The Potential Role of Lycopene for the Prevention and Therapy of Prostate Cancer: From Molecular Mechanisms to Clinical Evidence
Abstract
:1. Introduction
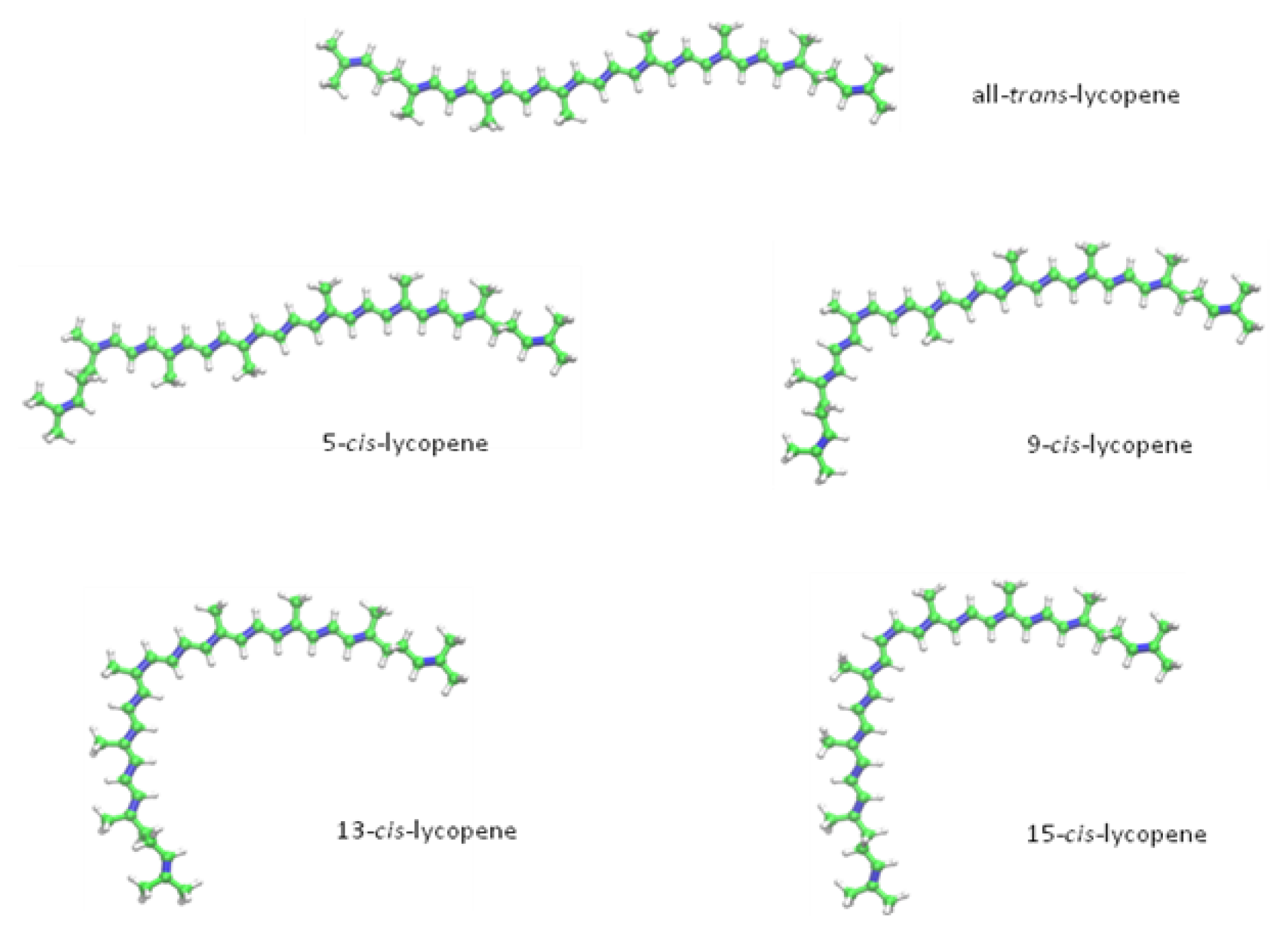
2. Chemical Properties of Lycopene
2.1. Anti-Oxidant Properties
2.2. Lycopene-Content in Different Sources
2.3. Bioavailability and Metabolism of Lycopene
3. Molecular Mechanisms of Lycopene on Prostate Cancer Cells: In Vitro Models
3.1. Prevention of DNA Damage
3.2. Effects on Tumour Cell Proliferation and Growth
3.3. Effects on the Cell-Cycle
3.4. Potential to Induce Apoptosis
3.5. Other Effects of Lycopene on Prostate Cancer Cells
4. In Vivo Models
5. Lycopene for the Prevention and Therapy of Prostate Cancer: Clinical Evidence
6. Conclusions
Conflict of Interest
References
- Bray, F.; Lortet-Tieulent, J.; Ferlay, J.; Forman, D.; Auvinen, A. Prostate cancer incidence and mortality trends in 37 European countries: An overview. Eur. J. Cancer 2010, 46, 3040–3052. [Google Scholar]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Klotz, L. Active surveillance for prostate cancer: Overview and update. Curr. Treat. Options Oncol 2013, 14, 97–108. [Google Scholar]
- Wilt, T.J.; MacDonald, R.; Hagerty, K.; Schellhammer, P.; Kramer, B.S. 5-alpha-reductase inhibitors for prostate cancer prevention. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Schmidt, K.; Pittler, M.H.; Ernst, E. A profile of journals of complementary and alternative medicine. Swiss Med. Wkly 2001, 131, 588–591. [Google Scholar]
- Foote, J.A.; Murphy, S.P.; Wilkens, L.R.; Hankin, J.H.; Henderson, B.E.; Kolonel, L.N. Factors associated with dietary supplement use among healthy adults of five ethnicities: The multiethnic cohort study. Am. J. Epidemiol 2003, 157, 888–897. [Google Scholar]
- Bishop, F.L.; Rea, A.; Lewith, H.; Chan, Y.K.; Saville, J.; Prescott, P.; von Elm, E.; Lewith, G.T. Complementary medicine use by men with prostate cancer: A systematic review of prevalence studies. Prostate Cancer Prostatic Dis 2011, 14, 1–13. [Google Scholar]
- Ponholzer, A.; Struhal, G.; Madersbacher, S. Frequent use of complementary medicine by prostate cancer patients. Eur. Urol 2003, 43, 604–608. [Google Scholar]
- Singh, H.; Maskarinec, G.; Shumay, D.M. Understanding the motivation for conventional and complementary/alternative medicine use among men with prostate cancer. Integr. Cancer Ther 2005, 4, 187–194. [Google Scholar]
- Chan, J.M.; Elkin, E.P.; Silva, S.J.; Broering, J.M.; Latini, D.M.; Carroll, P.R. Total and specific complementary and alternative medicine use in a large cohort of men with prostate cancer. Urology 2005, 66, 1223–1228. [Google Scholar]
- Engelmann, N.J.; Clinton, S.K.; Erdman, J.W., Jr. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv. Nutr. 2011, 2, 51–61. [Google Scholar]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Biotechnol 2000, 20, 293–334. [Google Scholar]
- Agarwal, S.; Rao, A.V. Carotenoids and chronic diseases. Drug Metab. Drug Interact 2000, 17, 189–210. [Google Scholar]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J 1995, 9, 1551–1558. [Google Scholar]
- Van Breemen, R.B.; Pajkovic, N. Multitargeted therapy of cancer by lycopene. Cancer Lett 2008, 269, 339–351. [Google Scholar]
- Clinton, S.K. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev 1998, 56, 35–51. [Google Scholar]
- Clinton, S.K.; Emenhiser, C.; Schwartz, S.J.; Bostwick, D.G.; Williams, A.W.; Moore, B.J.; Erdman, J.W., Jr. Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 823–833. [Google Scholar]
- Krinsky, N.I. The antioxidant and biological properties of the carotenoids. Ann. N.Y. Acad. Sci 1998, 854, 443–447. [Google Scholar]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys 1989, 274, 532–538. [Google Scholar]
- Chasse, G.A.; Mak, M.L.; Deretey, E.; Farkas, I.; Torday, L.L.; Papp, J.G.; Sarma, D.S.R.; Agarwal, A.; Chakravarthi, S.; Agarwal, S.; et al. An ab initio computational study on selected lycopene isomers. J. Mol. Struct. Theochem 2001, 571, 27–37. [Google Scholar]
- Sies, H.; Stahl, W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr 1995, 62, 1315S–1321S. [Google Scholar]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med 2003, 24, 345–351. [Google Scholar]
- Bast, A.; Haenen, G.R.; van den Berg, R.; van den Berg, H. Antioxidant effects of carotenoids. Int. J. Vitam. Nutr. Res 1998, 68, 399–403. [Google Scholar]
- Linnewiel, K.; Ernst, H.; Caris-Veyrat, C.; Ben-Dor, A.; Kampf, A.; Salman, H.; Danilenko, M.; Levy, J.; Sharoni, Y. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic. Biol. Med 2009, 47, 659–667. [Google Scholar]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp. Biol. Med 2002, 227, 845–851. [Google Scholar]
- Mangels, A.R.; Holden, J.M.; Beecher, G.R.; Forman, M.R.; Lanza, E. Carotenoid content of fruits and vegetables: An evaluation of analytic data. J. Am. Diet. Assoc 1993, 93, 284–296. [Google Scholar]
- Rao, A.V.; Ali, A. Biologically active phytochemicals in human health: Lycopene. Int. J. Food Prop 2007, 10, 279–288. [Google Scholar]
- Hart, D.J.; Scott, K.J. Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK. Food Chem 1995, 54, 101–111. [Google Scholar]
- Hallmann, E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types. J. Sci. Food Agric 2012, 92, 2840–2848. [Google Scholar]
- Rossi, F.; Godani, F.; Bertuzzi, T.; Trevisan, M.; Ferrari, F.; Gatti, S. Health-promoting substances and heavy metal content in tomatoes grown with different farming techniques. Eur. J. Nutr 2008, 47, 266–272. [Google Scholar]
- Caris-Veyrat, C.; Amiot, M.J.; Tyssandier, V.; Grasselly, D.; Buret, M.; Mikolajczak, M.; Guilland, J.C.; Bouteloup-Demange, C.; Borel, P. Influence of organic versus conventional agricultural practice on the antioxidant microconstituent content of tomatoes and derived purees; Consequences on antioxidant plasma status in humans. J. Agric. Food Chem 2004, 52, 6503–6509. [Google Scholar]
- Ordonez-Santos, L.; Vazquez-Oderiz, M.; Romero-Rodriguez, M. Micronutrient contents in organic and conventional tomatoes (Solanum lycopersicum L.). Int. J. Food Sci. Technol 2011, 46, 1561–1568. [Google Scholar]
- Tonucci, L.H.; Holden, J.M.; Beecher, G.R.; Khachik, F.; Davis, C.S.; Mulokozi, G. Carotenoid content of thermally processed tomato-based food-products. J. Agric. Food Chem 1995, 43, 579–586. [Google Scholar]
- Stahl, W.; Sies, H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J. Nutr 1992, 122, 2161–2166. [Google Scholar]
- Parker, R.S. Absorption, metabolism, and transport of carotenoids. FASEB J 1996, 10, 542–551. [Google Scholar]
- Rao, A.V.; Agarwal, S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutr. Res 1999, 19, 305–323. [Google Scholar]
- Gartner, C.; Stahl, W.; Sies, H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am. J. Clin. Nutr 1997, 66, 116–122. [Google Scholar]
- Agarwal, A.; Shen, H.; Agarwal, S.; Rao, A.V. Lycopene content of tomato products: Its stability, bioavailability and in vivo antioxidant porperties. J. Med. Food 2001, 4, 9–15. [Google Scholar]
- Hussein, L.; E.M. Vitamin—A potency of carrot and spinach carotenes in human metabolic studies. Int. J. Vitam. Nutr. Res 1990, 60, 229–235. [Google Scholar]
- Boileau, A.C.; Merchen, N.R.; Wasson, K.; Atkinson, C.A.; Erdman, J.W., Jr. Cis-lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets. J. Nutr. 1999, 129, 1176–1181. [Google Scholar]
- Boileau, T.W.M.; Boileau, A.C.; Erdman, J.W. Bioavailability of all-trans and cis-isomers of lycopene. Exp. Biol. Med 2002, 227, 914–919. [Google Scholar]
- Re, R.; Fraser, P.D.; Long, M.; Bramley, P.M.; Rice-Evans, C. Isomerization of lycopene in the gastric milieu. Biochem. Biophys. Res. Commun 2001, 281, 576–581. [Google Scholar]
- Sicilia, T.; Bub, A.; Rechkemmer, G.; Kraemer, K.; Hoppe, P.P.; Kulling, S.E. Novel lycopene metabolites are detectable in plasma of preruminant calves after lycopene supplementation. J. Nutr 2005, 135, 2616–2621. [Google Scholar]
- Honest, K.N.; Zhang, H.W.; Zhang, L.F. Lycopene: Isomerization effects on bioavailability and bioactivity properties. Food Rev. Int 2011, 27, 248–258. [Google Scholar]
- Hoppe, P.P.; Kramer, K.; van den Berg, H.; Steenge, G.; van Vliet, T. Synthetic and tomato-based lycopene have identical bioavailability in humans. Eur. J. Nutr 2003, 42, 272–278. [Google Scholar]
- Cohn, W.; Thurmann, P.; Tenter, U.; Aebischer, C.; Schierle, J.; Schalch, W. Comparative multiple dose plasma kinetics of lycopene administered in tomato juice, tomato soup or lycopene tablets. Eur. J. Nutr 2004, 43, 304–312. [Google Scholar]
- Tang, G.; Ferreira, A.L.; Grusak, M.A.; Qin, J.; Dolnikowski, G.G.; Russell, R.M.; Krinsky, N.I. Bioavailability of synthetic and biosynthetic deuterated lycopene in humans. J. Nutr. Biochem 2005, 16, 229–235. [Google Scholar]
- Wertz, K. Lycopene effects contributing to prostate health. Nutr. Cancer 2009, 61, 775–783. [Google Scholar]
- Pathak, S.K.; Sharma, R.A.; Steward, W.P.; Mellon, J.K.; Griffiths, T.R.; Gescher, A.J. Oxidative stress and cyclooxygenase activity in prostate carcinogenesis: Targets for chemopreventive strategies. Eur. J. Cancer 2005, 41, 61–70. [Google Scholar]
- Goo, Y.A.; Li, Z.; Pajkovic, N.; Shaffer, S.; Taylor, G.; Chen, J.; Campbell, D.; Arnstein, L.; Goodlett, D.R.; van Breemen, R.B. Systematic investigation of lycopene effects in LNCaP cells by use of novel large-scale proteomic analysis software. Proteom. Clin. Appl 2007, 1, 513–523. [Google Scholar]
- Qiu, X.; Yuan, Y.; Vaishnav, A.; Tessel, M.A.; Nonn, L.; van Breemen, R.B. Effects of lycopene on protein expression in human primary prostatic epithelial cells. Cancer Prev. Res. 2013, 6. [Google Scholar] [CrossRef]
- Matos, H.R.; di Mascio, P.; Medeiros, M.H. Protective effect of lycopene on lipid peroxidation and oxidative DNA damage in cell culture. Arch. Biochem. Biophys 2000, 383, 56–59. [Google Scholar]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar]
- Poirier, M.C.; Santella, R.M.; Weston, A. Carcinogen macromolecular adducts and their measurement. Carcinogenesis 2000, 21, 353–359. [Google Scholar]
- Hwang, E.S.; Bowen, P.E. Effects of lycopene and tomato paste extracts on DNA and lipid oxidation in LNCaP human prostate cancer cells. BioFactors 2005, 23, 97–105. [Google Scholar]
- Lowe, G.M.; Booth, L.A.; Young, A.J.; Bilton, R.F. Lycopene and β-carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly lose this capacity at higher doses. Free Radic. Res 1999, 30, 141–151. [Google Scholar]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr 2001, 131, 3303–3306. [Google Scholar]
- Hwang, E.S.; Bowen, P.E. Effects of tomato paste extracts on cell proliferation, cell-cycle arrest and apoptosis in LNCaP human prostate cancer cells. BioFactors 2005, 23, 75–84. [Google Scholar]
- Ivanov, N.I.; Cowell, S.P.; Brown, P.; Rennie, P.S.; Guns, E.S.; Cox, M.E. Lycopene differentially induces quiescence and apoptosis in androgen-responsive and -independent prostate cancer cell lines. Clin. Nutr 2007, 26, 252–263. [Google Scholar]
- Ford, N.A.; Elsen, A.C.; Zuniga, K.; Lindshield, B.L.; Erdman, J.W., Jr. Lycopene and apo-12′-lycopenal reduce cell proliferation and alter cell cycle progression in human prostate cancer cells. Nutr. Cancer 2011, 63, 256–263. [Google Scholar]
- Yang, C.M.; Lu, I.H.; Chen, H.Y.; Hu, M.L. Lycopene inhibits the proliferation of androgen-dependent human prostate tumor cells through activation of PPARγ-LXRα-ABCA1 pathway. J. Nutr. Biochem 2012, 23, 8–17. [Google Scholar]
- Mueller, E.; Smith, M.; Sarraf, P.; Kroll, T.; Aiyer, A.; Kaufman, D.S.; Oh, W.; Demetri, G.; Figg, W.D.; Zhou, X.P.; et al. Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc. Natl. Acad. Sci. USA 2000, 97, 10990–10995. [Google Scholar]
- Olefsky, J.M. Nuclear receptor minireview series. J. Biol. Chem 2001, 276, 36863–36864. [Google Scholar]
- Yang, C.M.; Lu, Y.L.; Chen, H.Y.; Hu, M.L. Lycopene and the LXRα agonist T0901317 synergistically inhibit the proliferation of androgen-independent prostate cancer cells via the PPARγ-LXRα-ABCA1 pathway. J. Nutr. Biochem 2012, 23, 1155–1162. [Google Scholar]
- Burgess, L.C.; Rice, E.; Fischer, T.; Seekins, J.R.; Burgess, T.P.; Sticka, S.J.; Klatt, K. Lycopene has limited effect on cell proliferation in only two of seven human cell lines (both cancerous and noncancerous) in an in vitro system with doses across the physiological range. Toxicol. In Vitro 2008, 22, 1297–1300. [Google Scholar]
- Liu, A.G.; Erdman, J.W., Jr. Lycopene and apo-10′-lycopenal do not alter DNA methylation of GSTP1 in LNCaP cells. Biochem. Biophys. Res. Commun. 2011, 412, 479–482. [Google Scholar]
- Diehl, J.A. Cycling to cancer with cyclin d1. Cancer Biol. Ther 2002, 1, 226–231. [Google Scholar]
- Palozza, P.; Colangelo, M.; Simone, R.; Catalano, A.; Boninsegna, A.; Lanza, P.; Monego, G.; Ranelletti, F.O. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis 2010, 31, 1813–1821. [Google Scholar]
- Hantz, H.L.; Young, L.F.; Martin, K.R. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp. Biol. Med 2005, 230, 171–179. [Google Scholar]
- Lim, M.L.; Lum, M.G.; Hansen, T.M.; Roucou, X.; Nagley, P. On the release of cytochrome c from mitochondria during cell death signaling. J. Biomed. Sci 2002, 9, 488–506. [Google Scholar]
- Kanagaraj, P.; Vijayababu, M.R.; Ravisankar, B.; Anbalagan, J.; Aruldhas, M.M.; Arunakaran, J. Effect of lycopene on insulin-like growth factor-I, IGF binding protein-3 and IGF type-I receptor in prostate cancer cells. J. Cancer Res. Clin. Oncol 2007, 133, 351–359. [Google Scholar]
- Teodoro, A.J.; Oliveira, F.L.; Martins, N.B.; Maia Gde, A.; Martucci, R.B.; Borojevic, R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Neil, B.; Chen, X. Effect of lycopene on androgen receptor and prostate-specific antigen velocity. Chin. Med. J 2010, 123, 2231–2236. [Google Scholar]
- Bureyko, T.; Hurdle, H.; Metcalfe, J.B.; Clandinin, M.T.; Mazurak, V.C. Reduced growth and integrin expression of prostate cells cultured with lycopene, Vitamin E and fish oil in vitro. Br. J. Nutr 2009, 101, 990–997. [Google Scholar]
- Tang, Y.; Parmakhtiar, B.; Simoneau, A.R.; Xie, J.; Fruehauf, J.; Lilly, M.; Zi, X. Lycopene enhances docetaxel’s effect in castration-resistant prostate cancer associated with insulin-like growth factor i receptor levels. Neoplasia 2011, 13, 108–119. [Google Scholar]
- Lin, C.Y.; Huang, C.S.; Hu, M.L. The use of fetal bovine serum as delivery vehicle to improve the uptake and stability of lycopene in cell culture studies. Br. J. Nutr 2007, 98, 226–232. [Google Scholar]
- Liu, A.; Pajkovic, N.; Pang, Y.; Zhu, D.W.; Calamini, B.; Mesecar, A.L.; van Breemen, R.B. Absorption and subcellular localization of lycopene in human prostate cancer cells. Mol. Cancer Ther 2006, 5, 2879–2885. [Google Scholar]
- O’Sullivan, S.M.; Woods, J.A.; O’Brien, N.M. Use of tween 40 and tween 80 to deliver a mixture of phytochemicals to human colonic adenocarcinoma cell (CaCo-2) monolayers. Br. J. Nutr 2004, 91, 757–764. [Google Scholar]
- Forssberg, A.; Lingen, C.; Ernster, L.; Lindberg, O. Modification of the X-irradiation syndrome by lycopene. Exp. Cell Res 1959, 16, 7–14. [Google Scholar]
- Lingen, C.; Ernster, L.; Lindberg, O. The promoting effect of lycopene on the non-specific resistance of animals. Exp. Cell Res 1959, 16, 384–393. [Google Scholar]
- Ilic, D.; Forbes, K.M.; Hassed, C. Lycopene for the prevention of prostate cancer. Cochrane Database Syst. Rev. 2011, 11. [Google Scholar] [CrossRef]
- Boileau, T.W.; Liao, Z.; Kim, S.; Lemeshow, S.; Erdman, J.W., Jr.; Clinton, S.K. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J. Natl. Cancer Inst 2003, 95, 1578–1586. [Google Scholar]
- Canene-Adams, K.; Lindshield, B.L.; Wang, S.; Jeffery, E.H.; Clinton, S.K.; Erdman, J.W., Jr. Combinations of tomato and broccoli enhance antitumor activity in dunning R3327-H prostate adenocarcinomas. Cancer Res. 2007, 67, 836–843. [Google Scholar]
- Guttenplan, J.B.; Chen, M.; Kosinska, W.; Thompson, S.; Zhao, Z.; Cohen, L.A. Effects of a lycopene-rich diet on spontaneous and benzo[a]pyrene-induced mutagenesis in prostate, colon and lungs of the lacZ mouse. Cancer Lett 2001, 164, 1–6. [Google Scholar]
- Imaida, K.; Tamano, S.; Kato, K.; Ikeda, Y.; Asamoto, M.; Takahashi, S.; Nir, Z.; Murakoshi, M.; Nishino, H.; Shirai, T. Lack of chemopreventive effects of lycopene and curcumin on experimental rat prostate carcinogenesis. Carcinogenesis 2001, 22, 467–472. [Google Scholar]
- Kavanaugh, C.J.; Trumbo, P.R.; Ellwood, K.C. The U.S. Food and drug administration’s evidence-based review for qualified health claims: Tomatoes, lycopene, and cancer. J. Natl. Cancer Inst 2007, 99, 1074–1085. [Google Scholar]
- Siler, U.; Barella, L.; Spitzer, V.; Schnorr, J.; Lein, M.; Goralczyk, R.; Wertz, K. Lycopene and vitamin E interfere with autocrine/paracrine loops in the dunning prostate cancer model. FASEB J 2004, 18, 1019–1021. [Google Scholar]
- Siler, U.; Herzog, A.; Spitzer, V.; Seifert, N.; Denelavas, A.; Hunziker, P.B.; Barella, L.; Hunziker, W.; Lein, M.; Goralczyk, R.; et al. Lycopene effects on rat normal prostate and prostate tumor tissue. J. Nutr 2005, 135, 2050S–2052S. [Google Scholar]
- Venkateswaran, V.; Fleshner, N.E.; Sugar, L.M.; Klotz, L.H. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res 2004, 64, 5891–5896. [Google Scholar]
- Yang, C.M.; Yen, Y.T.; Huang, C.S.; Hu, M.L. Growth inhibitory efficacy of lycopene and β-carotene against androgen-independent prostate tumor cells xenografted in nude mice. Mol. Nutr. Food Res 2011, 55, 606–612. [Google Scholar]
- Tang, L.; Jin, T.; Zeng, X.; Wang, J.S. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c nude mice. J. Nutr 2005, 135, 287–290. [Google Scholar]
- Konijeti, R.; Henning, S.; Moro, A.; Sheikh, A.; Elashoff, D.; Shapiro, A.; Ku, M.; Said, J.W.; Heber, D.; Cohen, P.; et al. Chemoprevention of prostate cancer with lycopene in the TRAMP model. Prostate 2010, 70, 1547–1554. [Google Scholar]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W., Jr. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar]
- Rackley, J.D.; Clark, P.E.; Hall, M.C. Complementary and alternative medicine for advanced prostate cancer. Urol. Clin. N. Am 2006, 33, 237–246. [Google Scholar]
- Etminan, M.; Takkouche, B.; Caamano-Isorna, F. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol. Biomark. Prev 2004, 13, 340–345. [Google Scholar]
- Kirsh, V.A.; Mayne, S.T.; Peters, U.; Chatterjee, N.; Leitzmann, M.F.; Dixon, L.B.; Urban, D.A.; Crawford, E.D.; Hayes, R.B. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol. Biomark. Prev 2006, 15, 92–98. [Google Scholar]
- Kristal, A.R.; Till, C.; Platz, E.A.; Song, X.; King, I.B.; Neuhouser, M.L.; Ambrosone, C.B.; Thompson, I.M. Serum lycopene concentration and prostate cancer risk: Results from the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev 2011, 20, 638–646. [Google Scholar]
- Peters, U.; Leitzmann, M.F.; Chatterjee, N.; Wang, Y.; Albanes, D.; Gelmann, E.P.; Friesen, M.D.; Riboli, E.; Hayes, R.B. Serum lycopene, other carotenoids, and prostate cancer risk: A nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol. Biomark. Prev 2007, 16, 962–968. [Google Scholar]
- Ilic, D.; Misso, M. Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: A systematic review. Maturitas 2012, 72, 269–276. [Google Scholar]
- Bunker, C.H.; McDonald, A.C.; Evans, R.W.; de la Rosa, N.; Boumosleh, J.M.; Patrick, A.L. A randomized trial of lycopene supplementation in tobago men with high prostate cancer risk. Nutr. Cancer 2007, 57, 130–137. [Google Scholar]
- Mohanty, N.K.; Saxena, S.; Singh, U.P.; Goyal, N.K.; Arora, R.P. Lycopene as a chemopreventive agent in the treatment of high-grade prostate intraepithelial neoplasia. Urol. Oncol 2005, 23, 383–385. [Google Scholar]
- Schwarz, S.; Obermuller-Jevic, U.C.; Hellmis, E.; Koch, W.; Jacobi, G.; Biesalski, H.K. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J. Nutr 2008, 138, 49–53. [Google Scholar]
- Van Breemen, R.B.; Sharifi, R.; Viana, M.; Pajkovic, N.; Zhu, D.; Yuan, L.; Yang, Y.; Bowen, P.E.; Stacewicz-Sapuntzakis, M. Antioxidant effects of lycopene in African American men with prostate cancer or benign prostate hyperplasia: A randomized, controlled trial. Cancer Prev. Res 2011, 4, 711–718. [Google Scholar]
- Ansari, M.S.; Gupta, N.P. A comparison of lycopene and orchidectomy vs. orchidectomy alone in the management of advanced prostate cancer. BJU Int 2003, 92, 375–378. [Google Scholar]
- Grainger, E.M.; Schwartz, S.J.; Wang, S.; Unlu, N.Z.; Boileau, T.W.; Ferketich, A.K.; Monk, J.P.; Gong, M.C.; Bahnson, R.R.; DeGroff, V.L.; et al. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr. Cancer 2008, 60, 145–154. [Google Scholar]
- Kucuk, O.; Sarkar, F.H.; Sakr, W.; Djuric, Z.; Pollak, M.N.; Khachik, F.; Li, Y.W.; Banerjee, M.; Grignon, D.; Bertram, J.S.; et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomark. Prev 2001, 10, 861–868. [Google Scholar]
- Vaishampayan, U.; Hussain, M.; Banerjee, M.; Seren, S.; Sarkar, F.H.; Fontana, J.; Forman, J.D.; Cher, M.L.; Powell, I.; Pontes, J.E.; et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr. Cancer 2007, 59, 1–7. [Google Scholar]
- Sporn, M.B.; Liby, K.T. Is lycopene an effective agent for preventing prostate cancer? Cancer Prev. Res 2013, 6, 384–386. [Google Scholar]

| Lycopene content [mg/100 g wet basis] | Source |
|---|---|
| 0.7–20 | Fresh tomatoes |
| 3.7 | Cooked tomatoes |
| 2.3–7.2 | Fresh watermelon |
| 2.0–5.3 | Fresh papaya |
| 5.2–5.5 | Pink guava |
| 0.4–3.4 | Pink grapefruit |
| 0.01–0.05 | Apricot |
| Lycopene content (mg/100 g) ± SD | Source |
|---|---|
| 9.27 ± 1.02 | Raw tomatoes |
| 17.23 ± 2.18 | Ketchup |
| 15.99 ± 0.90 | Spaghetti sauce |
| 55.45 ± 4.33 | Tomato paste |
| 16.67 | Tomato puree |
| 17.98 ± 1.47 | Tomato sauce |
| Author | Year | Strain | Tumour model | Lycopene formulation and source | Outcome |
|---|---|---|---|---|---|
| Imaida et al. [85] | 2001 | 6-week-old male F344 rats | Chemically induced with 3,2′-dimethyl-4-aminobiphenol | Lycopene purified from tomato extracts (99.9%, LycoRed™, Beer-Sheva, Israel) | Significantly decreased incidence of prostatic intraepithelial neoplasia and carcinoma of the ventral prostate |
| Guttenplan et al. [84] | 2001 | 6-week-old male lacZ mice | Chemically induced with benzo[a]pyrene | Suspension of 3.7% Lycopene, 0.3% phytofluene, 0.44% Z-carotene, 0.47% 2,6-cyclolycopene-1,5-diol and 1.2% β-carotene extracted from tomatoes (Cognis Corporation, LaGrange, IL, USA) | Carcinogen-induced mutagenesis in prostates from the lycopene treated groups was decreased compared to a control group without lycopene supplementation |
| Boileau et al. [82] | 2003 | 5-week-old male Wistar rats | Chemically induced with cyproterone, testosterone and N-methyl-N-nitrosourea | Beadlets of synthetic lycopene (Hoffman-La Roche, Basel, Switzerland) or powder derived from heat-processed tomato paste with skin and seeds (Armour/Del Monte Foods, San Francisco, CA, USA) | Tomato powder, but not synthetic lycopene, was able to increase prostate cancer-specific survival |
| iler et al. [87] | 2004, 2005 | 8–10-week-old male Copenhagen rats | Orthotopic implantation of MatLyLu cells into the ventral prostate | Beadlets of synthetic lycopene (Lycopene 5% TG, DSM Nutritional Products, Basel, Switzerland) | MRI revealed a significant increase of necrotic area in tumours of lycopene treated animals. Anti-androgen and anti-inflammatory effects on cancerous and healthy prostate tissue |
| Venkateswaran et al. [89] | 2004 | 4–5-week-old transgenic male Lady mice | Transgenic adenocarcinoma of the mouse prostate (TRAMP) model | Mixture of antioxidants containing 800 IU vitamin E, 200 μg Selenium and 50 mg lycopene (no information about the supplier or the formulation was given) | The mixture of micronutrients was able to inhibit prostate cancer development and to increase the disease-free survival of the animals. |
| Tang et al. [91] | 2005 | 4–6-week-old male BALB/c nude mice | Subcutaneous xenotransplantation of human prostate cancer cells (DU-145) along with Matrigel™ | >95% pure lycopene with 6% lycopene oleoresin purified from tomato extracts (no information about the supplier was given) | Decrease in tumour growth by 55.6% and 75.8% in mice treated with 100 mg and 300 mg lycopene, respectively |
| Canene-Adams et al. [83] | 2007 | 4-week-old male Copenhagen rats | Subcutaneous implantation of minced Dunning R3327-H tumour tissue suspended in Matrigel™ | Beadlets of synthetic lycopene (Lycopene 5% TG, DSM Nutritional Products, Basel, Switzerland) or powder derived from tomatoes (Gilroy Foods, Gilroy, CA, USA) | Synthetic lycopene insignificantly reduced tumour weight, whereas the reduction of tumour weight was significant for tomato powder. |
| Konijeti et al. [92] | 2010 | male transgenic TRAMP mice at weaning age | TRAMP model | Beadlets of synthetic lycopene (Lycopene 10%, DSM Nutritional Products, Parsippany, NJ, USA) or tomato paste without skin and seeds (Campbell’s Soup Company, Camden, NJ, USA) | Prostate cancer incidence was significantly decreased in the lycopene beadlet group, whereas the difference between the tomato paste group and the control group was not significant. |
| Tang et al. [75] | 2011 | NCR-nu/nu mice (no information about the age) | Subcutaneous xenotransplantation of human prostate cancer cells (DU-145) | Microencapsulated synthetic lycopene (LycoVit™ 10% CWD, BASF Corporation, Shreveport, LA, USA) alone or in combination with docetaxel i.p. | Synthetic lycopene alone had no significant effects on tumour regression or survival. Docetaxel plus lycopene led to a significant tumour regression and increase in survival when compared to docetaxel alone. |
| Yang et al. [90] | 2011 | 6–8-week-old male athymic nude mice | Subcutaneous xenotransplantation of human prostate cancer cells (PC-3) | Lycopene purified from tomato extracts (97%, Wako Pure Chemical Industries, Japan) or β-carotene | Both lycopene and β-carotene significantly decreased tumour volume and weight. |
| Author | Year | Patients | Follow-up | Lycopene formulation and source | Outcome |
|---|---|---|---|---|---|
| Mohanty et al. [101] | 2005 | 40 patients with high-grade intraepithelial neoplasia (intervention group: 20, control group: 20) | 2 years | 4 mg lycopene (Lyc-O-Mato™, LycoRed Natural Products Industries, Ltd., Beer-Sheva, Israel) twice a day for one year; Lyc-O-Mato™ is a tomato-oleoresin extracted from tomatoes, which contains a high amount of lycopene as well as other natural tomato phytonutrients, such as tocopherols, phytoene, phytofluene, β-carotene, phospholipids and phytosterols. | The incidence of prostate cancer was 10% in the intervention and 30% in the control group. PSA levels in the lycopene group decreased from a mean level of 6.07 to 3.5 ng/mL and increased in the control group from 6.55 to 8.06 ng/mL. Accordingly, the serum lycopene increased in the treatment group from 360 to 680 ng/mL and decreased in the control group from 378 to 180 ng/mL. |
| Bunker et al. [100] | 2007 | 80 patients with high-grade intraepithelial neoplasia, atypical foci or more than one non-cancerous biopsy (intervention group: 40, control group: 40; both groups were treated with a multivitamin mixture during the study) | 4 months | 15 mg lycopene (Lyc-O-Mato™, LycoRed Natural Products Industries, Ltd., Beer-Sheva, Israel) twice a day for the duration of the follow-up | No difference in PSA levels between groups were reported, neither at one month, nor four months after initiation of the intervention. Serum lycopene levels doubled in the intervention group. Incidence of prostate cancer was not reported. |
| Schwarz et al. [102] | 2008 | 40 patients with histologically proven benign prostate hyperplasia (intervention group: 20, control group: 20) | 6 months | 15 mg microencapsulated synthetic lycopene (LycoVit™ 10%, BASF, Ludwigshafen, Germany) once a day for the duration of the follow-up | Significant reduction of PSA levels in the intervention group, but not in the control group (p < 0.05). Accordingly, serum lycopene was increased in the intervention, but not in the control group (p < 0.0001). Prostate enlargement could be detected in the control group via trans-urethral ultrasonography (p < 0.05) and digital rectal examination (p < 0.01), whereas the prostate did not enlarge in the intervention group. Incidence of prostate cancer was not reported. |
| Van Breemen et al. [103] | 2011 | 131 patients scheduled for prostate cancer biopsy as a result of elevated PSA levels and an abnormal digital rectal examination or ultrasonography (intervention group: 69, control group 62) | 21 days | 15 mg lycopene (Lyc-O-Mato™, LycoRed Natural Products Industries, Ltd., Beer-Sheva, Israel) twice a day for 21 days | Significant increase of lycopene levels in the serum (p < 0.0001) and prostate tissue (p = 0.005) in the intervention group. No significant changes in the DNA oxidation product 8-oxo-desoxyguanosine and the lipid peroxidation product, malondialdehyde, in prostate tissue or plasma. No significant difference in prostate cancer incidence. |
| Author | Year | Patients | Follow-up | Lycopene formulation and source | Outcome |
|---|---|---|---|---|---|
| Kucuk et al. [106] | 2001 | 26 patients with clinically diagnosed prostate cancer prior to scheduled prostatectomy (intervention group: 15, control group: 11) | 3 weeks | 15 mg lycopene (Lyc-O-Mato™, LycoRed Natural Products Industries, Ltd., Beer-Sheva, Israel) once a day for 3 weeks | PSA serum levels decreased by 18% in the intervention group and increased by 14% in the control group (p = 0.25). 73% of the intervention and 18% of the control group had microscopically free resection margins (p = 0.02). 84% of the intervention and 45% of the control group had tumour volumes >4 mL (p = 0.222). |
| Ansari et Gupta [104] | 2003 | 54 patients with prostate cancer metastases and orchidectomy (intervention group: 27, control group: 27) | 24–28 months | 2 mg lycopene twice a day (no information about the supplier or the formulation) | After 2 years, the PSA serum levels were significantly lower in the intervention group (p < 0.001). The survival rate measured by the Kaplan-Meier method was significantly higher in the intervention group (p < 0.001). There was a significantly improvement in urinary peak flow rate in the lycopene group (p = 0.04) |
| Vaishampayan et al. [107] | 2007 | 71 patients with histologically proven prostate cancer and rising serum PSA levels following local therapy or during hormone therapy (intervention group: 38, control group: 33) | 5.5–6 months | 15 mg lycopene (Lyc-O-Mato™, LycoRed Natural Products Industries, Ltd., Beer-Sheva, Israel) twice a day for 6 months in the intervention group. The control group was treated with the same dosage of lycopene and soy isoflavone 40 mg twice a day for 5.5 months. | No decline in serum PSA level in either group. However, in both therapeutic arms, there was a significant decline in the rate of PSA increase from pre-therapy to post-therapy. |
| Grainger et al. [105] | 2008 | 41 patients with recurrent prostate cancer (intervention group: 20, control group: 21) | 8 weeks | The diet in the intervention group consisted of tomato and/or tomato products containing at least 25 mg lycopene per day given for 4 weeks. The control group was treated with soy protein. After 4 weeks, both groups were treated with lycopene 25 mg and soy protein for another 4 weeks. | Serum lycopene levels increased significantly in both groups after 8 weeks of dietary intervention. There was no statistically significant difference in PSA serum levels between the groups. |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The Potential Role of Lycopene for the Prevention and Therapy of Prostate Cancer: From Molecular Mechanisms to Clinical Evidence. Int. J. Mol. Sci. 2013, 14, 14620-14646. https://doi.org/10.3390/ijms140714620
Holzapfel NP, Holzapfel BM, Champ S, Feldthusen J, Clements J, Hutmacher DW. The Potential Role of Lycopene for the Prevention and Therapy of Prostate Cancer: From Molecular Mechanisms to Clinical Evidence. International Journal of Molecular Sciences. 2013; 14(7):14620-14646. https://doi.org/10.3390/ijms140714620
Chicago/Turabian StyleHolzapfel, Nina Pauline, Boris Michael Holzapfel, Simon Champ, Jesper Feldthusen, Judith Clements, and Dietmar Werner Hutmacher. 2013. "The Potential Role of Lycopene for the Prevention and Therapy of Prostate Cancer: From Molecular Mechanisms to Clinical Evidence" International Journal of Molecular Sciences 14, no. 7: 14620-14646. https://doi.org/10.3390/ijms140714620






