Identification of MicroRNA 395a in 24-Epibrassinolide-Regulated Root Growth of Arabidopsis thaliana Using MicroRNA Arrays
Abstract
:1. Introduction
2. Results and Discussion
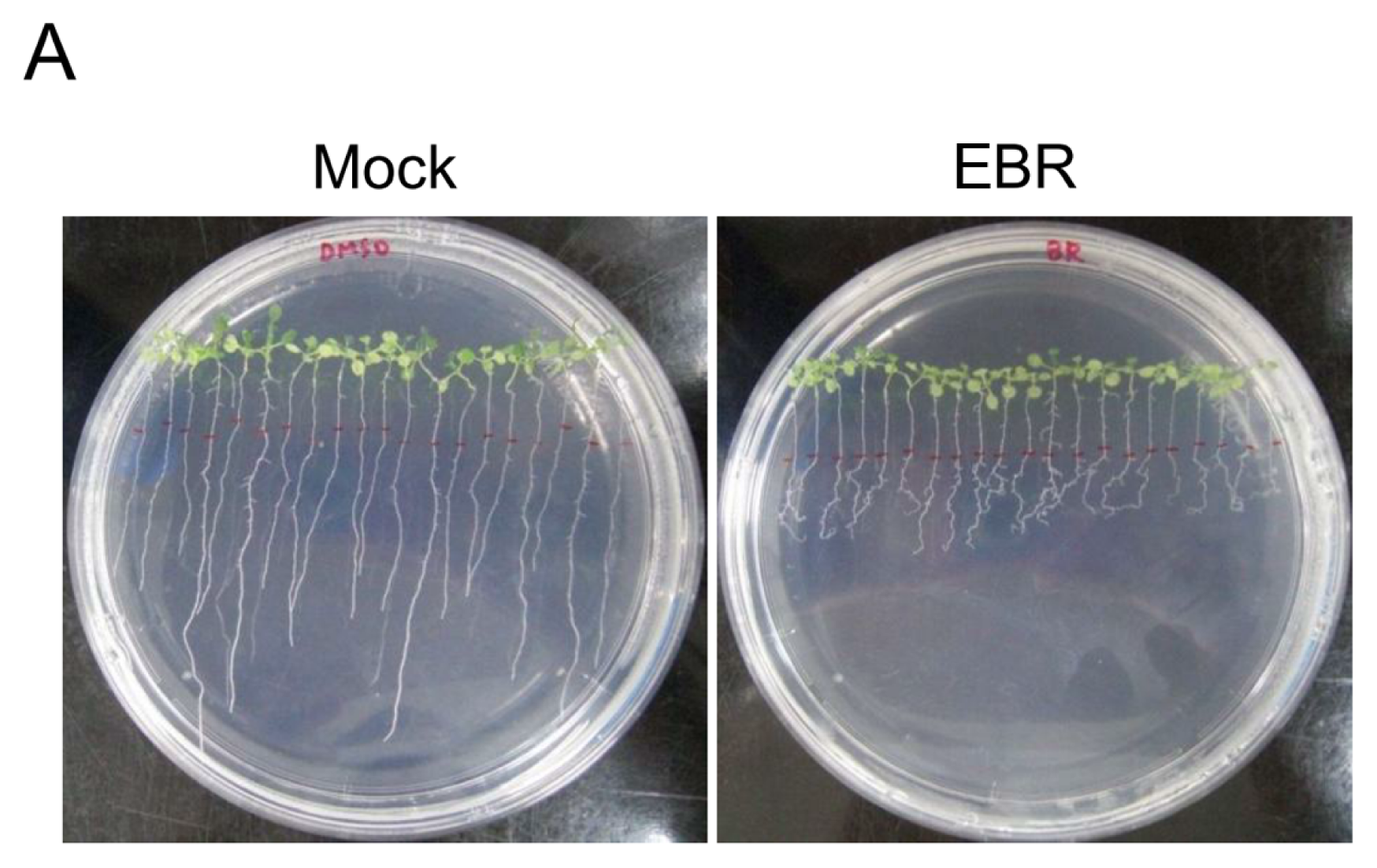
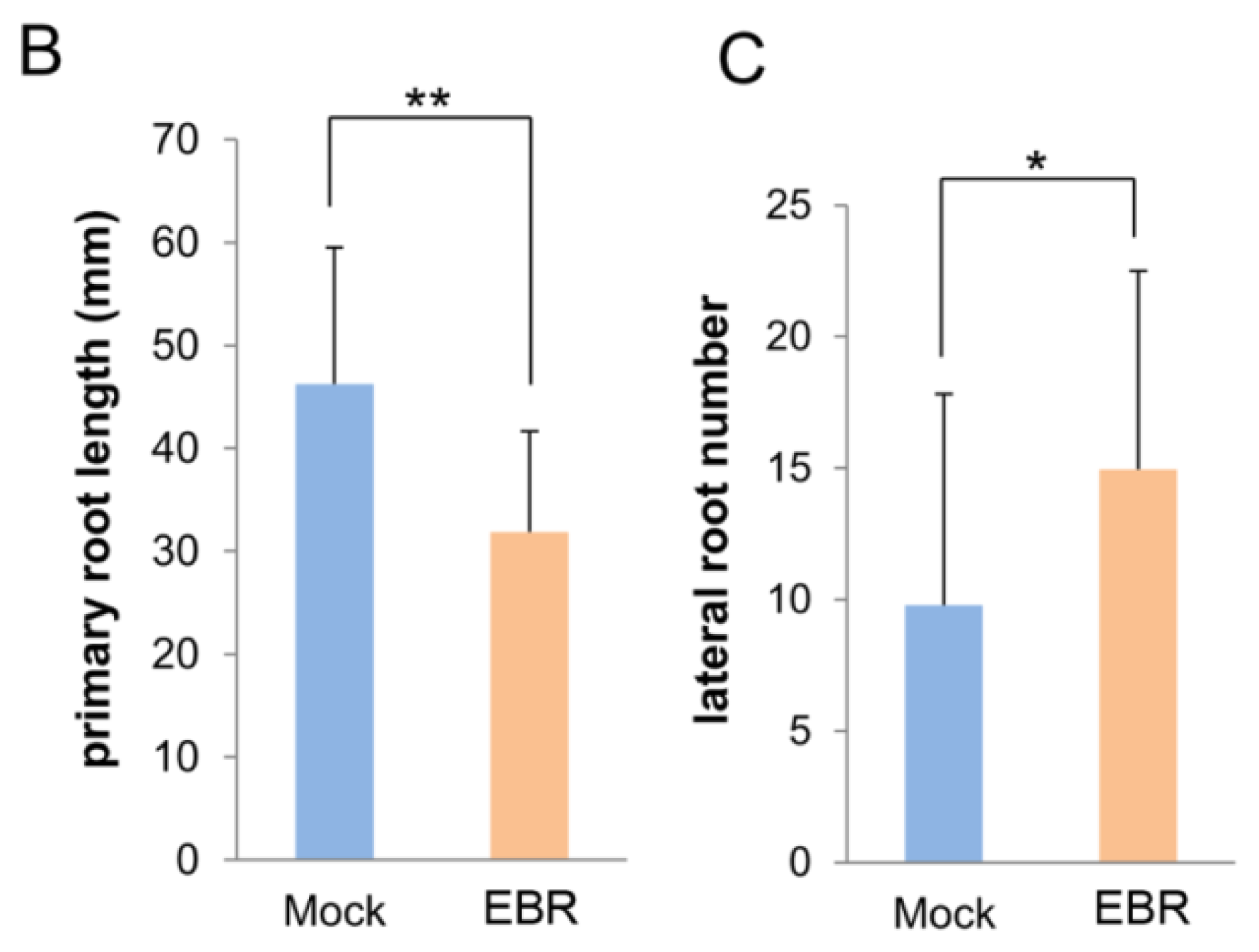
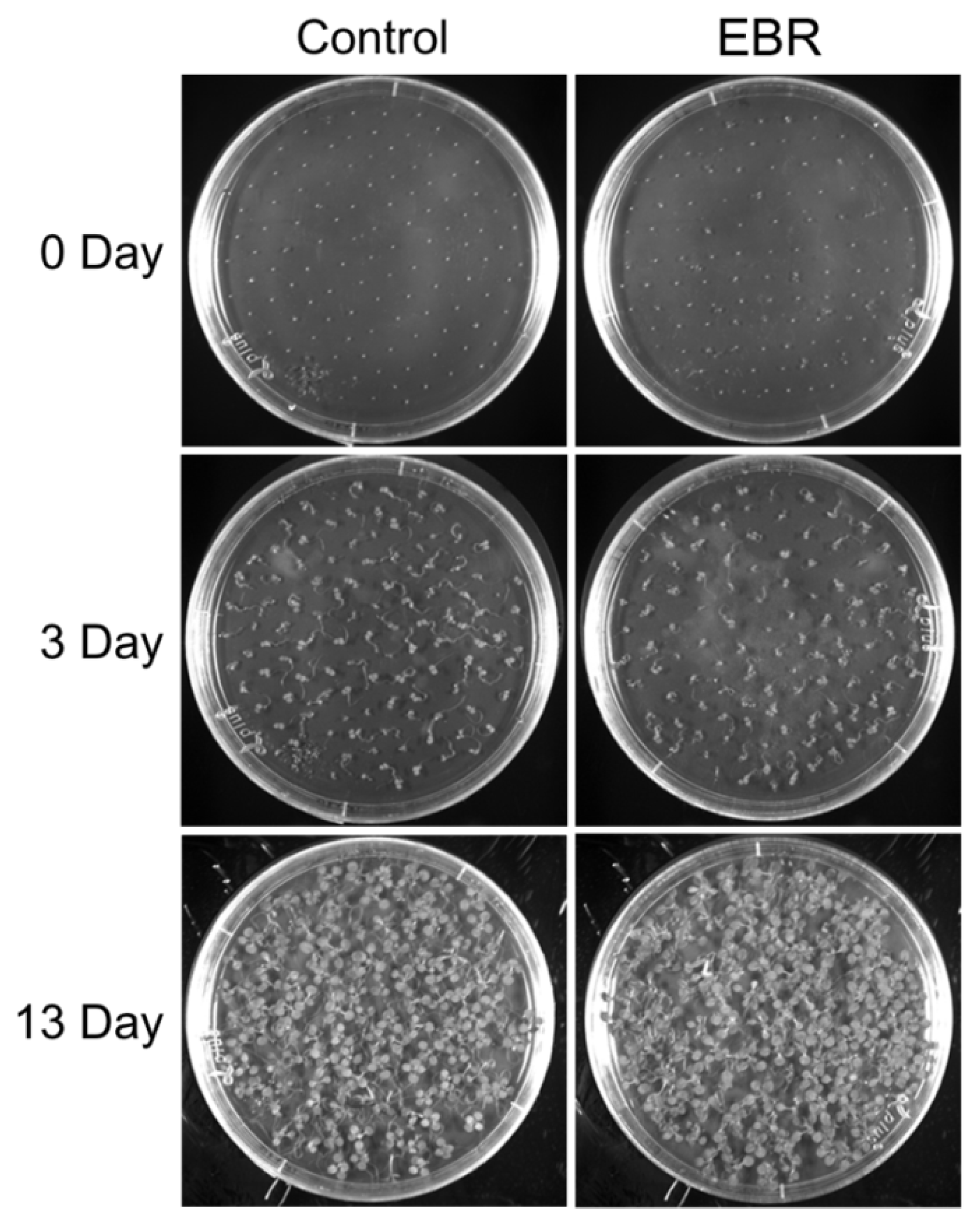
2.1. EBR Regulates the Root Development of Arabidopsis
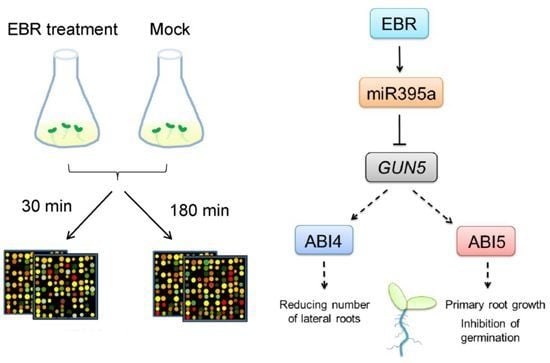
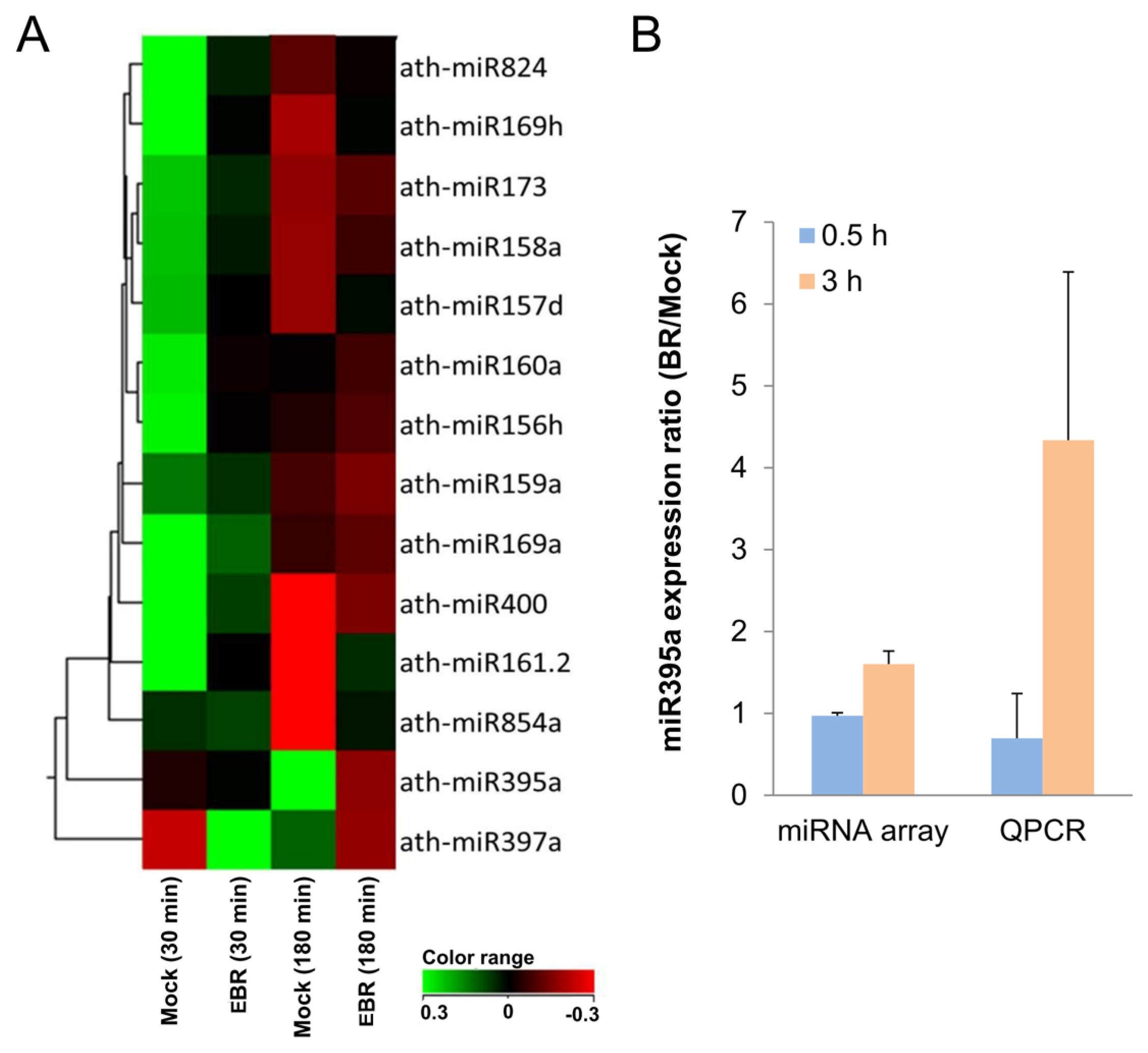
2.2. Identification of EBR-Regulated miRNAs in Arabidopsis
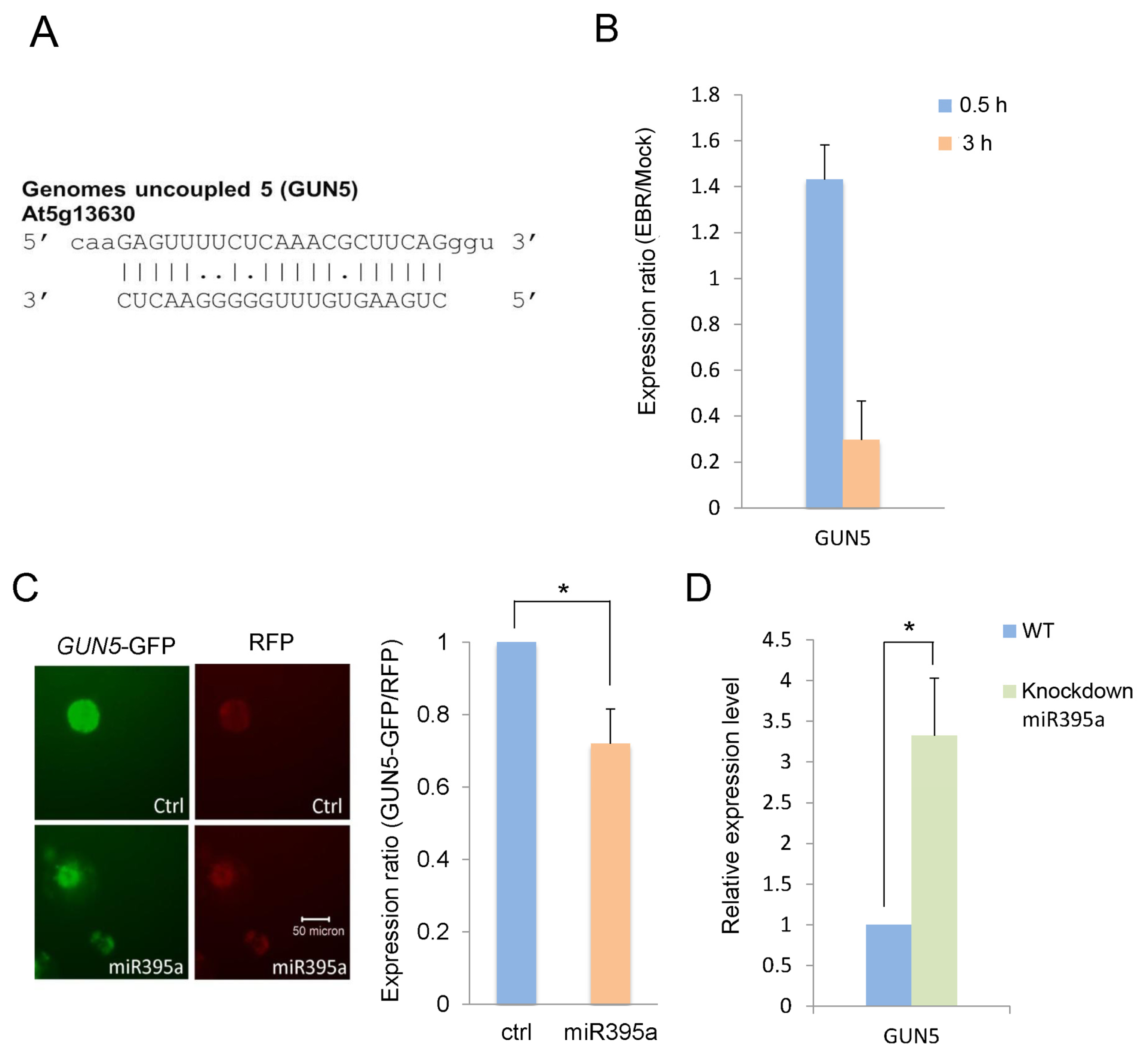
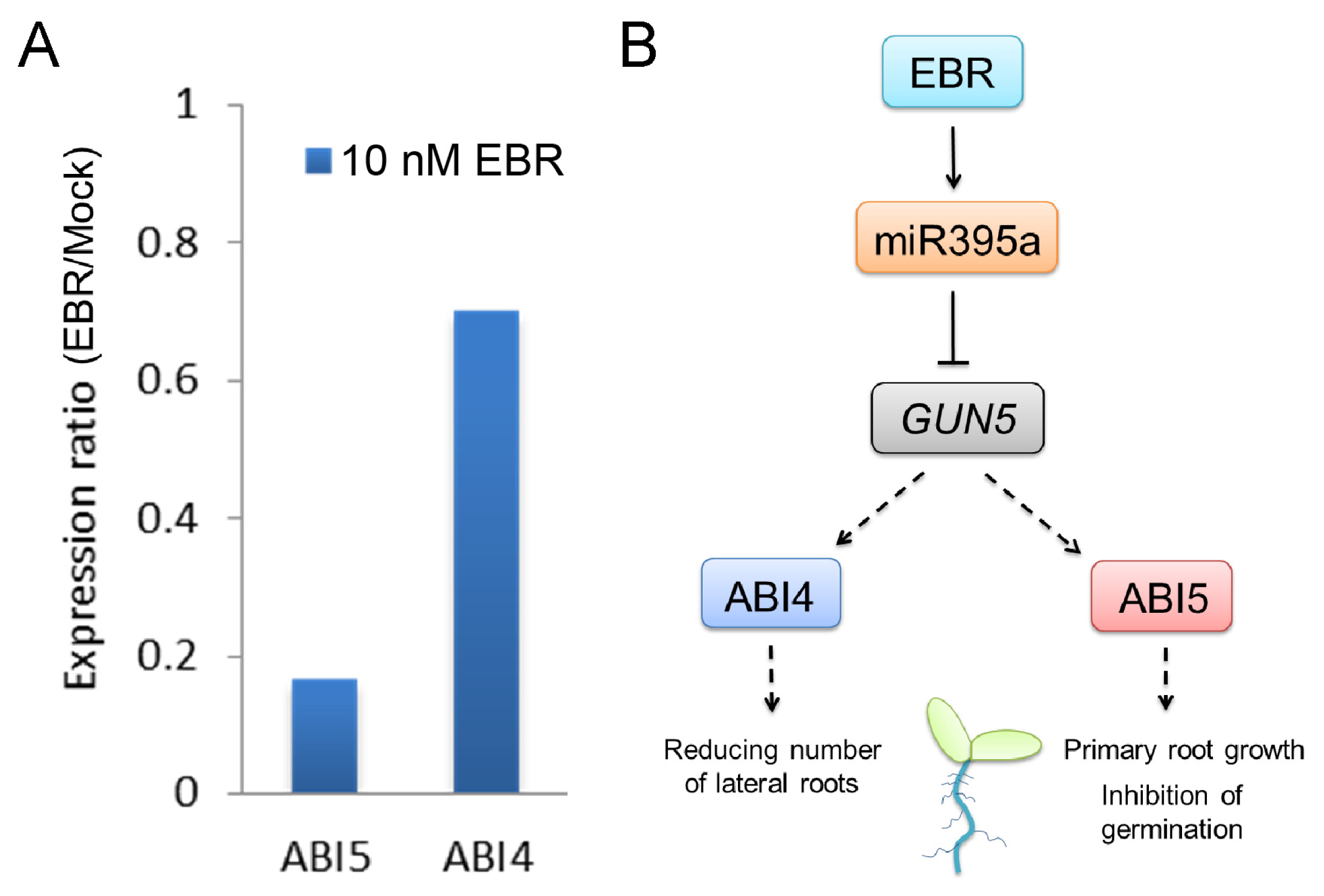
2.3. GUN5 Is a Novel Target of miR395a
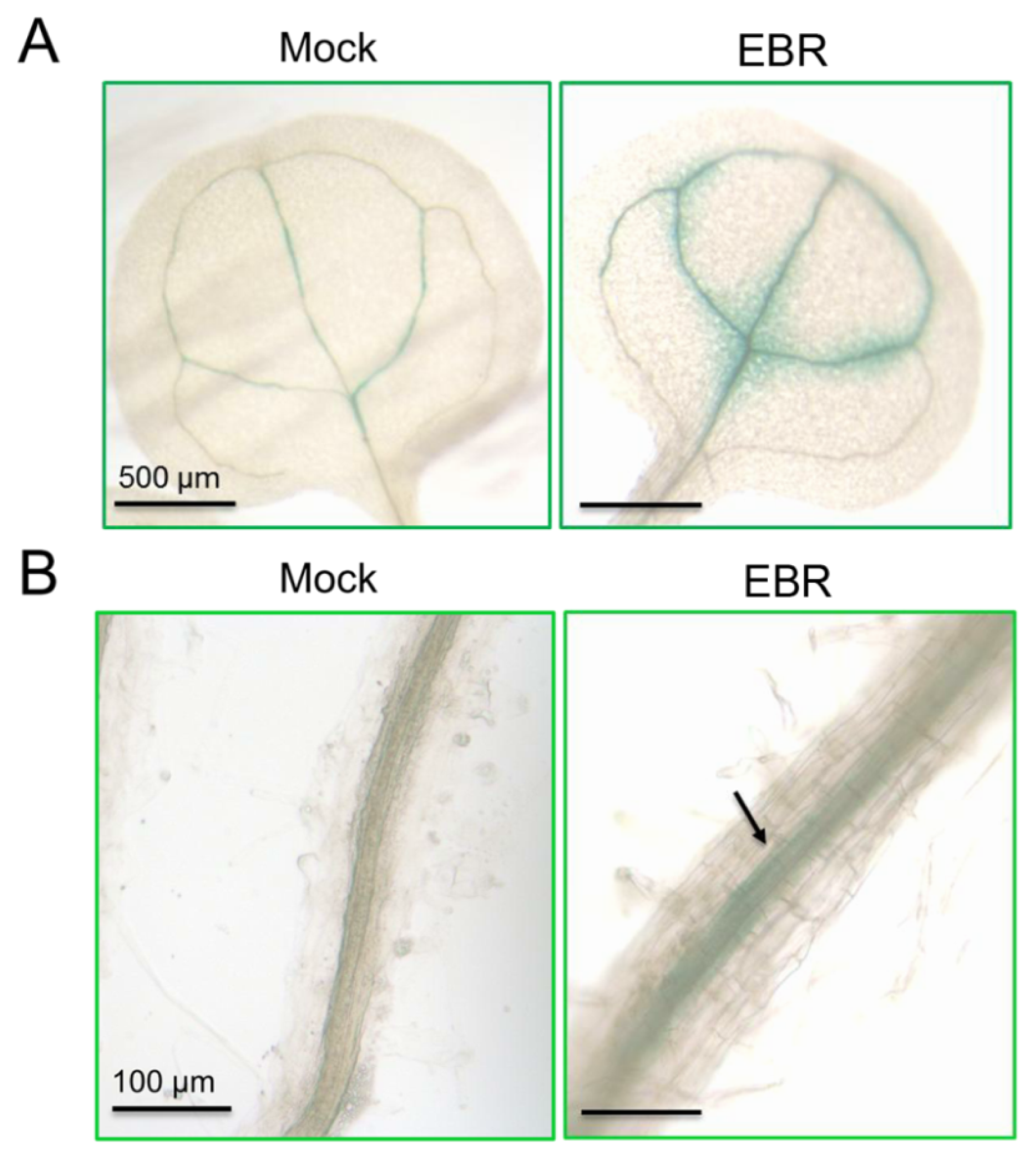
2.4. Distribution of miR395a in Vascular Bundles, Leaf Veins and Roots of Arabidopsis
2.5. Discussion
- (a)
- miR824 was down-regulated in BR-treated seedlings. It is involved in stomatal development by targeting AGL16, through which it causes a decrease in the number of stomata [46]. This suggests an increased stomata number in BR-treated plants [46,47]. Proper amounts and distributions of stomata are essential for successful gas exchange [46], and so an increase in the stomata number might therefore contribute to greater metabolic efficiency in plants.
- (b)
- miR169a, which can regulate adaptive responses to nutrient deprivation [48], was also up-regulated in our miRNA profiles. This suggests that miR169a might have acted in this capacity of adaptation to environmental change when we supplied exogenous BR.
- (c)
- miR160 mediates agravitropic roots with disorganized root caps as well as lateral root development, primary root growth, floral organs in carpels, and germination [40,42,49]. Our miRNA arrays indicated that the up-regulation of miR160a might have resulted in the expression of the phenotype observed in the present study. Since the lateral root formation caused by miR160 was similar to the morphology of BR-treated seedlings, we suspect miR160 might play an important role in lateral root development in BR-supplied plants.
- (d)
- miR156 has been shown in recent studies to increase leaf initiation, phase change, floral induction, and phosphate homeostasis, to decrease apical dominance, and to delay flowering time [40,42,49]. As suspected, miR156h was up-regulated in the miRNA profiles, suggesting a crucial function in promoting growth and development.
- (e)
3. Experimental Section
3.1. Plant Material and Growth Conditions
3.2. Germination Assay
3.3. Root Growth Assay
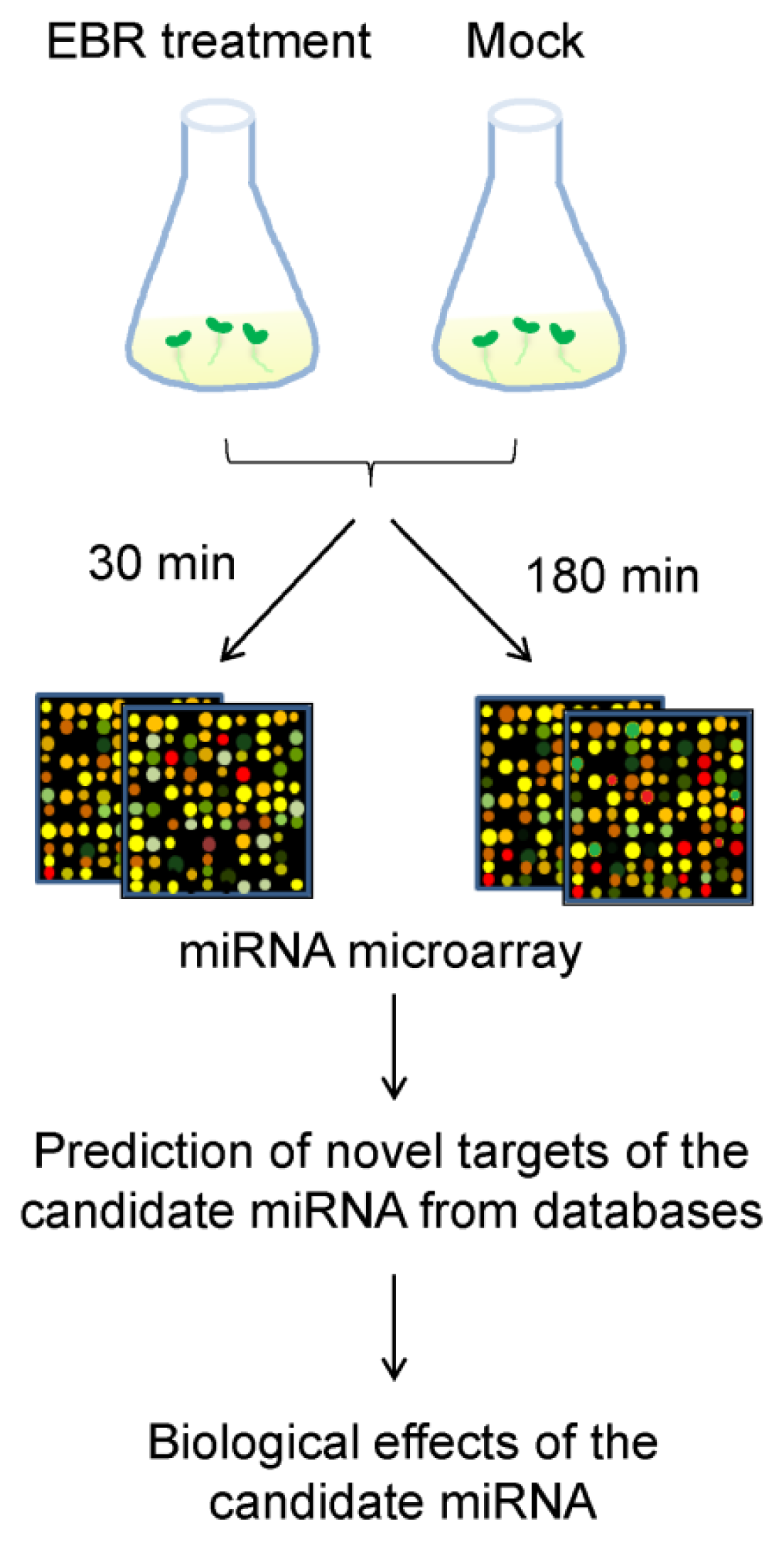
3.4. MicroRNA Microarray Hybridization and Analysis
3.5. Real-Time RT-PCR
3.6. Prediction of Novel miRNA Target Genes
3.7. Vector Construction
3.8. Fluorescence Assay for Validating miR395a and GUN5
3.9. Detection of the Expression Pattern of miR395a in Arabidopsis thaliana
3.10. Statistical Analysis
4. Conclusions
Supporting Information
ijms-14-14270-s001.pdfAcknowledgments
Conflicts of Interest
References
- Mitohell, J.W.; Mandava, N.; Worley, J.F.; Plimmer, J.R.; Smith, M.V. Brassins-a new family of plant hormone from rape pollen. Nature 1970, 225, 1065–1066. [Google Scholar]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K. Brassinolide, a plant growth-promoting steroid isolated. Nature 1979, 281, 216–217. [Google Scholar]
- Altmann, T. Molecular physiology of brassinosteroids revealed by the analysis of mutants. Planta 1999, 208, 1–11. [Google Scholar]
- Hategan, L.; Godza, B.; Russinova, M. Regulation of Brassinoteroid Metabolism. In Brassinosteroids: A Class of Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Heidelberg, Germany, 2011; p. 67. [Google Scholar]
- Yokota, T. The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci 1997, 2, 137–143. [Google Scholar]
- Clouse, S.D.; Langford, M.; McMorris, T.C. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 1996, 111, 671–678. [Google Scholar]
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol 1998, 49, 427–251. [Google Scholar]
- Clouse, S.D. Brassinosteroid signal transduction: Clarifying the pathway from ligand perception to gene expression. Mol. Cell 2002, 10, 973–982. [Google Scholar]
- Steber, C.M.; McCourt, P. A role for brassinosteroids in germination in Arabidopsis. Plant Physiol 2001, 125, 763–769. [Google Scholar]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 2007, 225, 353–364. [Google Scholar]
- Divi, U.K.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene AtDWF4 in Arabidopsis seeds overcomes abscisic acid-induced inhibition of germination and increases cold tolerance in transgenic seedlings. J. Plant Growth Regul 2010, 29, 385–393. [Google Scholar]
- Gomes, M.M.A. Physiological Effects Regulated to Brassinoteroid Application in Plants. In Brassinoteroids: A class of Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Heidelberg, Germany, 2011; pp. 193–204. [Google Scholar]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 2011, 39, D152–D157. [Google Scholar]
- Voinnet, O. Origin, biogenesis, and activity of plant microRNAs. Cell 2009, 136, 669–687. [Google Scholar]
- Bartel, D.P. microRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar]
- Liu, H.H.; Tian, X.; Li, Y.J.; Wu, C.A.; Zheng, C.C. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 2008, 14, 836–843. [Google Scholar]
- Fujii, H.; Chiou, T.J.; Lin, S.I.; Aung, K.; Zhu, J.K. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr. Biol 2005, 15, 2038–2043. [Google Scholar]
- Deng, Z.; Zhang, X.; Tang, W.; Oses-Prieto, J.A.; Suzuki, N.; Gendron, J.M.; Chen, H.; Guan, S.; Chalkley, R.J.; Peterman, T.K.; et al. A proteomics study of brassinosteroid response in Arabidopsis. Mol. Cell. Proteomics 2007, 6, 2058–2071. [Google Scholar]
- Tang, W.; Deng, Z.; Oses-Prieto, J.A.; Suzuki, N.; Zhu, S.; Zhang, X.; Burlingame, A.L.; Wang, Z.-Y. Proteomics studies of brassinosteroid signal transduction using prefractionation and two-dimensional DIGE. Mol. Cell. Proteomics 2008, 7, 728–738. [Google Scholar]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 2006, 126, 467–475. [Google Scholar]
- Goda, H.; Shimada, Y.; Asami, T.; Fujioka, S.; Yoshida, S. Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 2002, 130, 1319–1334. [Google Scholar]
- Mussig, C.; Shin, G.H.; Altmann, T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol 2003, 133, 1261–1271. [Google Scholar]
- Bao, F.; Shen, J.; Brady, S.R.; Muday, G.K.; Asami, T.; Yang, Z. Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol 2004, 134, 1624–1631. [Google Scholar]
- Fukaki, H.; Tasaka, M. Hormone interactions during lateral root formation. Plant Mol. Biol 2009, 69, 437–499. [Google Scholar]
- Zhang, Y. miRU: An automated plant miRNA target prediction server. Nucleic Acids Res 2005, 33, W701–W704. [Google Scholar]
- Schwab, R.; Ossowski, S.; Riester, M.; Warthmann, N.; Weigel, D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 2006, 18, 1121–1133. [Google Scholar]
- Dai, X.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server. Nucleic Acids Res 2011, 39, W155–W159. [Google Scholar]
- Mochizuki, N.; Brusslan, J.A.; Larkin, R.; Nagatani, A.; Chory, J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl. Acad. Sci. USA 2000, 98, 2053–2058. [Google Scholar]
- Rodermel, S.; Park, S. Pathways of intracellular communication: Tetrapyrroles and plastid-to-nucleus signaling. BioEssays 2003, 25, 631–636. [Google Scholar]
- Finkelstein, R.R.; Gampala, S.S.; Rock, C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell 2002, 14, S15–S45. [Google Scholar]
- Zhang, H.; Han, W.; de Smet, I.; Talboys, P.; Loya, R.; Hassan, A.; Rong, H.; Jurgens, G.; Paul Knox, J.; Wang, M.H. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J 2010, 64, 764–774. [Google Scholar]
- De Smet, I.; Signora, L.; Beeckman, T.; Inze, D.; Foyer, C.H.; Zhang, H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J 2003, 33, 543–555. [Google Scholar]
- Jenks, M.A.; Wood, A.J. Genes for Plant Abiotic Stress, 1st ed.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2009. [Google Scholar]
- Nakano, T.; Kimura, T.; Kaneko, I.; Nagata, N.; Matsuyama, T.; Asami, T.; Yoshida, S. Molecular mechanism of chloroplast development regulated by plant hormones. RIKEN Rev 2001, 41, 86–87. [Google Scholar]
- Shkolnik-Inbar, D.; Bar-Zvi, D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 2010, 22, 3560–3573. [Google Scholar]
- Lopez-Molina, L.; Mongrand, S.; McLachlin, D.T.; Chait, B.T.; Chua, N.H. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J 2002, 32, 317–328. [Google Scholar]
- Miura, K.; Lee, J.; Jin, J.B.; Yoo, C.Y.; Miura, T.; Hasegawa, P.M. Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 5418–5423. [Google Scholar]
- Ye, Q.; Zhu, W.; Li, L.; Zhang, S.; Yin, Y.; Ma, H.; Wang, X. Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. USA 2010, 107, 6100–6105. [Google Scholar]
- Clouse, S.D. Molecular genetic analysis of brassinosteroid action. Physiol. Plant 1997, 100, 702–709. [Google Scholar]
- Nonogaki, H. microRNA gene regulation cascades during early stages of plant development. Plant Cell Physiol 2010, 51, 1840–1846. [Google Scholar]
- Poethig, R.S. Small RNAs and developmental timing in plants. Curr. Opin. Genet. Dev 2009, 19, 374–378. [Google Scholar]
- Meng, Y.; Ma, X.; Chen, D.; Wu, P.; Chen, M. microRNA-mediated signaling involved in plant root development. Biochem. Biophys. Res. Commun 2010, 393, 345–349. [Google Scholar]
- Kawashima, C.G.; Yoshimoto, N.; Maruyama-Nakashita, A.; Tsuchiya, Y.N.; Saito, K.; Takahashi, H.; Dalmay, T. Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J 2009, 57, 313–321. [Google Scholar]
- Liang, G.; Yang, F.; Yu, D. microRNA395 mediates regulation of sulfate accumulation and allocation in Arabidopsis thaliana. Plant J 2010, 62, 1046–1057. [Google Scholar]
- Yamasaki, H.; Hayashi, M.; Fukazawa, M.; Kobayashi, Y.; Shikanai, T. SQUAMOSA promoter binding protein-like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell 2009, 21, 347–361. [Google Scholar]
- Kutter, C.; Schob, H.; Stadler, M.; Meins, F., Jr; Si-Ammour, A. microRNA-mediated regulation of stomatal development in Arabidopsis. Plant Cell 2007, 19, 2417–2429. [Google Scholar]
- Petti, F.B.; Liguori, A.; Ippoliti, F. Study on cytokines IL-2, IL-6, IL-10 in patients of chronic allergic rhinitis treated with acupuncture. J. Tradit. Chin. Med 2002, 22, 104–111. [Google Scholar]
- Zhao, M.; Ding, H.; Zhu, J.-K.; Zhang, F.; Li, W.-X. Involvement of miR169 in the nitrogen-starvation responses in Arabidopsis. New Phytol 2011, 190, 906–915. [Google Scholar]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. microRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol 2006, 57, 19–53. [Google Scholar]
- Achard, P.; Herr, A.; Baulcombe, D.C.; Harberd, N.P. Modulation of floral development by a gibberellin-regulated microRNA. Development 2004, 131, 3357–3365. [Google Scholar]
- Strand, A.; Asami, T.; Alonso, J.; Ecker, J.R.; Chory, J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 2003, 421, 79–83. [Google Scholar]
- McCourt, P.; Creelman, R. The ABA receptors—We report you decide. Curr. Opin. Plant Biol 2008, 11, 474–478. [Google Scholar]
- Rasband, W.S. ImageJ, U.S. ImageJ software; National Institutes of Health: Bethesda, MD, USA, 1997–2012. [Google Scholar]
- Miao, Y.; Jiang, L. Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat. Protoc 2007, 2, 2348–2353. [Google Scholar]
| microRNAs | Fold change (EBR/DMSO) | |
|---|---|---|
| 30 min | 180 min | |
| ath-miR824 | 1.27 | 0.93 |
| ath-miR169h | 1.37 | 0.86 |
| ath-miR173 | 1.15 | 0.95 |
| ath-miR158a | 1.16 | 0.92 |
| ath-miR157d | 1.18 | 0.87 |
| ath-miR160a | 1.25 | 1.06 |
| ath-miR156h | 1.25 | 1.04 |
| ath-miR159a | 1.07 | 1.05 |
| ath-miR169a | 1.26 | 1.04 |
| ath-miR400 | 1.21 | 0.89 |
| ath-miR161.2 | 1.31 | 0.71 |
| ath-miR854a | 0.98 | 0.78 |
| ath-miR395a | 0.97 | 1.60 |
| ath-miR397a | 0.53 | 1.25 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, L.-L.; Wu, C.-C.; Huang, H.-C.; Chen, H.-J.; Hsieh, H.-L.; Juan, H.-F. Identification of MicroRNA 395a in 24-Epibrassinolide-Regulated Root Growth of Arabidopsis thaliana Using MicroRNA Arrays. Int. J. Mol. Sci. 2013, 14, 14270-14286. https://doi.org/10.3390/ijms140714270
Lin L-L, Wu C-C, Huang H-C, Chen H-J, Hsieh H-L, Juan H-F. Identification of MicroRNA 395a in 24-Epibrassinolide-Regulated Root Growth of Arabidopsis thaliana Using MicroRNA Arrays. International Journal of Molecular Sciences. 2013; 14(7):14270-14286. https://doi.org/10.3390/ijms140714270
Chicago/Turabian StyleLin, Li-Ling, Chia-Chi Wu, Hsuan-Cheng Huang, Huai-Ju Chen, Hsu-Liang Hsieh, and Hsueh-Fen Juan. 2013. "Identification of MicroRNA 395a in 24-Epibrassinolide-Regulated Root Growth of Arabidopsis thaliana Using MicroRNA Arrays" International Journal of Molecular Sciences 14, no. 7: 14270-14286. https://doi.org/10.3390/ijms140714270