Monocyte Chemotactic Protein-1 Promotes the Myocardial Homing of Mesenchymal Stem Cells in Dilated Cardiomyopathy
Abstract
:1. Introduction
2. Methods
2.1. Generation of Doxorubicin-Induced DCM
2.2. Expansion and Transplantation of Bone Marrow MSCs
2.3. Echocardiographic and Hemodynamic Studies
2.4. Fibrosis Determination
2.5. RNA Extraction and Real-Time Polymerase Chain Reactions (PCRs) Analysis
2.6. Western Blot Analysis
2.7. Flow Cytometry Analysis
2.8. In Vitro Migration Assay
2.9. In Vivo Migration Assessment
2.10. Statistical Analysis
3. Results
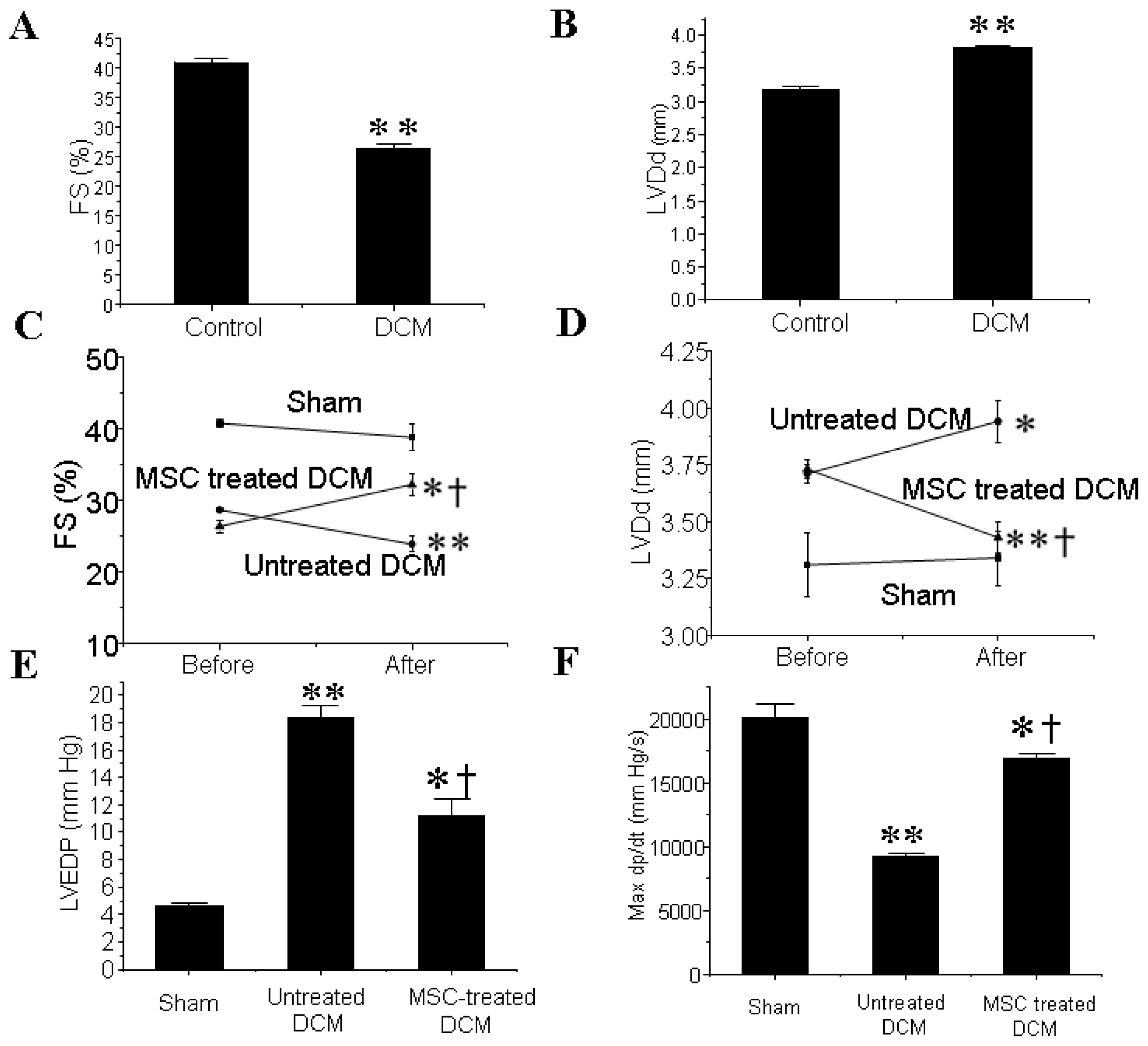
3.1. MSCs Transplantation Improves the Cardiac Function of DCM Mice
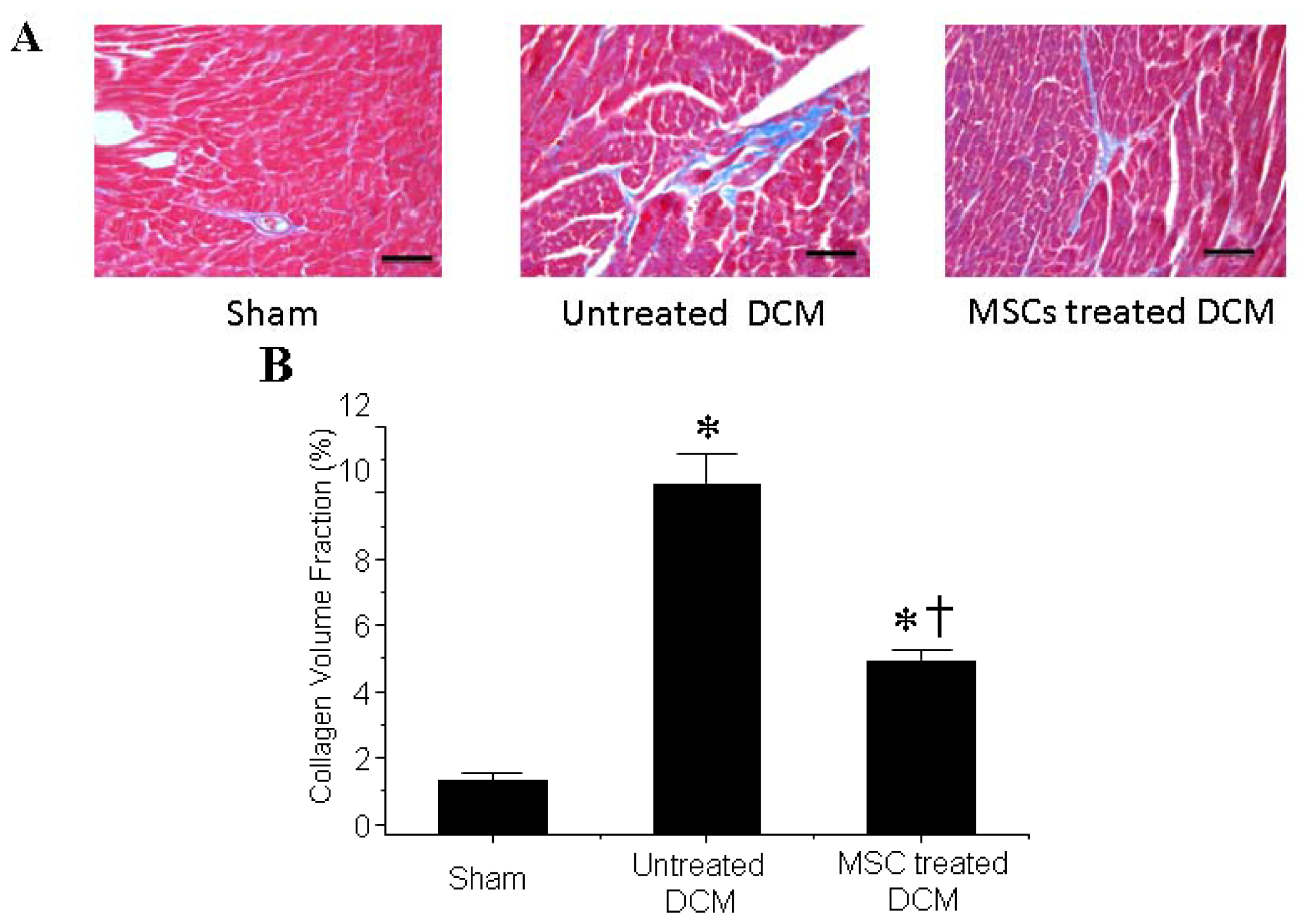
3.2. MSCs Transplantation Reduces Myocardial fibrosis
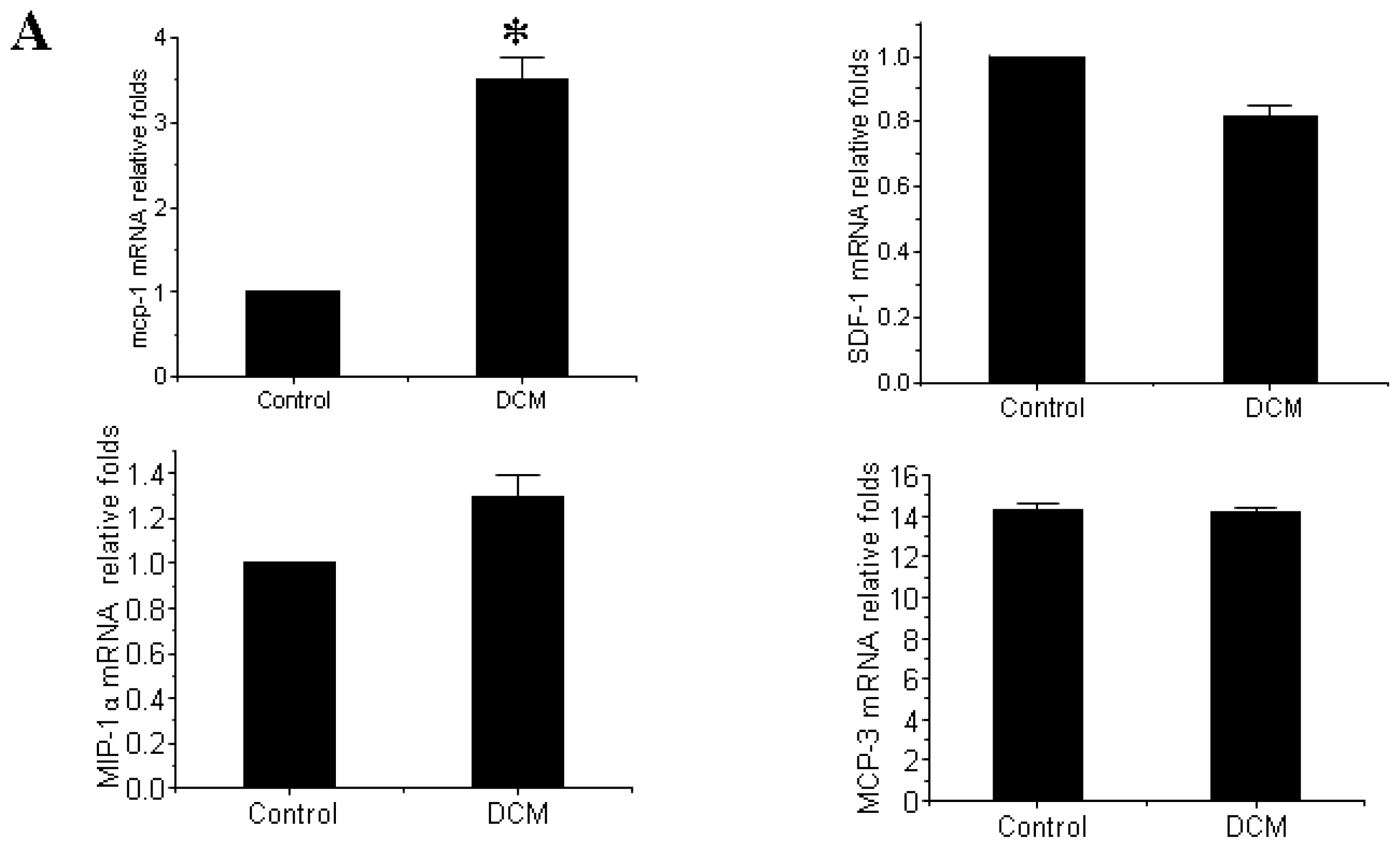
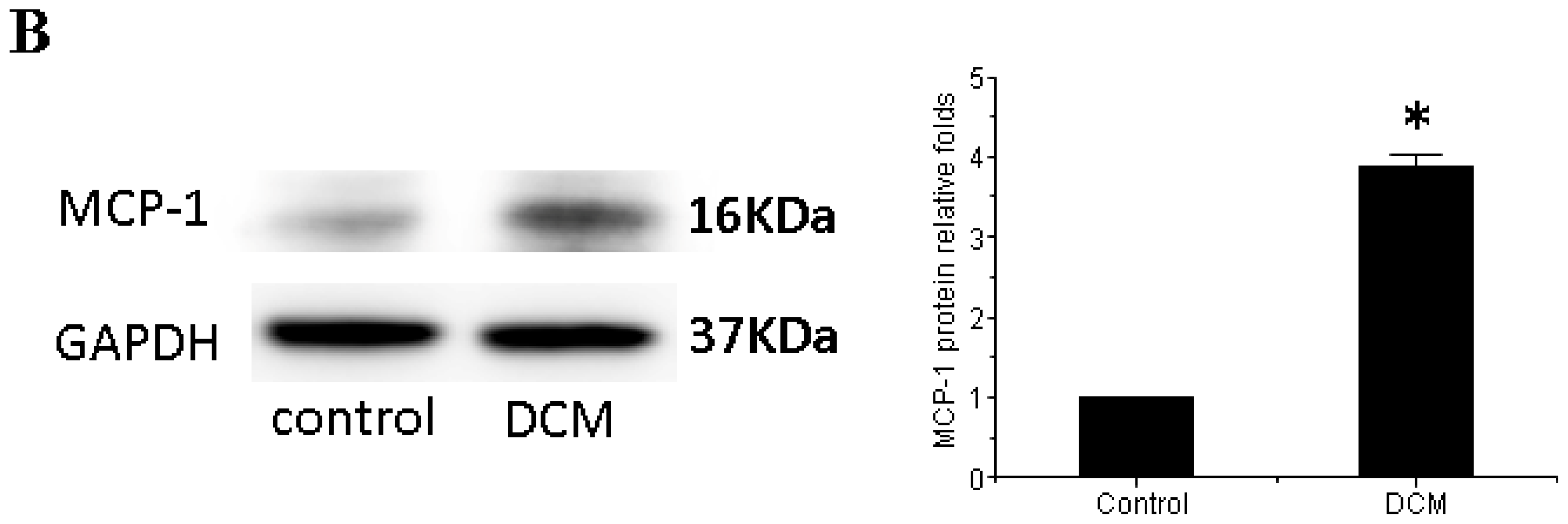
3.3. MCP-1 Is Up-regulated in Dilated Myocardial Tissue
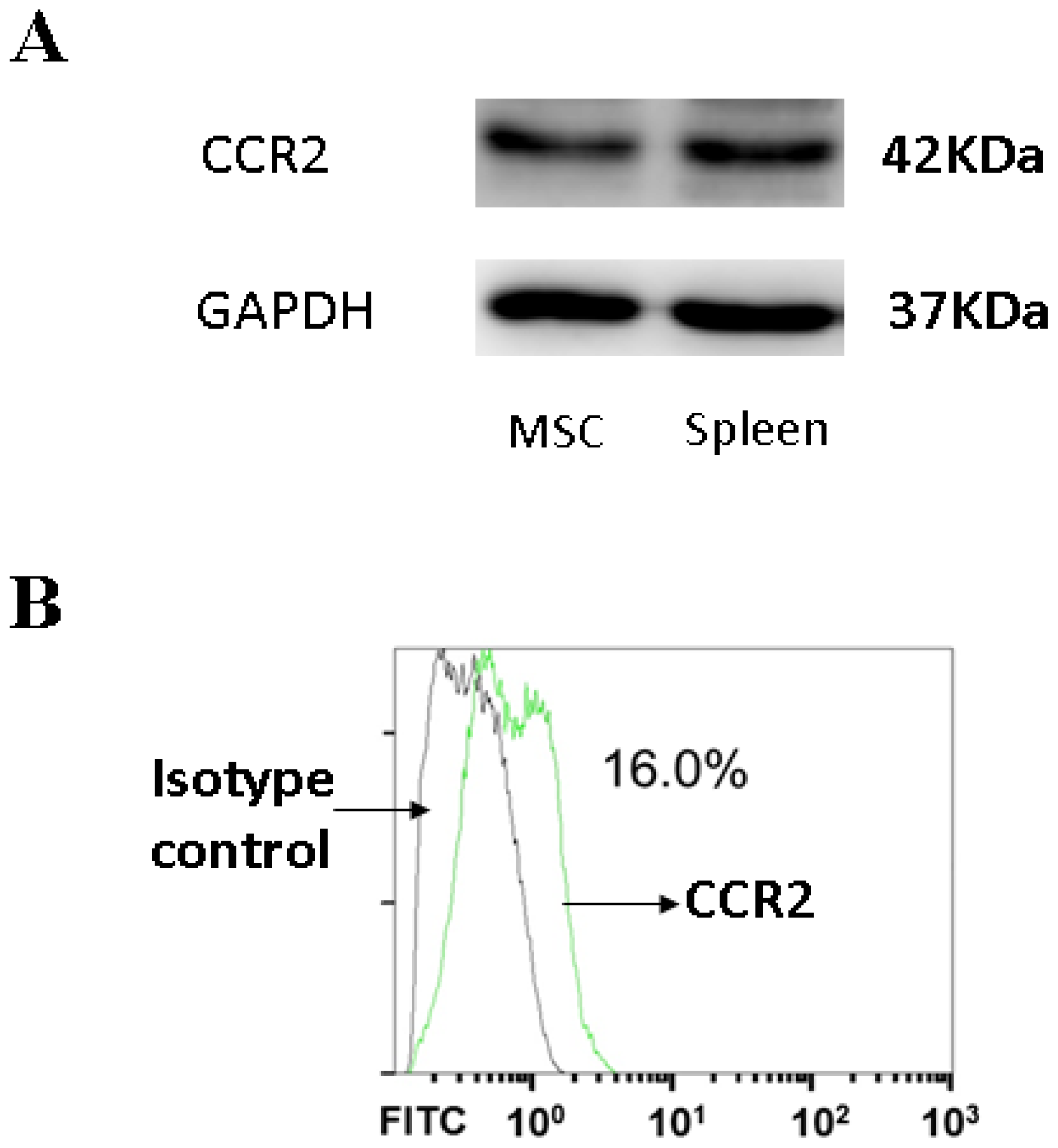
3.4. CCR2, a MCP-1 Receptor, Is Present in MSCs
3.5. MCP-1 Promotes MSCs Migration in Vitro
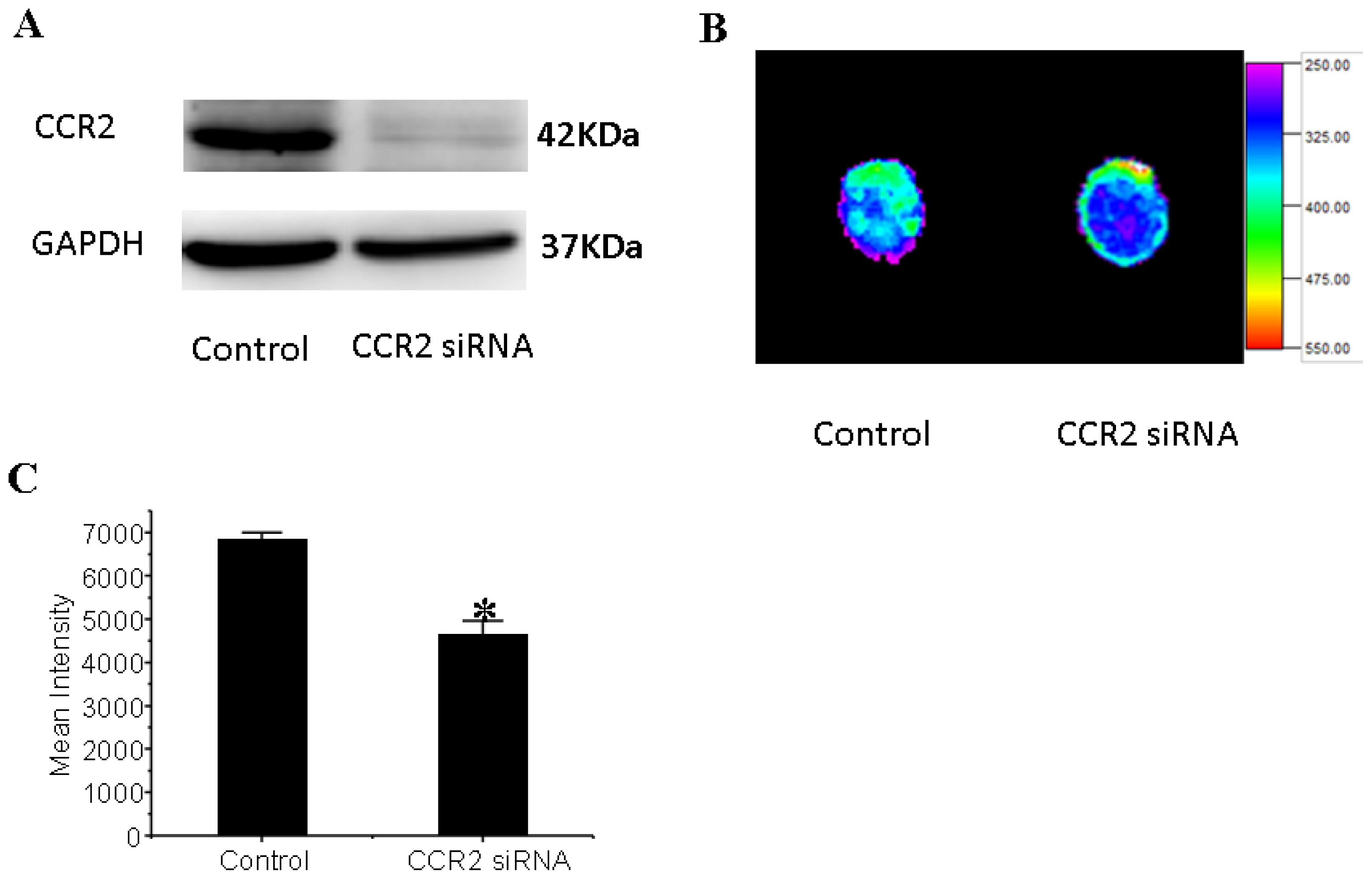
3.6. CCR2 Inhibition Decreases MSCs Migration to the Dilated Heart
4. Discussion
Acknowledgments
Conflict of Interests
References
- Mozid, A.M.; Arnous, S.; Sammut, E.C.; Mathur, A. Stem cell therapy for heart diseases. Br. Med. Bull 2011, 98, 143–159. [Google Scholar]
- Cowie, M.R.; Zaphiriou, A. Management of chronic heart failure. BMJ 2002, 325, 422–425. [Google Scholar]
- Fox, K.F.; Cowie, M.R.; Wood, D.A.; Coats, A.J.; Gibbs, J.S.; Underwood, S.R.; Turner, R.M.; Poole-Wilson, P.A.; Davies, S.W.; Sutton, G.C. Coronary artery disease as the cause of incident heart failure in the population. Eur. Heart J 2001, 22, 228–236. [Google Scholar]
- Cowie, M.R.; Mosterd, A.; Wood, D.A.; Deckers, J.W.; Poole-Wilson, P.A.; Sutton, G.C.; Grobbee, D.E. The epidemiology of heart failure. Eur. Heart J 1997, 18, 208–225. [Google Scholar]
- Baba, S.; Heike, T.; Yoshimoto, M.; Umeda, K.; Doi, H.; Iwasa, T.; Lin, X.; Matsuoka, S.; Komeda, M.; Nakahata, T. Flk1(+) cardiac stem/progenitor cells derived from embryonic stem cells improve cardiac function in a dilated cardiomyopathy mouse model. Cardiovasc. Res 2007, 76, 119–131. [Google Scholar]
- Schenk, S.; Mal, N.; Finan, A.; Zhang, M.; Kiedrowski, M.; Popovic, Z.; McCarthy, P.M.; Penn, M.S. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells 2007, 25, 245–251. [Google Scholar]
- Kocher, A.A.; Schuster, M.D.; Szabolcs, M.J.; Takuma, S.; Burkhoff, D.; Wang, J.; Homma, S.; Edwards, N.M.; Itescu, S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat. Med 2001, 7, 430–436. [Google Scholar]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410, 701–705. [Google Scholar]
- Makino, S.; Fukuda, K.; Miyoshi, S.; Konishi, F.; Kodama, H.; Pan, J.; Sano, M.; Takahashi, T.; Hori, S.; Abe, H.; et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Invest 1999, 103, 697–705. [Google Scholar]
- Shake, J.G.; Gruber, P.J.; Baumgartner, W.A.; Senechal, G.; Meyers, J.; Redmond, J.M.; Pittenger, M.F.; Martin, B.J. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann. Thorac. Surg 2002, 73, 1919–1925. [Google Scholar]
- Nagaya, N.; Kangawa, K.; Itoh, T.; Iwase, T.; Murakami, S.; Miyahara, Y.; Fujii, T.; Uematsu, M.; Ohgushi, H.; Yamagishi, M.; et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 2005, 112, 1128–1135. [Google Scholar]
- Mu, Y.; Cao, G.; Zeng, Q.; Li, Y. Transplantation of induced bone marrow mesenchymal stem cells improves the cardiac function of rabbits with dilated cardiomyopathy via upregulation of vascular endothelial growth factor and its receptors. Exp. Biol. Med 2011, 236, 1100–1107. [Google Scholar]
- Yoo, K.J.; Li, R.K.; Weisel, R.D.; Mickle, D.A.; Jia, Z.Q.; Kim, E.J.; Tomita, S.; Yau, T.M. Heart cell transplantation improves heart function in dilated cardiomyopathic hamsters. Circulation 2000, 102, III204–III209. [Google Scholar]
- Seth, S.; Narang, R.; Bhargava, B.; Ray, R.; Mohanty, S.; Gulati, G.; Kumar, L.; Reddy, K.S.; Venugopal, P. Percutaneous intracoronary cellular cardiomyoplasty for nonischemic cardiomyopathy: Clinical and histopathological results: The first-in-man ABCD (Autologous Bone Marrow Cells in Dilated Cardiomyopathy) trial. J. Am. Col Cardiol 2006, 48, 2350–2351. [Google Scholar]
- Fischer-Rasokat, U.; Assmus, B.; Seeger, F.H.; Honold, J.; Leistner, D.; Fichtlscherer, S.; Schachinger, V.; Tonn, T.; Martin, H.; Dimmeler, S.; et al. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: Final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circ. Heart Fail 2009, 2, 417–423. [Google Scholar]
- Smart, N.; Riley, P.R. The stem cell movement. Cir. Res 2008, 102, 1155–1168. [Google Scholar]
- Zhou, Y.L.; Zhang, H.F.; Li, X.L.; Di, R.M.; Yao, W.M.; Li, D.F.; Feng, J.L.; Huang, J.; Cao, K.J.; Fu, M. Increased stromal-cell-derived factor 1 enhances the homing of bone marrow derived mesenchymal stem cells in dilated cardiomyopathy in rats. Chin. Med. J 2010, 123, 3282–3287. [Google Scholar]
- GenBank sequence database. Available online: http://www.ncbi.nlm.nih.gov (accessed on 10 April 2013).
- Primer3 software. Available online: http://fokker.wi.mit.edu/primer3/input.htm (accessed on 10 April 2013).
- Bot, I.; Guo, J.; van Eck, M.; van Santbrink, P.J.; Groot, P.H.; Hildebrand, R.B.; Seppen, J.; van Berkel, T.J.; Biessen, E.A. Lentiviral shRNA silencing of murine bone marrow cell CCR2 leads to persistent knockdown of CCR2 function in vivo. Blood 2005, 106, 1147–1153. [Google Scholar]
- Zhuang, Y.; Chen, X.; Xu, M.; Zhang, L.Y.; Xiang, F. Chemokine stromal cell-derived factor 1/CXCL12 increases homing of mesenchymal stem cells to injured myocardium and neovascularization following myocardial infarction. Chin. Med. J 2009, 122, 183–187. [Google Scholar]
- Abbott, J.D.; Huang, Y.; Liu, D.; Hickey, R.; Krause, D.S.; Giordano, F.J. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 2004, 110, 3300–3305. [Google Scholar]
- Zhou, H.R.; Kim, E.K.; Kim, H.; Claycombe, K.J. Obesity-associated mouse adipose stem cell secretion of monocyte chemotactic protein-1. Am. J. Physiol 2007, 293, E1153–E1158. [Google Scholar]
- Min, J.Y.; Huang, X.; Xiang, M.; Meissner, A.; Chen, Y.; Ke, Q.; Kaplan, E.; Rana, J.S.; Oettgen, P.; Morgan, J.P. Homing of intravenously infused embryonic stem cell-derived cells to injured hearts after myocardial infarction. J. Thorac. Cardiovasc. Surg 2006, 131, 889–897. [Google Scholar]
- Lin, Y.C.; Leu, S.; Sun, C.K.; Yen, C.H.; Kao, Y.H.; Chang, L.T.; Tsai, T.H.; Chua, S.; Fu, M.; Ko, S.F.; et al. Early combined treatment with sildenafil and adipose-derived mesenchymal stem cells preserves heart function in rat dilated cardiomyopathy. J. Trans. Med 2010, 8, 88. [Google Scholar]
- Askari, A.T.; Unzek, S.; Popovic, Z.B.; Goldman, C.K.; Forudi, F.; Kiedrowski, M.; Rovner, A.; Ellis, S.G.; Thomas, J.D.; DiCorleto, P.E.; et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003, 362, 697–703. [Google Scholar]
- Chamberlain, G.; Wright, K.; Rot, A.; Ashton, B.; Middleton, J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: Comparison with human. PLoS One 2008, 3, e2934. [Google Scholar]
- Dwyer, R.M.; Potter-Beirne, S.M.; Harrington, K.A.; Lowery, A.J.; Hennessy, E.; Murphy, J.M.; Barry, F.P.; O’Brien, T.; Kerin, M.J. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin. Cancer Res 2007, 13, 5020–5027. [Google Scholar]
- Gerard, C.; Rollins, B.J. Chemokines and disease. Nat. Immunol 2001, 2, 108–115. [Google Scholar]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu. Rev. Immunol 2004, 22, 891–928. [Google Scholar]
- Gu, L.; Okada, Y.; Clinton, S.K.; Gerard, C.; Sukhova, G.K.; Libby, P.; Rollins, B.J. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 1998, 2, 275–281. [Google Scholar]
- Zhong, L.; Chen, W.Q.; Ji, X.P.; Zhang, M.; Zhao, Y.X.; Yao, G.H.; Zhang, P.F.; Zhang, C.; Zhang, Y. Dominant-negative mutation of monocyte chemoattractant protein-1 prevents vulnerable plaques from rupture in rabbits independent of serum lipid levels. J. Cell Mol. Med 2008, 12, 2362–2371. [Google Scholar]
- Birdsall, H.H.; Green, D.M.; Trial, J.; Youker, K.A.; Burns, A.R.; MacKay, C.R.; LaRosa, G.J.; Hawkins, H.K.; Smith, C.W.; Michael, L.H.; et al. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation 1997, 95, 684–692. [Google Scholar]
- Hayashidani, S.; Tsutsui, H.; Shiomi, T.; Ikeuchi, M.; Matsusaka, H.; Suematsu, N.; Wen, J.; Egashira, K.; Takeshita, A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 2003, 108, 2134–2140. [Google Scholar]
- Boyle, A.J.; Yeghiazarians, Y.; Shih, H.; Hwang, J.; Ye, J.; Sievers, R.; Zheng, D.; Palasubramaniam, J.; Palasubramaniam, D.; Karschimkus, C.; et al. Myocardial production and release of MCP-1 and SDF-1 following myocardial infarction: Differences between mice and man. J. Transl. Med 2011, 9, 150. [Google Scholar]
- Fujiyama, S.; Amano, K.; Uehira, K.; Yoshida, M.; Nishiwaki, Y.; Nozawa, Y.; Jin, D.; Takai, S.; Miyazaki, M.; Eqashira, K.; et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Cir. Res 2003, 93, 980–989. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guo, J.; Zhang, H.; Xiao, J.; Wu, J.; Ye, Y.; Li, Z.; Zou, Y.; Li, X. Monocyte Chemotactic Protein-1 Promotes the Myocardial Homing of Mesenchymal Stem Cells in Dilated Cardiomyopathy. Int. J. Mol. Sci. 2013, 14, 8164-8178. https://doi.org/10.3390/ijms14048164
Guo J, Zhang H, Xiao J, Wu J, Ye Y, Li Z, Zou Y, Li X. Monocyte Chemotactic Protein-1 Promotes the Myocardial Homing of Mesenchymal Stem Cells in Dilated Cardiomyopathy. International Journal of Molecular Sciences. 2013; 14(4):8164-8178. https://doi.org/10.3390/ijms14048164
Chicago/Turabian StyleGuo, Jing, Haifeng Zhang, Junjie Xiao, Jian Wu, Yong Ye, Zheng Li, Yunzeng Zou, and Xinli Li. 2013. "Monocyte Chemotactic Protein-1 Promotes the Myocardial Homing of Mesenchymal Stem Cells in Dilated Cardiomyopathy" International Journal of Molecular Sciences 14, no. 4: 8164-8178. https://doi.org/10.3390/ijms14048164




