504–513. [Google Scholar]
- He, J.; Benedito, V.A.; Wang, M.; Murray, J.D.; Zhao, P.X.; Tang, Y.; Udvardi, M.K. The Medicago truncatula gene expression atlas web server. BMC Bioinforma 2009, 10, 441. [Google Scholar]
- Li, X.; Brummer, C. Applied genetics and genomics in alfalfa breeding. Agronomy 2012, 2, 40–61. [Google Scholar]
- Queiroux, C.; Washburn, B.K.; Davis, O.M.; Stewart, J.; Brewer, T.E.; Lyons, M.R.; Jones, K.M. A comparative genomics screen identifies a Sinorhizobium meliloti 1021 sodM-like gene strongly expressed within host plant nodules. BMC Microbiol 2012, 12, 74. [Google Scholar]
- Sulieman, S.; Schulze, J. The efficiency of nitrogen fixation of the model legume Medicago truncatula (Jemalong A17) is low compared to Medicago sativa. J. Plant Physiol 2010, 167, 683–692. [Google Scholar]
- Terpolilli, J.J.; O’Hara, G.W.; Tiwari, R.P.; Dilworth, M.J.; Howieson, J.G. The model legume Medicago truncatula A17 is poorly matched for N2 fixation with the sequenced microsymbiont Sinorhizobium meliloti 1021. New Phytol 2008, 179, 62–66. [Google Scholar]
- Sulieman, S.; Fischinger, S.A.; Gresshoff, P.M.; Schulze, J. Asparagine as a major factor in the N-feedback regulation of N2 fixation in Medicago truncatula. Physiol. Plant 2010, 140, 21–31. [Google Scholar]
- Parsons, R.; Baker, A. Cycling of amino compounds in symbiotic lupin. J. Exp. Bot 1996, 47, 421–429. [Google Scholar]
- Shi, L.; Twary, S.N.; Yoshioka, H.; Gregerson, R.G.; Miller, S.S.; Samac, D.A.; Gantt, J.S.; Unkefer, P.J.; Vance, C.P. Nitrogen assimilation in alfalfa: Isolation and characterization of an asparagine synthetase gene showing enhanced expression in root nodules and dark-adapted leaves. Plant Cell 1997, 9, 1339–1356. [Google Scholar]
- O’Rourke, J.A.; Yang, S.S.; Miller, S.S.; Bucciarelli, B.; Liu, J.; Rydeen, A.; Bozsoki, Z.; Uhde-Stone, C.; Tu, Z.J.; Allan, D.; et al. An RNA-seq transcriptome analysis of orthophosphate-deficient white lupin reveals novel insights into phosphorus acclimation in plants. Plant Physiol 2013, 161, 705–724. [Google Scholar]
- Ha, S.; Tran, L.S. Understanding plant responses to phosphorus starvation for improvement of plant tolerance to phosphorus deficiency by biotechnological approaches. Crit. Rev. Biotechnol. 2013, in press. [Google Scholar]
- Schulze, J.; Drevon, J.J. P-deficiency increases the O2 uptake per N2 reduced in alfalfa. J. Exp. Bot 2005, 56, 1779–1784. [Google Scholar]
- Moreau, D.; Voisin, A.S.; Salon, C.; Munier-Jolain, N. The model symbiotic association between Medicago truncatula cv. Jemalong and Rhizobium meliloti strain 2011 leads to N-stressed plants when symbiotic N2 fixation is the main N source for plant growth. J. Exp. Bot 2008, 59, 3509–3522. [Google Scholar]
- Fischinger, S.A.; Schulze, J. The importance of nodule CO2 fixation for the efficiency of symbiotic nitrogen fixation in pea at vegetative growth and during pod formation. J. Exp. Bot 2010, 61, 2281–2291. [Google Scholar]
- Sulieman, S.; Tran, L.S. Asparagine: An amide of particular distinction in the regulation of symbiotic nitrogen fixation of legumes. Crit. Rev. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Fischinger, S.A.; Hristozkova, M.; Mainassara, Z.A.; Schulze, J. Elevated CO2 concentration around alfalfa nodules increases N2 fixation. J. Exp. Bot 2010, 61, 121–130. [Google Scholar]
- Larrainzar, E.; Wienkoop, S.; Scherling, C.; Kempa, S.; Ladrera, R.; Arrese-Igor, C.; Weckwerth, W.; González, E.M. Carbon metabolism and bacteroid functioning are involved in the regulation of nitrogen fixation in Medicago truncatula under drought and recovery. Mol. Plant Microbe Interact 2009, 22, 1565–1576. [Google Scholar]
- Fougère, F.; Le Rudulier, D.; Streeter, J.G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol 1991, 96, 1228–1236. [Google Scholar]
- Almeida, J.P.; Hartwig, U.A.; Frehner, M.; Nösberger, J.; Lüscher, A. Evidence that P deficiency induces N feedback regulation of symbiotic N2 fixation in white clover (Trifolium repens L.). J. Exp. Bot 2000, 51, 1289–1297. [Google Scholar]
- Hartwig, U.A.; Trommler, J. Increase in the concentrations of amino acids in the vascular tissue of white clover and white lupin after defoliation: An indication of a N feedback regulation of symbiotic N2 fixation. Agronomie 2001, 21, 615–620. [Google Scholar]
- Fischinger, S.A.; Drevon, J.J.; Claassen, N.; Schulze, J. Nitrogen from senescing lower leaves of common bean is re-translocated to nodules and might be involved in a N-feedback regulation of nitrogen fixation. J. Plant Physiol 2006, 163, 987–995. [Google Scholar]
- González, E.M.; Gálvez, L.; Royuela, M.; Aparicio-Tejo, P.M.; Arrese-Igor, C. Insights into the regulation of nitrogen fixation in pea nodules: Lessons from drought, abscisic acid and increased photoassimilate availability. Agronomie 2001, 21, 607–613. [Google Scholar]
- Jeudy, C.; Ruffel, S.; Freixes, S.; Tillard, P.; Santoni, A.L.; Morel, S.; Journet, E.P.; Duc, G.; Gojon, A.; Lepetit, M.; et al. Adaptation of Medicago truncatula to nitrogen limitation is modulated via local and systemic nodule developmental responses. New Phytol 2010, 185, 817–828. [Google Scholar]
- Sulieman, S. Does GABA increase the efficiency of symbiotic N2 fixation in legumes? Plant Signal. Behav 2011, 6, 32–36. [Google Scholar]
- Drevon, J.-J.; Hartwig, U.A. Phosphorus deficiency increases the argon-induced decline of nodule nitrogenase activity in soybean and alfalfa. Planta 1997, 201, 463–469. [Google Scholar]
- Sulieman, S.; Schulze, J. Phloem-derived γ-aminobutyric acid (GABA) is involved in upregulating nodule N2 fixation efficiency in the model legume Medicago truncatula. Plant Cell Environ 2010, 33, 2162–2172. [Google Scholar]
- Blumenthal, J.M.; Russelle, M.P.; Vance, C.P. Nitrogenase activity is affected by reduced partial pressures of N2 and NO3−. Plant Physiol 1997, 114, 1405–1412. [Google Scholar]
- Chen, R.F.; Scott, C.; Trepman, E. Fluorescence properties of o-phthaldialdehyde derivatives of amino acids. Biochim. Biophys. Acta 1979, 576, 440–455. [Google Scholar]
- Scheffer, F.; Pajenkamp, H. Phosphatbestimmung in Pflanzenaschen nach der Molybdän-Vanadin-Methode. Z. Pflanzenernähr. Düngung Bodenk 1952, 56, 2–8. [Google Scholar]
| M. truncatula | M. sativa | |||
|---|---|---|---|---|
| Deficient-P | Control | Deficient-P | Control | |
| DM (g plant−1) | ||||
| Shoot | 0.17 ± 0.01 b | 0.71 ± 0.11 c | 0.40 ± 0.06 b | 2.67 ± 0.23 a |
| Root | 0.20 ± 0.01 b | 0.48 ± 0.01 b | 0.43 ± 0.07 b | 1.62 ± 0.18 a |
| Total | 0.39 ± 0.02 b | 1.22 ± 0.10 c | 0.85 ± 0.13 b | 4.33 ± 0.39 a |
| Shoot/Root (g g−1) | 0.84 ± 0.02 b | 1.50 ± 0.27 a | 0.93 ± 0.02 b | 1.67 ± 0.11 a |
| Nodule number plant−1 | 13.0 ± 1.6 a,b | 17.0 ± 1.3 b | 21.0 ± 3.2 a | 27.0 ± 3.7 a |
| Nodule DM (mg plant−1) | 11.8 ± 1.6 b | 29.4 ± 2.9 c | 16.1 ± 1.8 b | 41.8 ± 1.8 a |
| Individual nodule DM (mg) | 0.9 ± 0.1 b | 1.7 ± 0.1 a | 0.9 ± 0.2 b | 1.6 ± 0.2 a |
| M. truncatula | M. sativa | |||
|---|---|---|---|---|
| Deficient-P | Control | Deficient-P | Control | |
| Nitrogenase activity: | ||||
| ANA (μmol H2 plant−1 h−1) | 0.36 ± 0.04 d | 0.78 ± 0.03 c | 1.85 ± 0.08 b | 2.99 ± 0.24 a |
| TNA (μmol H2 plant−1 h−1] | 0.99 ± 0.08 c | 1.66 ± 0.08 c | 3.53 ± 0.30 b | 7.40 ± 0.49 a |
| EAC | 0.63 ± 0.02 d | 0.52 ± 0.01 a,c | 0.47 ± 0.03 b | 0.60 ± 0.02 a |
| Fixed-N per plant (mg N 24 h−1) | 0.14 ± 0.01 c | 0.20 ± 0.01 c | 0.38 ± 0.05 b | 0.99 ± 0.08 a |
| Specific fixed-N (μg N mg nodule−1 h−1) | 12.96 ± 2.71 c | 6.94 ± 1.01 b | 25.62 ± 7.08 a | 23.63 ± 1.66 a |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sulieman, S.; Schulze, J.; Tran, L.-S.P. Comparative Analysis of the Symbiotic Efficiency of Medicago truncatula and Medicago sativa under Phosphorus Deficiency. Int. J. Mol. Sci. 2013, 14, 5198-5213. https://doi.org/10.3390/ijms14035198
Sulieman S, Schulze J, Tran L-SP. Comparative Analysis of the Symbiotic Efficiency of Medicago truncatula and Medicago sativa under Phosphorus Deficiency. International Journal of Molecular Sciences. 2013; 14(3):5198-5213. https://doi.org/10.3390/ijms14035198
Chicago/Turabian StyleSulieman, Saad, Joachim Schulze, and Lam-Son Phan Tran. 2013. "Comparative Analysis of the Symbiotic Efficiency of Medicago truncatula and Medicago sativa under Phosphorus Deficiency" International Journal of Molecular Sciences 14, no. 3: 5198-5213. https://doi.org/10.3390/ijms14035198




