A Central Role for Thiols in Plant Tolerance to Abiotic Stress
Abstract
:1. Introduction
2. Sulphur-Containing Amino Acids
2.1. Cysteine Biosynthesis and Free Cysteine Accumulation
2.2. Methionine
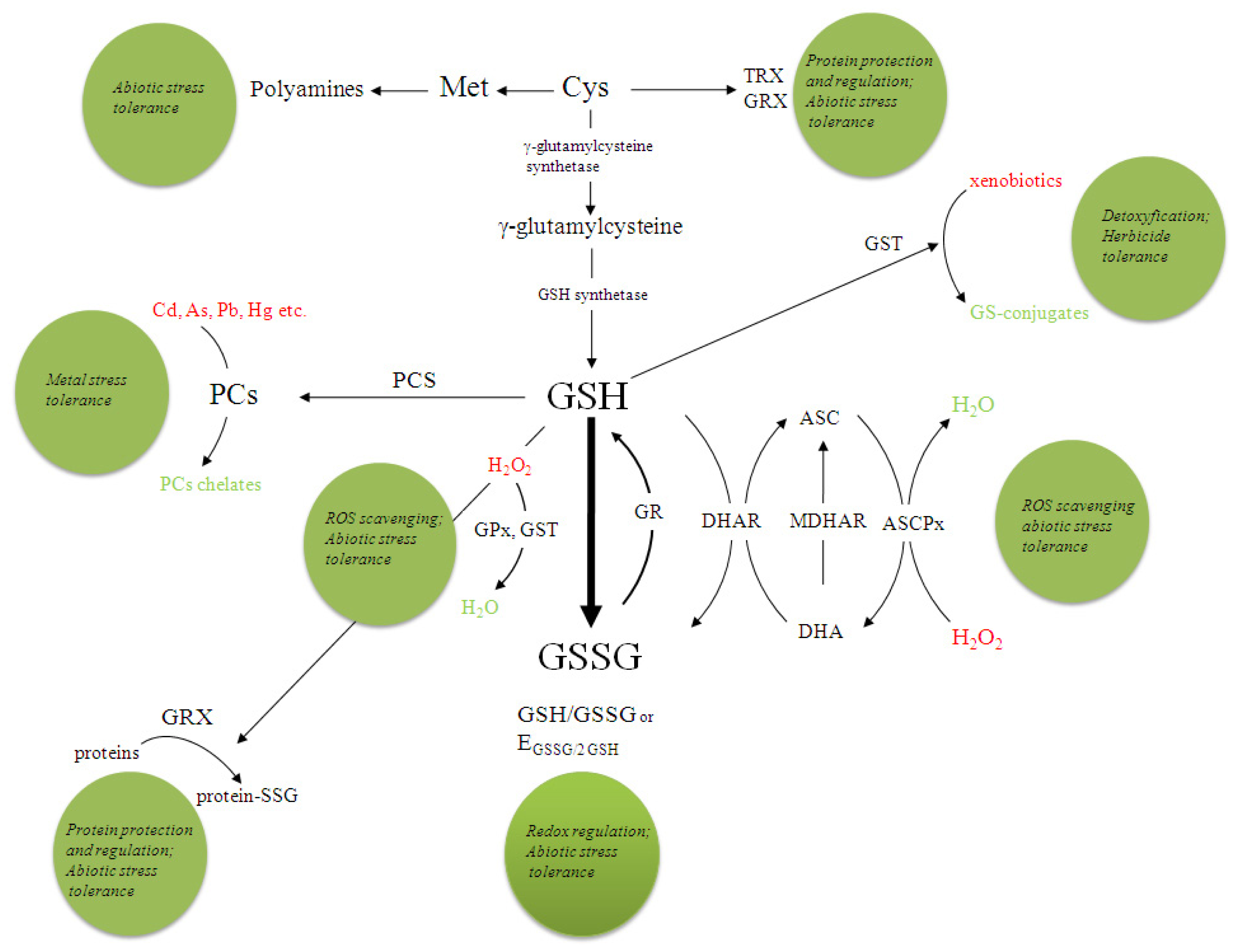
3. Glutathione
3.1. GSH in Plant Stress Response
3.2. Glutathione Redox State
3.3. The Ascorbate-Glutathione Cycle
3.4. Glutathione-S-Transferases
3.5. Phytochelatins
3.6. The Involvement of GSH in Transcriptional Control
4. Protein Thiols
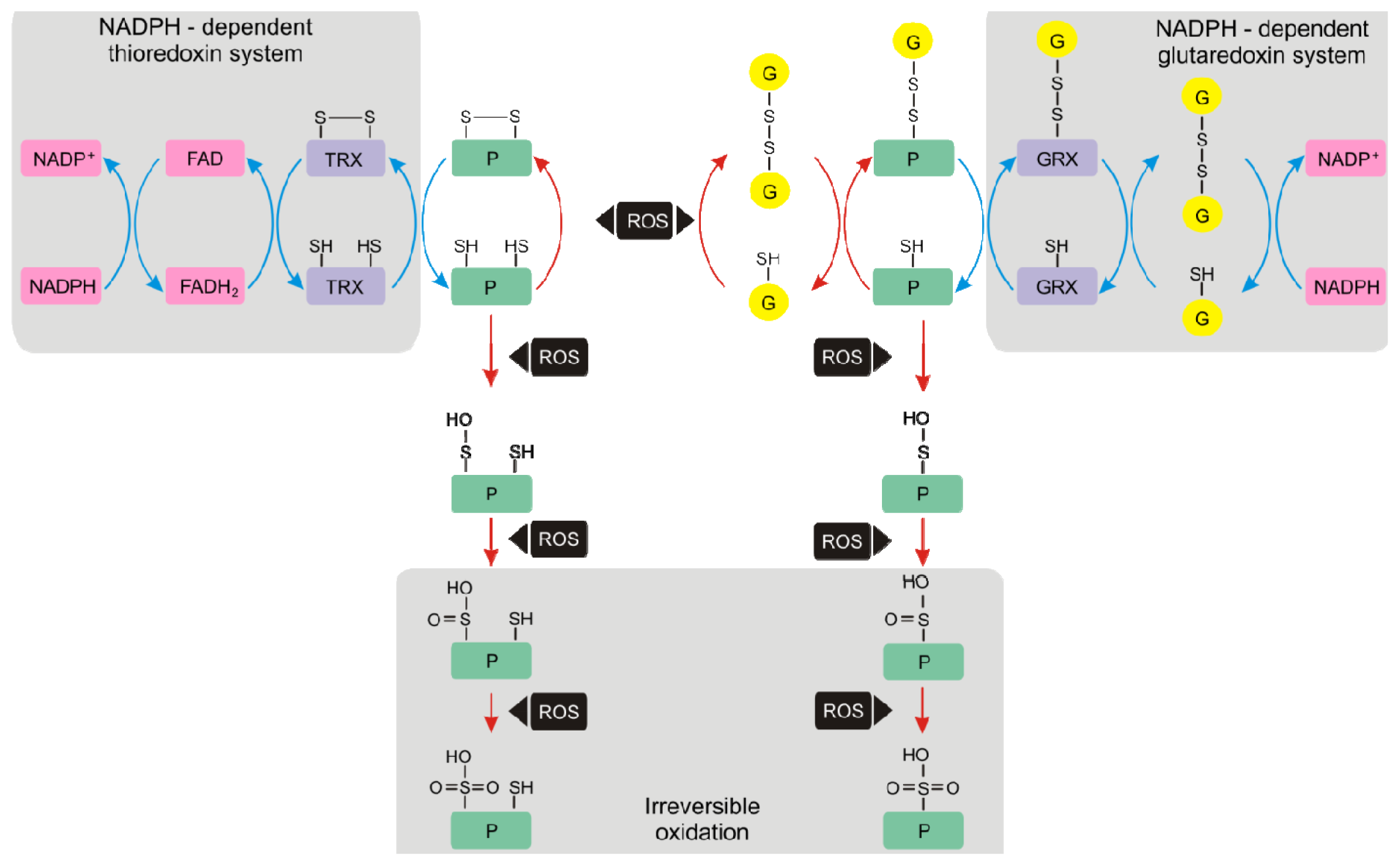
4.1. Thioredoxins and Glutaredoxins
4.2. Glutathionylation
5. Conclusions and Future Perspectives
Acknowledgments
Abbreviations
| ABA | abscisic acid |
| ASC | ascorbate |
| ASCPx | ascorbate peroxidase |
| Cys | cysteine |
| CySS | cystine, cysteine-disulphide |
| DHA | dehydroascorbate |
| DHAR | dehydroascorbate reductase |
| EGSSG/2 GSH | half-cell reduction potential of the glutathione disulphide-glutathione redox couple |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GRX | glutaredoxin |
| GSH | glutathione |
| GSNO | S-nitrosoglutathione |
| GSSG | glutathione disulphide |
| GST | glutathione-S-transferase |
| hGSH | homoglutathione |
| hGSSG | homoglutathione disulphide |
| HSP | heat shock protein |
| MDHAR | monodehydroascorbate reductase |
| Met | methionine |
| MSR | methionine sulfoxide reductase |
| NTRC | thioredoxin reductase type C |
| PC | phytochelatins |
| PCS | phytochelatin synthetase |
| PRX | thiol peroxidase |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SAM | S-adenosyl methionine |
| SAT | serine acetyltransferase |
| SOD | superoxide dismutase |
| TRX | thioredoxin |
| TTL | tetratricopeptide thioredoxin-like |
Conflict of Interest
References
- Urano, K.; Kurihara, Y.; Seki, M.; Shinozaki, K. “Omics” analyses of regulatory networks in plant abiotic stress responses. Curr. Opin. Plant Biol 2010, 13, 132–138. [Google Scholar]
- Todaka, D.; Nakashima, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Towards understanding transcriptional regulatory networks in abiotic stress responses and tolerance in rice. Rice 2012, 5, 1–9. [Google Scholar]
- Evers, D.; Legay, S.; Lamoureux, D.; Hausman, J.F.; Hoffmann, L.; Renaut, J. Towards a synthetic view of potato cold and salt stress response by transcriptomic and proteomic analyses. Plant Mol. Biol 2012, 78, 503–514. [Google Scholar]
- Sarhadi, E.; Bazargani, M.M.; Sajise, A.G.; Abdolahi, S.; Vispo, N.A.; Arceta, M.; Nejad, G.M.; Singh, R.K.; Salekdeh, G.H. Proteomic analysis of rice anthers under salt stress. Plant Physiol. Bioch 2012, 58, 280–287. [Google Scholar]
- Cheesman, J.M. Mechanisms of salinity tolerance in plants. Plant Physiol 1988, 87, 547–550. [Google Scholar]
- Hell, R. Molecular physiology of plant sulfur metabolism. Planta 1997, 202, 138–148. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol 1998, 49, 249–279. [Google Scholar]
- Bowne, J.; Bacic, A.; Tester, M.; Roessner, U. Abiotic Stress and Metabolomics. In Annual Plant Reviews 43: Biology of Plant Metabolomics; Hall, R.D., Ed.; Wiley-Blackwell: Oxford, UK, 2011. [Google Scholar]
- Pastori, J.M.; Foyer, C.H. Common components, networks, and pathways of cross-tolerance to stress. The central role of “redox” and abscisic acid-mediated controls. Plant Physiol 2002, 129, 460–468. [Google Scholar]
- Chinnusamy, V.; Zhu, J.-K.; Sunkar, R. Gene regulation during cold stress acclimation in plants. Method Mol. Biol 2010, 639, 39–55. [Google Scholar]
- Keunen, E.; Remans, T.; Bohler, S.; Vangronsveld, J.; Cuypers, A. Metal-induced oxidative stress and plant mitochondria. Int. J. Mol. Sci 2011, 12, 6894–6918. [Google Scholar]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 2010, 33, 453–467. [Google Scholar]
- Meyer, A.J.; Hell, R. Glutathione homeostasis and redox regulation by sulfhydryl groups. Photosynth. Res 2005, 86, 435–457. [Google Scholar]
- Colville, L.; Kranner, I. Desiccation tolerant plants as model systems to study redox regulation of protein thiols. Plant Growth Regul 2010, 62, 241–255. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: an integrated overview. Plant Cell Environ 2012, 35, 454–484. [Google Scholar]
- Hell, R.; Wirtz, M. Molecular biology, biochemistry and cellular physiology of cysteine metabolism in Arabidopsis thaliana. Arabidopsis Book 2011, 9, e0154. [Google Scholar]
- Lewandowska, M.; Liszewska, L.; Wirtz, M.; Hell, R. Subcellular Compartmentation of Cysteine Synthesis in Plants—One Step More. In Sulfur Metabolism in Plants; de Kok, L.J., Tabe, L., Tausz, M., Hawkesford, M.J., Hoefgen, R., McManus, M.T., Norton, R.M., Rennenberg, H., Saito, K., Schnug, E., Eds.; Springer: Dordrecht, Netherlands, 2012; Volume 1, pp. 71–75. [Google Scholar]
- Kawashima, C.G.; Noji, M.; Nakamura, M.; Ogra, Y.; Suzuki, K.T.; Saito, K. Heavy metal tolerance of transgenic tobacco plants over-expressing cysteine synthase. Biotechnol. Lett 2004, 26, 153–157. [Google Scholar]
- Nakamura, M.; Kuramata, M.; Kasugai, I.; Abe, M.; Youssefian, S. Increased thiol biosynthesis of transgenic poplar expressing a wheat O-acetylserine(thiol) lyase enhances resistance to hydrogen sulfide and sulfur dioxide toxicity. Plant Cell Rep 2009, 28, 313–323. [Google Scholar]
- Wirtz, M.; Hell, R. Functional analysis of the cysteine synthase protein complex from plants: Structural, biochemical and regulatory properties. J. Plant Physiol 2006, 163, 273–286. [Google Scholar]
- Tabe, L.; Wirtz, M.; Molvig, L.; Droux, M.; Hell, R. Overexpression of serine acetlytransferase produced large increases in O-acetylserine and free cysteine in developing seeds of a grain legume. J. Exp. Bot 2010, 61, 721–733. [Google Scholar]
- Harms, K.; von Ballmoos, P.; Brunold, C.; Höfgen, R.; Hesse, H. Expression of a bacterial serine acetyltransferase in transgenic potato plants leads to increased levels of cysteine and glutathione. Plant J 2000, 22, 335–343. [Google Scholar]
- Ruiz, J.; Blumwald, E. Salinity-induced glutathione synthesis in Brassica napus. Planta 2002, 214, 965–969. [Google Scholar]
- Bashir, H.; Ahmad, J.; Bagheri, R.; Nauman, M.; Qureshi, M.I. Limited sulfur resource forces Arabidopsis thalianato shift towards non-sulfur tolerance under cadmium stress. Environ. Exp. Bot 2012. [Google Scholar] [CrossRef]
- Romano, A.H.; Nickerson, W.J. Cystine reductase of pea seeds and yeasts. J. Biol. Chem 1954, 208, 409–416. [Google Scholar]
- Jeelani, G.; Husain, A.; Sato, D.; Ali, V.; Suematsu, M.; Soga, T.; Nozaki, T. Two atypical L-cysteine-regulated NADPH-dependent oxidoreductases involved in redox maintenance, L-cystine and iron reduction, and metronidazole activation in the enteric protozoan Entamoeba histolytica. J. Biol. Chem 2010, 285, 26889–26899. [Google Scholar]
- Helmann, J.D. Bacillithiol, a new player in bacterial redox homeostasis. Antioxid. Redox Sign 2011, 15, 123–133. [Google Scholar]
- Iyer, S.S.; Ramirez, A.M.; Ritzenthaler, J.D.; Torres-Gonzalez, E.; Roser-Page, S.; Mora, A.L.; Brigham, K.L.; Jones, D.P.; Roman, J.; Rojas, M. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, 37–45. [Google Scholar]
- Krauth-Siegel, R.L.; Leroux, A.E. Low-molecular-mass antioxidants in parasites. Antioxid. Redox Sign 2012, 17, 583–607. [Google Scholar]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. Redox state of low-molecular-weight thiols and disulphides during somatic embryogenesis of salt-treated suspension cultures of Dactylis glomerata L. Free Radic. Res 2012, 46, 656–664. [Google Scholar]
- Olm, E.; Fernandes, A.P.; Hebert, C.; Rundlöf, A.K.; Larsen, E.H.; Danielsson, O.; Björnstedt, M. Extracellular thiol-assisted selenium uptake dependent on the x(c)–cystine transporter explains the cancer-specific cytotoxicity of selenite. Proc. Natl. Acad. Sci. USA 2009, 106, 11400–11405. [Google Scholar]
- Brown, D.M.; Upcroft, J.A.; Upcroft, P. A thioredoxin reductase-class of disulphide reductase in the protozoan parasite Giardia duodenalis. Mol. Biochem. Parasit 1996, 83, 211–220. [Google Scholar]
- Arias, D.G.; Marquez, V.E.; Beccaria, A.J.; Guerrero, S.A.; Iglesias, A.A. Purification and characterization of a glutathione reductase from Phaeodactylum tricornutum. Protist 2010, 161, 91–101. [Google Scholar]
- Dos Santos, C.V.; Cuiné, S.; Rouhier, N.; Rey, P. The Arabidopsis plastidic methionine sulfoxide reductase B proteins. Sequence and activity characteristics, comparison of the expression with plastidic methionine sulfoxide reductase A, and induction by photooxidative stress. Plant Physiol 2005, 138, 909–922. [Google Scholar]
- Cabreiro, F.; Picot, C.R.; Friguet, B.; Petropoulos, I. Methionine sulfoxide reductases. Ann. N. Y. Acad. Sci 2006, 1067, 37–44. [Google Scholar]
- Good, A.G.; Zaplachinski, S.T. The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus. Physiol. Plantarum 1994, 90, 9–14. [Google Scholar]
- Gzik, A. Accumulation of proline and pattern of alpha-amino acids in sugar beet plants in response to osmotic, water and salt stress. Environ. Exp. Bot 1996, 36, 29–38. [Google Scholar]
- Hacham, Y.; Matityahu, I.; Schuster, G.; Amir, R. Overexpression of mutated forms of aspartate kinase and cystathionine γ-synthase in tobacco leaves resulted in the high accumulation of methionine and threonine. Plant J 2008, 54, 260–271. [Google Scholar]
- Nguyen, H.C.; Hoefgen, R.; Hesse, H. Improving the nutritive value of rice seeds: elevation of cysteine and methionine contents in rice plants by ectopic expression of a bacterial serine acetyltransferase. J. Exp. Bot 2012, 63, 5991–6001. [Google Scholar]
- Jozefczuk, S.; Klie, S.; Catchpole, G.; Szymanski, J.; Cuadros-Inostroza, A.; Steinhauser, D.; Selbig, J.; Willmitzer, L. Metabolomic and transcriptomic stress response of Escherichia coli. Mol. Syst. Biol 2010, 6, 1–16. [Google Scholar]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar]
- De Poel, B.V.; Bulens, I.; Oppermann, Y.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Sauter, M.; Geeraerd, A.H. S-adenosyl-l-methionine usage during climacteric ripening of tomato in relation to ethylene and polyamine biosynthesis and transmethylation capacity. Physiol. Plantarum 2012. early online. [Google Scholar]
- Kasukabe, Y.; He, L.; Watakabe, Y.; Otani, M.; Shimada, T.; Tachibana, S. Improvement of environmental stress tolerance of sweet potato by introduction of genes for spermidine synthase. Plant Biotechnol 2006, 23, 75–83. [Google Scholar]
- Groppa, M.D.; Benavides, M.P. Polyamines and abiotic stress: Recent advances. Amino Acids 2008, 34, 35–45. [Google Scholar]
- Martin-Tanguy, J. Metabolism and function of polyamines in plants: Recent development (new approaches). Plant Growth Regul 2001, 100, 675–688. [Google Scholar]
- Zechmann, B.; Müller, M. Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma 2010, 246, 15–24. [Google Scholar]
- Wachter, A.; Wolf, S.; Steininger, H.; Bogs, J.; Rausch, T. Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: Implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J. 2005, 41, 15–30. [Google Scholar]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 2012, 182, 112–120. [Google Scholar]
- Estrella-Gómez, N.E.; Sauri-Duch, E.; Zapata-Pérez, O.; Santamaría, J.M. Glutathione plays a role in protecting leaves of Salvinia minima from Pb2+ damage associated with changes in the expression of SmGS genes and increased activity of GS. Environ. Exp. Bot 2012, 75, 188–194. [Google Scholar]
- Pyngrope, S.; Bhoomika, K.; Dubey, R.S. Reactive oxygen species, ascorbate–glutathione pool, and enzymes of their metabolism in drought-sensitive and tolerant indica rice (Oryza sativa L.) seedlings subjected to progressing levels of water deficit. Protoplasma 2013, 250, 585–600. [Google Scholar]
- Kumar, S.; Asif, M.H.; Chakrabarty, D.; Tripathi, R.D.; Dubey, R.S.; Trivedi, P.K. Differential expression of rice lambda class GST gene family members during plant growth, development, and in response to stress conditions. Plant Mol. Biol. Rep. 2012. [Google Scholar] [CrossRef]
- Xiang, C.; Werner, B.L.; Christensen, E.M.; Oliver, D.J. The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol. 2001, 126, 564–574. [Google Scholar]
- Goldsbrough, P.B. Metal Tolerance in Plants: The Role of Phytochelatins and Metallothioneins. In Phytoremediation of Trace Elements; Terry, N., Banuelos, G.S., Eds.; CRC Press: Boca Raton, FL, USA, 1998; pp. 221–233. [Google Scholar]
- Li, Y.; Dhankher, O.P.; Carreira, L.; Balish, R.S.; Meagher, R.B. Arsenic and mercury tolerance and cadmium sensitivity in Arabidopsis plants expressing bacterial γ-glutamylcysteine synthetase. Environ. Toxicol. Chem 2005, 24, 1376–1386. [Google Scholar]
- LeBlanc, M.S.; Lima, A.; Montello, P.; Kim, T.; Meagher, R.B.; Merkle, S. Enhanced arsenic tolerance of transgenic Eastern cottonwood plants expressing gamma-glutamylcysteine synthetase. Int. J. Phytoremed 2011, 13, 657–673. [Google Scholar]
- Song, Y.; Cui, J.; Zhang, H.; Wang, G.; Zhao, F.J.; Shen, Z. Proteomic analysis of copper stress responses in the roots of two rice (Oryza sativaL.) varieties differing in Cu tolerance. Plant Soil 2012. [Google Scholar] [CrossRef]
- Sengupta, D.; Ramesh, G.; Mudalkar, S.; Kumar, K.R.R.; Kirti, P.B.; Reddy, A.R. Molecular cloning and characterization of γ-glutamyl cysteine synthetase (VrγECS) from roots of Vigna radiata (L.) Wilczek under progressive drought stress and recovery. Plant Mol. Biol. Rep 2012, 30, 894–903. [Google Scholar]
- Nazar, R.; Iqbal, N.; Masood, A.; Syeed, S.; Khan, N.A. Understanding the significance of sulfur in improving salinity tolerance in plants. Environ. Exp. Bot 2011, 70, 80–87. [Google Scholar]
- Lim, B.; Meyer, A.J.; Cobbett, C.S. Development of glutathione-deficient embryos in Arabidopsis is influenced by the maternal level of glutathione. Plant Biol 2011, 13, 693–697. [Google Scholar]
- El Msehli, S.; Lambert, A.; Baldacci-Cresp, F.; Hopkins, J.; Boncompagni, E.; Smiti, S.A.; Hérouart, D.; Frendo, P. Crucial role of (homo)glutathione in nitrogen fixation in Medicago truncatula nodules. New Phytol 2011, 192, 496–506. [Google Scholar]
- Sobrino-Plata, J.; Ortega-Villasante, C.; Flores-Cáceres, M.L.; Escobar, C.; Del Campo, F.F.; Hernández, L.E. Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere 2009, 77, 946–954. [Google Scholar]
- Loscos, J.; Matamoros, M.A.; Becana, M. Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiol 2008, 146, 1282–1292. [Google Scholar]
- Cruz de Carvalho, M.H.; Brunet, J.; Bazin, J.; Kranner, I.; d′ Arcy-Lameta, A.; Zuily-Fodil, Y.; Contour-Ansel, D. Homoglutathione synthetase and glutathione synthetase in drought-stressed cowpea leaves: Expression patterns and accumulation of low-molecular-weight thiols. J. Plant Physiol 2010, 167, 480–487. [Google Scholar]
- Becana, M.; Matamoros, M.A.; Udvardi, M.; Dalton, D.A. Recent insights into antioxidant defenses of legume root nodules. New Phytol 2010, 188, 960–976. [Google Scholar] [Green Version]
- Diaz Vivancos, P.; Driscoll, S.P.; Bulman, C.A.; Ying, L.; Emami, K.; Treumann, A.; Mauve, C.; Noctor, G.; Foyer, C.F. Perturbations of amino acid metabolism associated with glyphosate-dependent inhibition of shikimic acid metabolism affect cellular redox homeostasis and alter the abundance of proteins involved in photosynthesis and photorespiration. Plant Physiol 2011, 157, 256–268. [Google Scholar]
- Vázquez, S.; Goldsbrough, P.; Carpena, R.O. Comparative analysis of the contribution of phytochelatins to cadmium and arsenic tolerance in soybean and white lupin. Plant Physiol. Bioch 2009, 47, 63–67. [Google Scholar]
- Clemente, M.R.; Bustos-Sanmamed, P.; Loscos, J.; James, E.K.; Pérez-Rontomé, C.; Navascués, J.; Gay, M.; Becana, M. Thiol synthetases of legumes: Immunogold localization and differential gene regulation by phytohormones. J. Exp. Bot 2012, 63, 3923–3934. [Google Scholar]
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The Ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int. J. Mol. Sci 2012, 13, 4458–4483. [Google Scholar]
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What is stress? Concepts, definitions and applications in seed science. New Phytol 2010, 188, 655–673. [Google Scholar]
- Szalai, G.; Kellos, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant Growth Regul 2009, 28, 66–80. [Google Scholar]
- Kocsy, G.; Kobrehel, K.; Szalai, G.; Duviau, M.P.; Buzás, Z.; Galiba, G. Abiotic stress-induced changes in glutathione and thioredoxin h levels in maize. Environ. Exp. Bot 2004, 52, 101–112. [Google Scholar]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med 2001, 30, 1191–1212. [Google Scholar]
- Kranner, I.; Birtić, S.; Anderson, K.; Pritchard, H.W. Glutathione half-cell reduction potential: A universal stress marker and modulator of programmed cell death? Free Radic. Biol. Med 2006, 40, 2155–2165. [Google Scholar]
- Seal, C.E.; Zammit, R.; Scott, P.; Flowers, T.J.; Kranner, I. Glutathione half cell reduction potential and α-tocopherol as viability markers during the prolonged storage of Suaeda maritima seeds. Seed Sci. Res 2010, 20, 47–53. [Google Scholar]
- Seal, C.E.; Zammit, R.; Scott, P.; Nyamongo, D.O.; Daws, M.I.; Kranner, I. Glutathione half-cell reduction potential as a seed viability marker of the potential oilseed crop Vernonia galamensis. Ind. Crop. Prod 2010, 32, 687–691. [Google Scholar]
- Birtić, S.; Colville, L.; Pritchard, H.W.; Pearce, S.R.; Kranner, I. Mathematically combined half-cell reduction potentials of low-molecular-weight thiols as markers of seed ageing. Free Radic. Res 2011, 45, 1093–1102. [Google Scholar]
- Cameron, J.C.; Pakrasi, H.B. Glutathione facilitates antibiotic resistance and photosystem I stability during exposure to gentamicin in Cyanobacteria. Appl. Environ. Microbiol 2011, 77, 3547–3550. [Google Scholar]
- Creissen, G.; Firmin, J.; Fryer, M.; Kular, B.; Leyland, N.; Reynolds, H.; Pastori, G.; Wellburn, F.; Baker, N.; Wellburn, A.; Mullineaux, P. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 1999, 11, 1277–1291. [Google Scholar]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 2012, 35, 259–270. [Google Scholar]
- Dubreuil-Maurizi, C.; Poinssot, B. Role of glutathione in plant signaling under biotic stress. Plant Signal. Behaviour 2012, 7, 210–212. [Google Scholar]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol 2011, 155, 2–18. [Google Scholar]
- Munné-Bosch, S.; Queval, G.; Foyer, C.H. The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol 2013, 161, 5–19. [Google Scholar]
- Urano, J.; Nakagawa, T.; Maki, Y.; Masumura, T.; Tanaka, K.; Murata, N.; Ushimaru, T. Molecular cloning and characterization of a rice dehydroascorbate reductase. FEBS Lett 2000, 466, 107–111. [Google Scholar]
- Eltelib, H.A.; Badejo, A.A.; Fujikawa, Y.; Esaka, M. Gene expression of monodehydroascorbate reductase and dehydroascorbate reductase during fruit ripening and in response to environmental stresses in acerola (Malpighia glabra). J. Plant Physiol 2011, 168, 619–627. [Google Scholar]
- Yin, L.; Wang, S.; Eltayeb, A.E.; Uddin, M.I.; Yamamoto, Y.; Tsuji, W.; Takeuchi, Y.; Tanaka, K. Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 2010, 231, 609–621. [Google Scholar]
- Kavitha, K.; George, S.; Venkataraman, G.; Parida, A. A salt-inducible chloroplastic monodehydroascorbate reductase from halophyte Avicennia marina confers salt stress tolerance on transgenic plants. Biochimie 2010, 92, 1321–1329. [Google Scholar]
- Eltelib, H.A.; Fujikawa, Y.; Esaka, M. Overexpression of the acerola (Malpighia glabra) monodehydroascorbate reductase gene in transgenic tobacco plants results in increased ascorbate levels and enhanced tolerance to salt stress. S. Afr. J. Bot 2012, 78, 295–301. [Google Scholar]
- Le Martret, B.; Poage, M.; Shiel, K.; Nugent, G.D.; Dix, P.J. Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol. J 2011, 9, 661–673. [Google Scholar]
- Srivastava, S.; D’Souza, S.F. Effect of variable sulfur supply on arsenic tolerance and antioxidant responses in Hydrilla verticillata (L.f.) Royle. Ecotox. Environ. Safe 2010, 73, 1314–1322. [Google Scholar]
- Wang, Z.; Xiao, Y.; Chen, W.; Tang, K.; Zhang, L. Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J. Int. Plant Biol 2010, 52, 400–409. [Google Scholar]
- Kabir, M.H.; Han, W.; Wang, M.H. Environmental stress response of a dehydroascorbate reductase gene from tomato, and its protective role in Escherichia coli. Hort. Environ. Biotech 2011, 52, 621–628. [Google Scholar]
- Kabir, M.H.; Wang, M.H. Expression pattern of two dehydroascorbate reductase genes from tomato (Solanum lycopersicum L.) in response to stress. J. Korean Soc. Appl. Biol. Chem 2010, 53, 668–676. [Google Scholar]
- Gallie, D.R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot 2013, 64, 433–443. [Google Scholar]
- Ding, S.; Lu, Q.; Zhang, Y.; Wen, X.; Zhang, L.; Lu, C. Enhanced sensitivity to oxidative stress in transgenic tobacco plants with decreased glutathione reductase activity leads to a decrease in ascorbate pool and ascorbate redox state. Plant Mol. Biol 2009, 69, 577–592. [Google Scholar]
- Yousuf, P.Y.; Hakeem, K.U.R.; Chandna, R.; Ahmad, P. Role of Glutathione Reductase in Plant Abiotic Stress. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 149–158. [Google Scholar]
- Melchiorre, M.; Robert, G.; Trippi, V.; Racca, R.; Lascano, H.R. Superoxide dismutase and glutathione reductase overexpression in wheat protoplast: Photooxidative stress tolerance and changes in cellular redox state. Plant Growth Regul 2009, 57, 57–68. [Google Scholar]
- Wu, H.; Wu, X.; Li, Z.; Duan, L.; Zhang, M. Physiological evaluation of drought stress tolerance and recovery in Cauliflower (Brassica oleracea L.) seedling treated with methyl jasmonate and coronatine. J. Plant Growth Regul 2012, 31, 113–123. [Google Scholar]
- Chi, Y.; Cheng, Y.; Vanitha, J.; Kumar, N.; Ramamoorthy, R.; Ramachandran, S.; Jiang, S.Y. Expansion mechanisms and functional divergence of the glutathione S-transferase family in Sorghum and other higher plants. DNA Res 2011, 18, 1–16. [Google Scholar]
- Dixon, D.P.; Lapthorn, A.; Edwards, R. Plant glutathione transferases. Genome Biol 2002, 3, 3004, :1–3004:10.. [Google Scholar]
- George, S.; Venkataraman, G.; Parida, A. A chloroplast-localized and auxin-induced glutathione S-transferase from phreatophyte Prosopis juliflora confer drought tolerance on tobacco. J. Plant Physiol 2010, 167, 311–318. [Google Scholar]
- Qi, Y.C.; Liu, W.Q.; Qiu, L.Y.; Zhang, S.M.; Ma, L.; Zhang, H. Overexpression of glutathione S-transferase gene increases salt tolerance of Arabidopsis. Russ. J. Plant Physiol 2010, 57, 233–240. [Google Scholar]
- Chen, J.H.; Jiang, H.W.; Hsieh, E.J.; Chen, H.Y.; Chien, C.T.; Hsieh, H.L.; Lin, T.P. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol 2012, 158, 340–351. [Google Scholar]
- Dixit, P.; Mukherjee, P.K.; Ramachandran, V.; Eapen, S. Glutathione transferase from Trichoderma virens enhances cadmium tolerance without enhancing its accumulation in transgenic Nicotiana tabacum. PLoS One 2011, 6, e16360. [Google Scholar]
- Huang, C.; Guo, T.; Zheng, S.C.; Feng, Q.L.; Liang, J.H.; Li, L. Increased cold tolerance in Arabidopsis thaliana transformed with Choristoneura fumiferana glutathione S-transferase gene. Biol. Plantarum 2009, 53, 183–187. [Google Scholar]
- Ji, W.; Zhu, Y.; Li, Y.; Yang, L.; Zhao, X.; Cai, H.; Bai, X. Over-expression of a glutathione S-transferase gene, GsGST, from wild soybean (Glycine soja) enhances drought and salt tolerance in transgenic tobacco. Biotech. Lett 2010, 32, 1173–1179. [Google Scholar]
- Zhang, Y.; Liu, J. Transgenic alfalfa plants co-expressing glutathione S-transferase (GST) and human CYP2E1 show enhanced resistance to mixed contaminates of heavy metals and organic pollutants. J. Hazard. Mater 2011, 189, 357–362. [Google Scholar]
- Hu, T.; Qy, X.; Xiao, G.; Huang, X. Enhanced tolerance to herbicide of rice plants by over-expression of a glutathione S-transferase. Mol. Breed 2009, 24, 409–418. [Google Scholar]
- Jha, B.; Sharma, A.; Mishra, A. Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Mol. Biol. Rep 2011, 38, 4823–4832. [Google Scholar]
- Kondo, N.; Imai, K.; Isobe, M.; Goto, T.; Murasugi, A.; Wada-Nakagawa, C.; Hayashi, Y. Cadystin A and B, major unit peptides comprising cadmium binding peptides induced in a fission yeast-separation, revision of structures and synthesis. Tetrahedron Lett 1984, 25, 3869–3872. [Google Scholar]
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins: The principal heavy-metal complexing peptides of higher plants. Science 1985, 230, 674–676. [Google Scholar]
- Pal, R.; Rai, J.P.N. Phytochelatins: Peptides involved in heavy metal detoxification. Appl. Biochem. Biotech 2010, 160, 945–963. [Google Scholar]
- Zhang, X.; Kalle Uroic, M.; Xie, W.Y.; Zhu, Y.G.; Chen, B.D.; McGrath, S.P.; Feldmann, J.; Zhao, F.J. Phytochelatins play a key role in arsenic accumulation and tolerance in the aquatic macrophyte Wolffia globosa. Environ. Pollut 2012, 165, 18–24. [Google Scholar]
- Dave, R.; Singh, P.K.; Tripathi, P.; Shri, M.; Dixit, G.; Dwivedi, S.; Chakrabarty, D.; Trivedi, P.K.; Sharma, Y.K.; Dhankher, O.P.; et al. Arsenite tolerance is related to proportional thiolic metabolite synthesis in rice (Oryza sativa L.). Arch. Environ. Con. Tox 2013, 64, 235–242. [Google Scholar]
- Tripathi, P.; Mishra, A.; Dwivedi, S.; Chakrabarty, D.; Trivedi, P.K.; Singh, R.P.; Tripathi, R.D. Differential response of oxidative stress and thiol metabolism in contrasting rice genotypes for arsenic tolerance. Ecotox. Environ. Saf. 2012, 79, 189–198. [Google Scholar]
- Lei, M.; Wan, X.M.; Li, X.W.; Chen, T.B.; Liu, Y.R.; Huang, Z.C. Impacts of sulfur regulation in vivo on arsenic accumulation and tolerance of hyperaccumulator Pteris vittata. Environ. Exp. Bot 2013, 85, 1–6. [Google Scholar]
- Mohamed, A.A.; Castagna, A.; Ranieri, A.; di Toppi, L.S. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Biochem 2012, 57, 15–22. [Google Scholar]
- Liu, Z.; Gu, C.; Chen, F.; Yang, D.; Wu, K.; Chen, S.; Jiang, J.; Zhang, Z. Heterologous expression of a Nelumbo nucifera phytochelatin synthase gene enhances cadmium tolerance in Arabidopsis thaliana. Appl. Biochem. Biotech 2012, 166, 722–734. [Google Scholar]
- Iori, V.; Pietrini, F.; Massacci, A.; Zacchini, A. Induction of metal binding compounds and antioxidative defence in callus cultures of two black poplar (P. nigra) clones with different tolerance to cadmium. Plant Cell Tiss. Org 2012, 108, 17–26. [Google Scholar]
- Son, K.H.; Kim, D.Y.; Koo, N.; Kim, K.R.; Kim, J.G.; Owens, G. Detoxification through phytochelatin synthesis in Oenothera odorata exposed to Cd solutions. Environ. Exp. Bot 2012, 75, 9–15. [Google Scholar]
- Hentz, S.; McComb, J.; Miller, G.; Begonia, M.; Begonia, G. Cadmium uptake, growth and phytochelatin contents of Triticum aestivum in response to various concentrations of cadmium. World Environ 2012, 2, 44–50. [Google Scholar]
- Najmanova, J.; Neumannova, E.; Leonhardt, T.; Zitka, O.; Kizek, R.; Macek, T.; Mackova, M.; Kotrba, P. Cadmium-induced production of phytochelatins and speciation of intracellular cadmium in organs of Linum usitatissimum seedlings. Ind. Crop. Prod 2012, 36, 536–542. [Google Scholar]
- Shukla, D.; Kesari, R.; Mishra, S.; Dwivedi, S.; Tripathi, R.D.; Nath, P.; Trivedi, P.K. Expression of phytochelatin synthase from aquatic macrophyte Ceratophyllum demersum L. enhances cadmium and arsenic accumulation in tobacco. Plant Cell Rep 2012, 31, 1687–1699. [Google Scholar]
- Huang, J.; Zhang, Y.; Peng, J.S.; Zhong, C.; Yi, H.Y.; Ow, D.W.; Gong, J.W. Fission yeast HMT1 lowers seed cadmium through phytochelatin-dependent vacuolar sequestration in Arabidopsis. Plant Physiol 2012, 158, 1779–1788. [Google Scholar]
- Yao, Y.; Xu, G.; Mou, D.; Wang, J.; Ma, J. Subcellular Mn compartation, anatomic and biochemical changes of two grape varieties in response to excess manganese. Chemosphere 2012, 89, 150–157. [Google Scholar]
- Zhang, H.; Xu, W.; Guo, J.; He, Z.; Ma, M. Coordinated responses of phytochelatins and metallothioneins to heavy metals in garlic seedlings. Plant Sci 2005, 169, 1059–1065. [Google Scholar]
- Bhargava, P.; Srivastava, A.K.; Urmil, S.; Rai, L.C. Phytochelatin plays a role in UV-B tolerance in N2-fixing cyanobacterium Anabaena doliolum. J. Plant Physiol 2005, 162, 1220–1225. [Google Scholar]
- Chaurasia, N.; Mishra, Y.; Rai, L.C. Cloning expression and analysis of phytochelatin synthase (pcs) gene from Anabaena sp. PCC 7120 offering multiple stress tolerance in Escherichia coli. Biochem. Bioph. Res. Commun 2008, 376, 225–230. [Google Scholar]
- Seki, M.; Narusaka, M.; Ishida, J.; Nanjo, T.; Fujita, M.; Oono, Y.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T.; et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 2002, 31, 279–292. [Google Scholar]
- Stroiński, A.; Chadzinikolau, T.; Giżewska, K.; Zielezińska, M. ABA or cadmium induced phytochelatin synthesis in potato tubers. Biol. Plantarum 2010, 54, 117–120. [Google Scholar]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol 2011, 14, 290–295. [Google Scholar]
- Gupta, D.K.; Huang, H.G.; Yang, X.E.; Razafindrabe, B.H.N.; Inouhe, M. The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J. Hazard. Mater 2010, 177, 437–444. [Google Scholar]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci 2012, 13, 3145–3175. [Google Scholar]
- Delalande, O.; Desvaux, H.; Godat, E.; Valleix, A.; Junot, C.; Labarre, J.; Boulard, Y. Cadmium – glutathione solution structures provide new insights into heavy metal detoxification. FEBS J 2010, 277, 5086–5096. [Google Scholar]
- Gusmão, R.; Ariño, C.; Díaz-Cruz, J.M.; Esteban, M. Electrochemical survey of the chain length influence in phytochelatins competitive binding by cadmium. Anal. Bioch 2010, 406, 61–69. [Google Scholar]
- Elviri, L.; Speroni, F.; Careri, M.; Mangia, A.; Sanità di Toppi, L.; Zottini, M. Identification of in vivo nitrosylated phytochelatins in Arabidopsis thaliana cells by liquid chromatography-direct electrospray-linear ion trap-mass spectrometry. J. Chromatogr. A 2010, 1217, 4120–4126. [Google Scholar]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; GwóŸdŸ, E.A. The message of nitric oxide in cadmium challenged plants. Plant Sci 2011, 181, 612–620. [Google Scholar]
- De Vos, C.H.R.; Vonk, M.J.; Vooijs, R.; Schat, H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol 1992, 98, 853–858. [Google Scholar]
- Seth, C.S.; Misra, V.; Chauhan, L.K.S. Accumulation, detoxification, and genotoxicity of heavy metals in indian mustard (Brassica juncea L.). Int. J. Phytoremediat 2012, 14, 1–13. [Google Scholar]
- Gao, L.; Peng, K.; Xia, Y.; Wang, G.; Niu, L.; Lian, C.; Shen, Z. Cadmium and manganese accumulation in Phytolacca americana L. and the roles of non-protein thiols and organic acids. Int. J. Phytoremed 2013, 15, 307–319. [Google Scholar]
- Wang, F.; Wang, Z.; Zhu, C. Heteroexpression of the wheat phytochelatin synthase gene (TaPCS1) in rice enhances cadmium sensitivity. Acta Biochim. Biophys. Sin 2012, 44, 886–893. [Google Scholar]
- Queval, G.; Foyer, C. Redox regulation of photosynthetic gene expression. Phil. Trans. R. Soc. B 2012, 367, 3475–3485. [Google Scholar]
- Vivancos, P.D.; Wolff, T.; Markovic, J.; Pallardó, F.V.; Foyer, C.H. A nuclear glutathione cycle within the cell cycle. Biochem. J 2010, 431, 169–178. [Google Scholar]
- Bellomo, G.; Palladini, G.; Vairetti, M. Intranuclear distribution, function and fate of glutathione and glutathione-S-conjugate in living rat hepatocytes studied by fluorescence microscopy. Microsc. Res. Tech 1997, 36, 243–252. [Google Scholar]
- García-Giménez, J.L.; Markovic, J.; Dasí, F.; Queval, G.; Foyer, C.H.; Pallardó, F.V. Nuclear glutathione. Biochim. Biophys. Acta 2012. [Google Scholar] [CrossRef]
- Markovic, J.; Mora, N.J.; Broseta, A.M.; Gimeno, A.; de-la-Concepción, N.; Vina, J.; Pallardo, F.V. The depletion of nuclear glutathione impairs cell proliferation in 3t3 fibroblasts. PLoS One 2009, 4, e6413. [Google Scholar]
- Zechmann, B.; Liou, L.-C.; Koffler, B.E.; Horvat, L.; Tomašić, A.; Fulgosi, H.; Zhang, Z. Subcellular distribution of glutathione and its dynamic changes under oxidative stress in the yeast Saccharomyces cerevisiae. FEMS Yeast Res 2011, 11, 631–642. [Google Scholar]
- Queval, G.; Jaillard, D.; Zechmann, B.; Noctor, G. Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ 2011, 34, 21–32. [Google Scholar]
- Vivancos, P.D.; Dong, Y.; Ziegler, K.; Markovic, J.; Pallardó, F.V.; Pellny, T.K.; Verrier, P.J.; Foyer, C.H. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J 2010, 64, 825–838. [Google Scholar]
- Guillas, I.; Zachowski, A.; Baudouin, E. A matter of fat: Interaction between nitric oxide and sphingolipid signaling in plant cold response. Plant Signal. Behav 2011, 6, 140–142. [Google Scholar]
- De Pinto, M.C.; Paradiso, A.; Leonetti, P.; de Gara, L. Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J 2006, 48, 784–795. [Google Scholar]
- Gaupels, F.; Kuruthukulangarakoola, G.T.; Durner, J. Upstream and downstream signals of nitric oxide in pathogen defence. Curr. Opin. Plant Biol 2011, 14, 707–714. [Google Scholar]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Gupta, D.K.; Archilla, A.; Romero-Puertas, M.C.; del Río, L.A. Reactive Oxygen Species and Nitric Oxide in Plants Under Cadmium Stress. In From Toxicity to Signaling Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 199–215. [Google Scholar]
- Arasimowicz, M.; Floryszak-Wieczorek, J.; Milczarek, G.; Jelonek, T. Nitric oxide, induced by wounding, mediates redox regulation in pelargonium leaves. Plant Biol 2009, 11, 650–663. [Google Scholar]
- Xu, J.; Wang, W.; Sun, J.; Zhang, Y.; Ge, Q.; Du, L.; Yin, H.; Liu, X. Involvement of auxin and nitric oxide in plant Cd-stress responses. Plant Soil 2011, 346, 107–119. [Google Scholar]
- Siddiqui, M.H.; Mohammad, F.; Khan, M.M.A.; Al-Whaibi, M.H. Cumulative effect of nitrogen and sulphur on Brassica juncea L. genotypes under NaCl stress. Protoplasma 2012, 249, 139–153. [Google Scholar]
- Corpas, F.J.; Leterrier, M.; Valderrama, R.; Airaki, M.; Chaki, M.; Palma, J.M.; Barroso, J.B. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci 2011, 181, 604–611. [Google Scholar]
- Sueishi, Y.; Hori, M.; Kita, M.; Kotake, Y. Nitric oxide (NO) scavenging capacity of natural antioxidants. Food Chem 2011, 129, 866–870. [Google Scholar]
- Stamler, J.S.; Lamas, S.; Fang, F.C. Nitrosylation: The prototypic redox-based signaling mechanism. Cell 2001, 106, 675–683. [Google Scholar]
- Baudouin, E. The language of nitric oxide signalling. Plant Biol 2011, 13, 233–242. [Google Scholar]
- Leterrier, M.; Chaki, M.; Airaki, M.; Valderrama, R.; Palma, J.M.; Barroso, J.B.; Corpas, F.J. Function of S-nitrosoglutathione reductase (GSNOR) in plant development and under biotic/abiotic stress. Plant Signal. Behaviour 2011, 6, 789–793. [Google Scholar]
- Lindermayr, C.; Sell, S.; Muller, B.; Leister, D.; Durner, J. Redox regulation of the NPR1–TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 2010, 22, 2894–2907. [Google Scholar]
- Yu, M.; Yun, B.W.; Spoel, S.H.; Loake, G.J. A sleigh ride through the SNO: Regulation of plant immune function by protein S-nitrosylation. Curr. Opin. Plant Biol 2012, 15, 424–430. [Google Scholar]
- Serpa, V.; Vernal, J.; Lamattina, L.; Grotewold, E.; Cassia, R.; Terenzi, H. Inhibition of AtMYB2 DNA-binding by nitric oxide involves cysteine S-nitrosylation. Biochem. Biophys. Res. Commun 2007, 361, 1048–1053. [Google Scholar]
- Espunya, M.C.; de Michele, R.; Gómez-Cadenas, A.; Martínez, M.C. S-Nitrosoglutathione is a component of wound- and salicylic acid-induced systemic responses in Arabidopsis thaliana. J. Exp. Bot 2012, 63, 3219–3227. [Google Scholar]
- Molassiotis, A.; Tanou, G.; Diamantidis, G. NO says more than ‘YES’ to salt tolerance: Salt priming and systemic nitric oxide signaling in plants. Plant Signal. Behaviour 2010, 5, 209–212. [Google Scholar]
- Wang, H.; Liang, X.; Wan, Q.; Wang, X.; Bi, Y. Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 2009, 230, 293–307. [Google Scholar]
- Meyer, Y.; Belin, C.; Delorme-Hinoux, V.; Reichheld, J.P.; Riondet, C. Thioredoxin and glutaredoxin systems in plants: Molecular mechanisms, crosstalks, and functional significance. Antioxid. Redox Sign 2012, 17, 1124–1160. [Google Scholar]
- Lemaire, S.D.; Michelet, L.; Zaffagnini, M.; Massot, V.; Issakidis-Bourguet, E. Thioredoxins in chloroplasts. Curr. Genet 2007, 51, 343–365. [Google Scholar]
- Schürmann, P.; Buchanan, B.B. The Ferredoxin/Thioredoxin system of oxygenic photosynthesis. Antioxid. Redox Sign 2008, 10, 1235–1274. [Google Scholar]
- Nuruzzaman, M.; Sharoni, A.M.; Satoh, K.; Al-Shammari, T.; Shimizu, T.; Sasaya, T.; Omura, T.; Kikuchi, S. The thioredoxin gene family in rice: Genome-wide identification and expression profiling under different biotic and abiotic treatments. Biochem. Biophys. Res. Commun 2012, 423, 417–423. [Google Scholar]
- Fatehi, F.; Hosseinzadeh, A.; Alizadeh, H.; Brimavandi, T. The proteome response of Hordeum spontaneumto salinity stress. Cereal Res. Commun 2012, 1–10. [Google Scholar]
- Sanz-Barrio, R.; Millán, A.F.S.; Carballeda, J.; Corral-Martínez, P.; Seguí-Simarro, J.M.; Farran, I. Chaperone-like properties of tobacco plastid thioredoxins f and m. J. Exp. Bot 2012, 63, 365–379. [Google Scholar]
- Lakhssassi, N.; Doblas, V.G.; Rosado, A.; del Valle, A.E.; Posé, D.; Jimenez, A.J.; Castillo, A.G.; Valpuesta, V.; Borsani, O.; et al. The Arabidopsis tetratricopeptide thioredoxin-like gene family is required for osmotic stress tolerance and male sporogenesis. Plant Physiol 2012, 158, 1252–1266. [Google Scholar]
- Prasad, B.D.; Goel, S.; Krishna, P. In silico identification of carboxylate clamp type tetratricopeptide repeat proteins in Arabidopsis and Rice as putative co-chaperones of Hsp90/Hsp70. PLoS One 2010, 5, e12761. [Google Scholar]
- Chae, H.B.; Moon, J.C.; Shin, M.R.; Chi, Y.H.; Jung, Y.J.; Lee, S.Y.; Nawkar, G.M.; Jung, H.S.; Hyun, J.K.; Kim, W.Y.; et al. Thioredoxin reductase type C (NTRC) orchestrates enhanced thermotolerance to Arabidopsis by its redox-dependent holdase chaperone function. Mol. Plant 2013, 6, 323–336. [Google Scholar]
- Rampitsch, C.; Bykova, N. V. Proteomics and plant disease: Advances in combating a major threat to the global food supply. Proteomics 2012, 12, 673–690. [Google Scholar]
- Chi, Y.H.; Kim, S.Y.; Jung, I.J.; Shin, M.R.; Jung, Y.J.; Park, J.H.; Lee, E.S.; Maibam, P.; Kim, K.S.; Park, J.H.; et al. Dual functions of Arabidopsis sulfiredoxin: Acting as a redox-dependent sulfinic acid reductase and as a redox-independent nuclease enzyme. FEBS Lett 2012, 586, 3493–3499. [Google Scholar]
- Kumar, B.; Singla-Pareek, S.L.; Sopory, S.K. Glutathione Homeostasis: Crucial for Abiotic Stress Tolerance in Plants. In Abiotic Stress Adaptation in Plants; Pareek, A., Sopory, S.K., Bohnert, H.J., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 263–282. [Google Scholar]
- Lemaire, S.D.; Guillon, B.; Maréchal, P.; Keryer, E.; Miginiac-Maslow, M.; Decottignies, P. New thioredoxin targets in the unicellular photosynthetic eukaryote Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2004, 101, 7475–7480. [Google Scholar]
- Zaffagnini, M.; Bedhomme, M.; Marchand, C.H.; Couturier, J.; Gao, X.H.; Rouhier, N.; Trost, P.; Lemaire, S.D. Glutaredoxin S12: Unique properties for redox signaling. Antioxid. Redox Sign. 2012, 16, 17–32. [Google Scholar]
- Mortimer, J.C.; Laohavisit, A.; Macpherson, N.; Webb, A.; Brownlee, C.; Battey, N.H.; Davies, J.M. Annexins: Multifunctional components of growth and adaptation. J. Exp. Bot 2008, 59, 533–544. [Google Scholar]
- Konopka-Postupolska, D.; Clark, G.; Goch, G.; Debski, J.; Floras, K.; Cantero, A.; Fijolek, B.; Roux, S.; Hennig, J. The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol 2009, 150, 1394–1410. [Google Scholar]
- Nadeau, P.J.; Charette, S.J.; Toledano, M.B.; Landry, J. Disulfide bond mediated multimerization of Ask1 and its reduction by thioredoxin-1 regulate H2O2-induced c-Jun NH2-terminal kinase activation and apoptosis. Mol. Biol. Cell 2007, 18, 3903–3913. [Google Scholar]
- Clark, G.; Konopka-Postupolska, D.; Hennig, J.; Roux, S. Is annexin 1 a multifunctional protein during stress responses? Plant Signal. Behav 2010, 5, 303–307. [Google Scholar]
| Type of stress | Notes | Species | Reference |
|---|---|---|---|
| Cd | GST gene from Trichoderma virens | Tobacco | [104] |
| Cold | GST gene from Choristoneura fumiferana | Arabidopsis | [105] |
| Drought | Expression of GST gene from Prosopis juliflora | Tobacco | [106] |
| Drought and salt | GST gene from Glycine soja | Tobacco | [101] |
| Heavy metals | Human GST and CYP2E1 genes | Alfalfa | [107] |
| Herbicide | Overexpression of GST | Rice | [108] |
| Salt | Overexpression of GST | Arabidopsis | [102] |
| Salt | GST gene from Salicornia brachiata | Tobacco | [109] |
| Metal tolerance | Plant species | Reference |
|---|---|---|
| As | Wolffia globosa | [113] |
| Oryza sativa | [114] | |
| Oryza sativa | [115] | |
| Pteris vittata | [116] | |
| Cd | Brassica juncea | [117] |
| Arabidopsis thaliana | [118]* | |
| Populus nigra | [119] | |
| Oenothera odorata | [120] | |
| Triticum aestivum | [121] | |
| Linum usitatissimum | [122] | |
| Cd, As | Nicotiana tabacum | [123]* |
| Cd, Cu, As, Zn | Arabidopsis thaliana | [124]^ |
| Mn | Vitis vinifera | [125] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. A Central Role for Thiols in Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2013, 14, 7405-7432. https://doi.org/10.3390/ijms14047405
Zagorchev L, Seal CE, Kranner I, Odjakova M. A Central Role for Thiols in Plant Tolerance to Abiotic Stress. International Journal of Molecular Sciences. 2013; 14(4):7405-7432. https://doi.org/10.3390/ijms14047405
Chicago/Turabian StyleZagorchev, Lyuben, Charlotte E. Seal, Ilse Kranner, and Mariela Odjakova. 2013. "A Central Role for Thiols in Plant Tolerance to Abiotic Stress" International Journal of Molecular Sciences 14, no. 4: 7405-7432. https://doi.org/10.3390/ijms14047405




